Abstract
The aim of this study was to explore the incidence and risk factors for posterior cage migration (PCM) following decompression and instrumented fusion for degenerative lumbar disorders, and hope to provide references in decision making and surgical planning for spine surgeons.
By retrieving the medical records from January 2011 to December 2015, 286 patients were retrospectively reviewed. According to the occurrence of PCM, patients were divided into 2 groups: PCM group and non-PCM (N-PCM). To investigate risk values for PCM, 3 categorized factors were analyzed statistically: patient characteristics: age, sex, body mass index, bone mineral density, duration of disease, diagnosis, comorbidity, smoke; surgical variables: surgery time, blood loss, surgical strategy, cage morphology, cage size, surgical segment, fusion number, source of bone graft, surgeon experience; radiographic parameters: preoperative lumbar lordosis, correction of lumbar lordosis, preoperative lumbar mobility, preoperative intervertebral height, change of intervertebral height, Modic changes, paraspinal muscle degeneration.
PCM was detected in 18 of 286 patients (6.3%) at follow-up. There was no statistically significant difference between the 2 groups in patient characteristics, except diagnosis, as lumbar spondylolisthesis was more prevalent in PCM group than that in N-PCM group. There was no difference between the 2 groups in surgical variables, except cage size and surgeon experience, as size of cage was smaller in PCM group than that in N-PCM group, and the surgeons with less experience (less than 3 years) were more prevalent in PCM group than that in N-PCM group. There was no statistically significant difference between 2 groups in radiographic parameters. Logistic regression model revealed that less than 3 years of surgeons’ experience, small cage size, and lumbar spondylolisthesis were independently associated with PCM.
For patients with lumbar spondylolisthesis, they should be fully informed about the risk of PCM before operation. While for spinal surgeons, large cage should be preferred, and careful manipulation should be adopted, especially for new learners with less than 3-year experience of fusion surgery.
Keywords: cage migration, degenerative lumbar disorder, spondylolisthesis
1. Introduction
Application of intervertebral fusion cages in the surgical treatment of degenerative lumbar disorders has many advantages, primarily involving improved spinal stability, extensive nerve root decompression, high fusion rate, restoration of disc space height.[1,2] Deng et al[3] indicated that cages could promote effective clinical and radiographic outcomes when used for degenerative lumbar diseases. Zhong et al[4] found that the interbody fusion cages can better preserve the intervertebral space and the foramen height than the autologous bone graft. However, intraoperative and postoperative complications are still encountered, as cage migration into the adjacent vertebral bodies and posterior dislocation into the spinal canal are well-known complications.[5–9] Aoki et al[10] followed up 125 patients with TLIF and reported that the incidence of cage migration was 3.2%. Although the incidence is not very high, the adverse effects cannot be underestimated. Cage migration might cause the loss of lumbar lordosis (LL), a narrowing of the disc space and foraminal, a lower fusion rate, and even directly compress the dural sac and nerve root, as well as progressive neurological deterioration. In the event of a cage migration, revision surgery, which is technically challenging, is frequently required for patients with neurological symptoms, while the revision outcome is varied.[5,6,11]
Although previous reports have alerted spine surgeons about the cage migration subsequent to lumbar decompression and interbody fusion, very few published reports describing specific risk factors that need to be taken into account to avoid this undesirable event. The aim of this study was therefore to explore the incidence and possible risk factors for posterior cage migration (PCM) following decompression and instrumented fusion for degenerative lumbar disorders, and hope to provide references in decision making and surgical planning for spine surgeons.
2. Methods
2.1. Subjects
A retrospective study was conducted; it was approved by the Institutional Review Board of the Third Hospital of HeBei Medical University before data collection and analysis. The inclusion criteria were diagnosis of lumbar disc herniation (LDH), lumbar spinal stenosis (LSS), and lumbar spondylolisthesis; surgical strategy including posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF); and complete radiological data, including lumbar antero-posterior (A/P) and lateral X-ray at preoperation, early postoperation, and final follow-up, computed tomography, or magnetic resonance imaging at preoperative period and final follow-up. The exclusion criteria were nondegenerative disorders, such as trauma, tumor, infection, inflammation, or isthmic spondylolisthesis; and patients treated by anterior lumbar fusion surgery, minimally invasive lumbar fusion surgery, discectomy, and laminectomy.
By retrieving the medical records from January 2011 to December 2015, 286 patients who met both the inclusion and exclusion criteria were retrospectively reviewed: 156 female and 130 male, with a mean age of 45.2 ± 5.5 years (range from 34 to 68 years). There were 162 cases diagnosed as LDH, 76 cases as LSS, and 48 cases as spondylolisthesis. One hundred sixty-one cases undertook PLIF (108 of them received 1-level PLIF and 53 patients received 2-level PLIF) and 125 cases undertook TLIF (86 of them received 1-level TLIF and 39 patients received 2-level TLIF).
2.2. Clinical and radiological evaluation
Data measurements were done 3 times with 200% magnification for accuracy by the first author, and the mean value was used for analysis. LL was measured from T12 inferior endplate to S1 superior endplate by the Cobb method on lateral X-ray (Fig. 1). Lumbar mobility was calculated as the change of LL between flexion-extension lateral radiographs (Fig. 2). Fatty infiltration rate (FIR) of paraspinal muscles was calculated by subtracting the muscle without the fat value from the total multifidus and erector spinae muscle value in the image processing software (Image J, version 1.48; National Institutes of Health) (Fig. 3). The Modic changes included type I changes that consist of reduced signal intensity (SI) in the vertebral end-plates on T1- and increased SI on T2-weighted sequences. Type II changes consist of increased SI on T1- and either increased SI or isointensity on T2-weighted sequences. Type III changes consist of reduced SI on both T1- and T2-weighted sequences due to subchondral sclerosis (Fig. 4).
Figure 1.
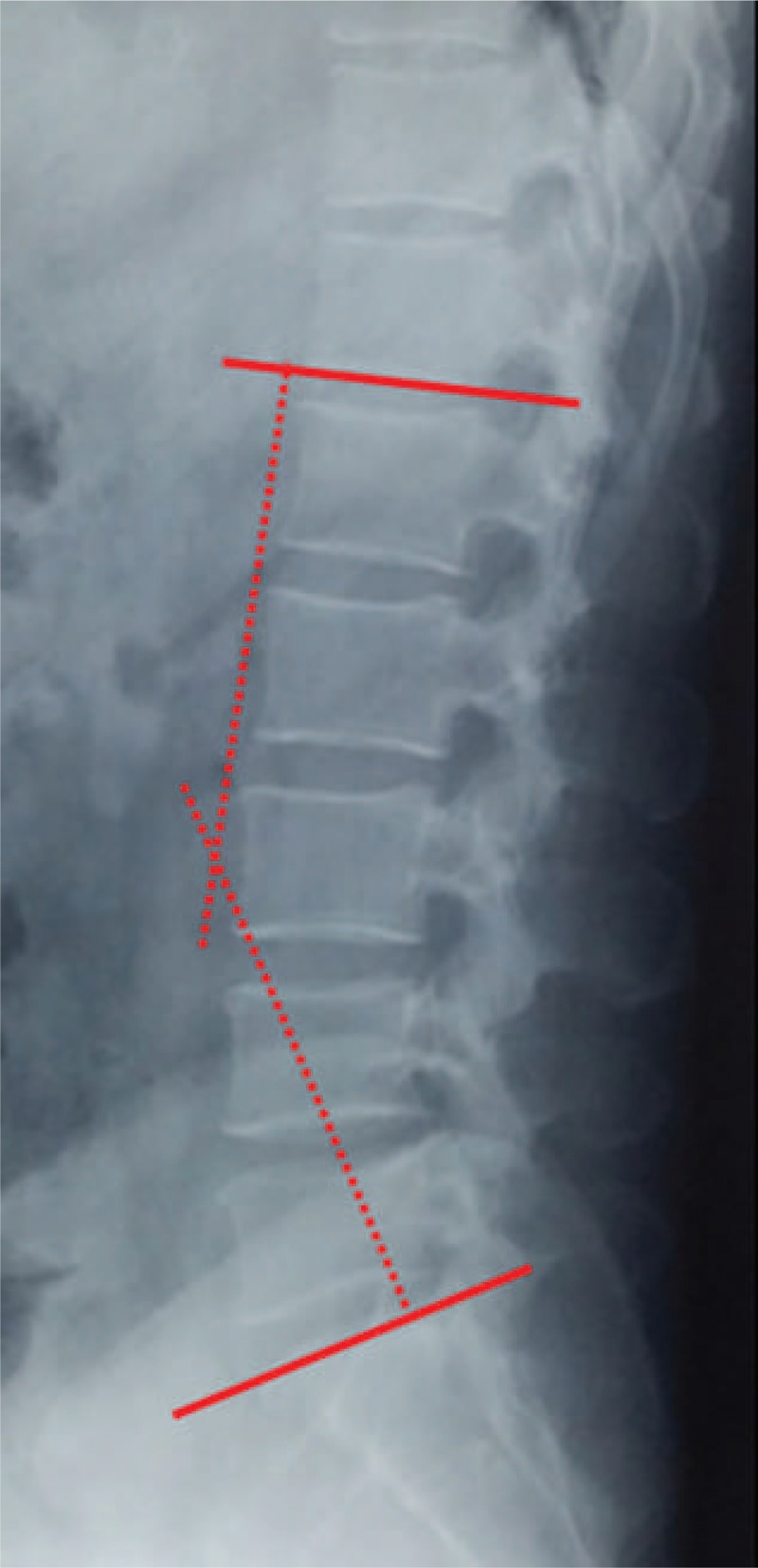
Lumbar lordosis was measured by the Cobb method.
Figure 2.
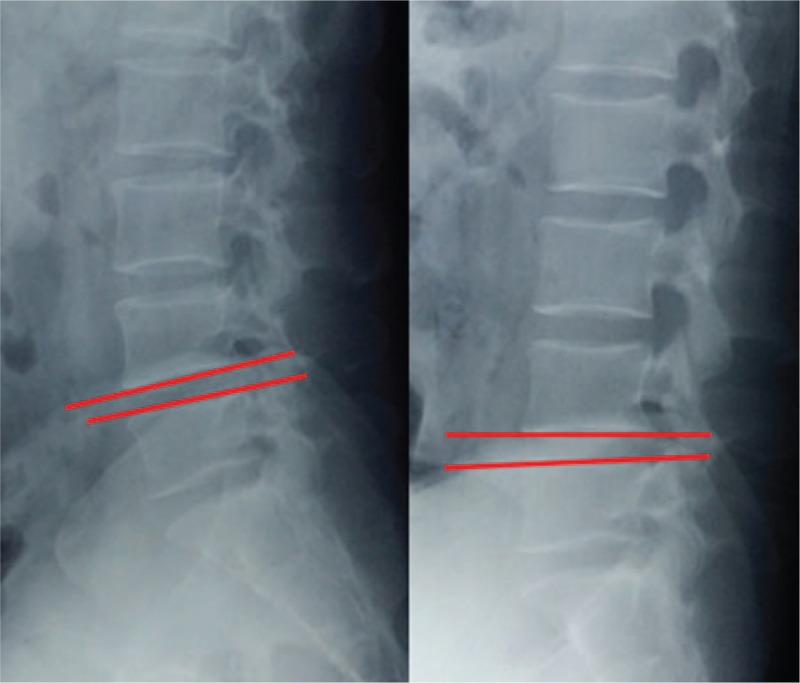
Lumbar mobility was calculated as the difference of LL on flexion and extension lateral radiographs.
Figure 3.

The method to evaluate fatty infiltration rate (FIR) of paraspinal muscles (multifidus and erector spinae).
Figure 4.
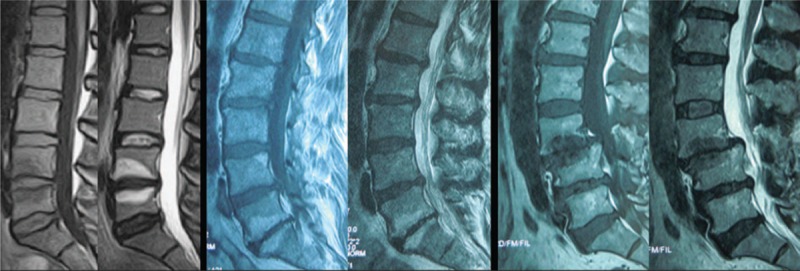
Modic changes were characterized on magnetic resonance imaging.
PCM was defined as posterior movement of the cage past the posterior wall of the vertebral body. According to the occurrence of PCM at follow-up, patients were divided into 2 groups: PCM group and non-PCM (N-PCM) group. To investigate risk values for the occurrence of PCM, 3 categorized factors were analyzed statistically: patient characteristics: age, gender, body mass index (BMI), bone mineral density (BMD), duration of disease (from first symptoms to operation), diagnosis, comorbidity (hypertension, diabetes, rheumatism, heart disease), smoke (yes vs no). Surgical variables: surgical strategy (TLIF vs PLIF), cage morphology (bullet-shaped vs box-shaped), size of cage, surgical segment, the number of fusion levels, source of bone graft (autogenous vs allograft bone), surgeon experience (>3 vs <3 years), surgery time, blood loss. Radiographic parameters: preoperative LL, correction of LL, preoperative lumbar mobility, preoperative intervertebral height, change of intervertebral height, Modic changes, preoperative paraspinal muscle degeneration (FIR).
2.3. Statistical analysis
All the data were analyzed using Statistical Product and Service Solutions software (version 13; SPSS, Chicago, IL). The continuous variables were expressed as mean ± standard deviation; the categorical variables were measured as frequency or percentages. An independent t test was performed for the analysis of the difference in continuous variables. A χ2 analysis or Fisher exact test was used to examine the differences among categorical variables. Variables with P values < .05 in the univariate analyses were entered into a multivariate logistic regression model.
3. Results
PCM was developed in 18 of 286 patients (6.3%) at follow-up and were enrolled as the PCM group; the follow-up duration from surgery to this complication range from 2 to 7 months (mean 4.3 months). Three patients experienced <6 mm PCM, 12 patients experienced 6 to 10 mm PCM, and 3 patients experienced >10 mm PCM.
There was no statistically significant difference between the 2 groups in age at operation, gender, BMI, BMD, duration, comorbidity, and smoke. The lumbar spondylolisthesis was more prevalent in PCM group than that in N-PCM group (Table 1). There was no difference between the 2 groups in surgery time, blood loss, surgical strategy, cage morphology, surgical segment, fusion number, or source of bone graft. The size of cage was smaller in PCM group than that in N-PCM group, and the surgeons with less experience (less than 3 years) were more prevalent in PCM group than that in N-PCM group (Table 2). There was no statistically significant difference between the 2 groups in preoperative LL, correction of LL, preoperative lumbar mobility, preoperative intervertebral height, change of intervertebral height, or Modic changes. The FIR was larger in PCM group than that in N-PCM group (Table 3).
Table 1.
Comparison of patient characteristics between PCM group and N-PCM group.
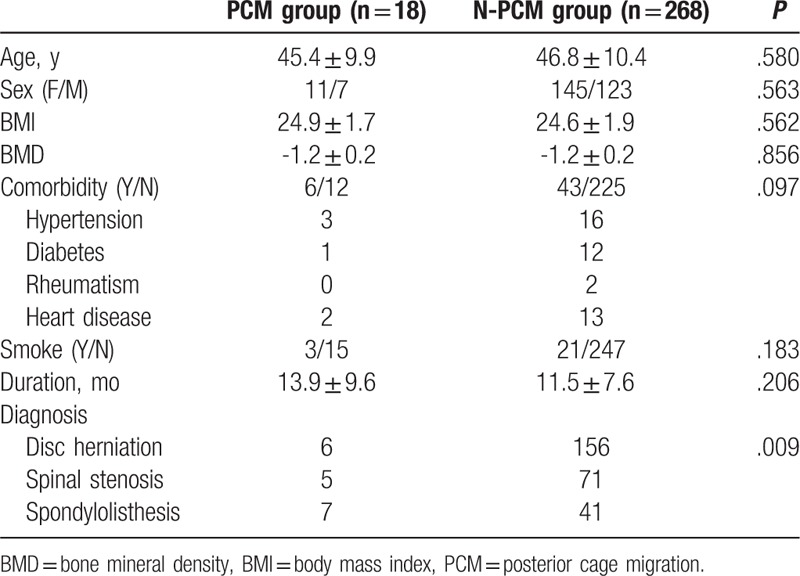
Table 2.
Comparison of surgical variables between PCM group and N-PCM group.
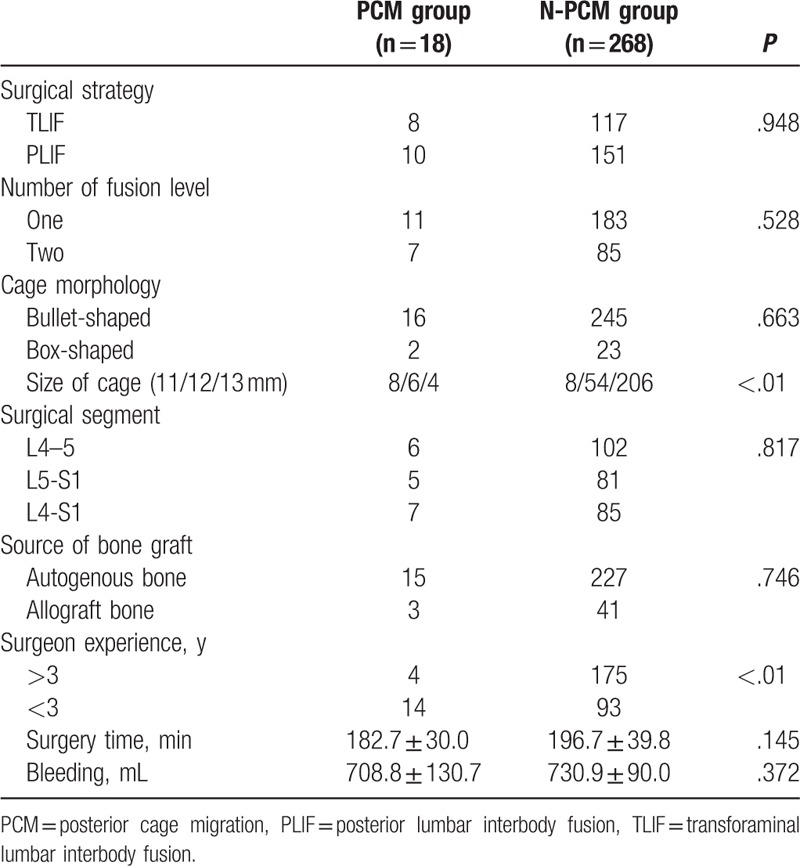
Table 3.
Comparison of radiographic parameters between PCM group and N-PCM group.
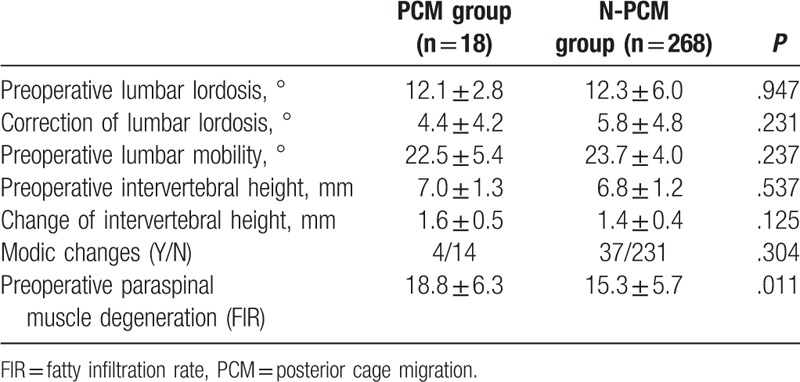
The following variables were entered into the multivariate model: age, gender, BMI, BMD, duration, diagnosis, comorbidity, smoke, surgery time, blood loss, surgical strategy, cage size, cage morphology, surgical segment, fusion number, source of bone graft, surgeon experience, preoperative LL, correction of LL, preoperative lumbar mobility, preoperative intervertebral height, change of intervertebral height, Modic changes, and FIR. Multivariate logistic regression model revealed that, less than 3 years of surgeon experience, small cage size, and lumbar spondylolisthesis were independently associated with cage migration (Table 4).
Table 4.
Risk factors for PCM, identified by logistic regression analysis.
4. Discussion
In the current study, 6.3% of the patients (18/286) experienced PCM. Instead, we found that less than 3-year surgeon experience and lumbar spondylolisthesis were significantly and independently associated with the PCM. This finding could be assessed before surgery. Moreover, small cage size was also associated with the occurrence of cage migration, and these results were not confounded by other variables that potentially affect PCM.
Spine surgeon experience of decompression and instrumented fusion for degenerative lumbar disorders is significantly associated with PCM, as new learners are responsible for the high incidence of PCM in the current study, and we suppose that there are 2 possible explanations may account for it. First, cage packed with cancellous bone is usually inserted into the disc space after meticulous endplate decortication.[12] The importance of preserving vertebral bone endplates to prevent cage migration has been emphasized by several authors.[13,14] Endplate preparation before fusion is a very important factor and a high demand technique: too little abrasion could prejudice fusion of the interface between cage-bone and endplates, whereas too much abrasion might damage the bony endplate and result in cage subsidence or posterior migration.[13] For new learners, appropriate preparation of the endplate needs guidance and practice. Second, care must be taken during cage insertion to decrease the possibility of cage migration. When the surgically treated segment presents bone hyperplasia of posterior vertebrae, removing the hyperplastic bone to provide space for cage insertion may be the priority for most new learners. While for experienced spinal surgeons, the cage should be inserted with the disc space distracted, using a lamina spreading device to avoid destruction of the posterior wall of the vertebral body. Moreover, when the decompression and instrumented fusion are finished, adequate compression needs to be applied by the pedicle screws to prevent PCM.[12]
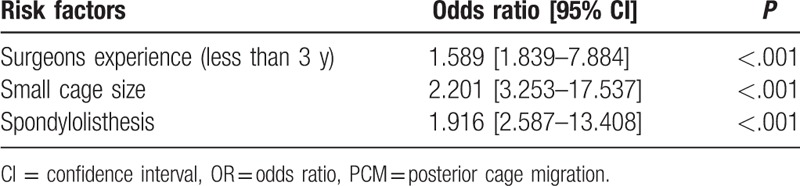
It has been shown that smaller cage size is correlated with migration and that larger size cages are recommended to increase the contact area between the cage and endplate to increase the failure load.[15,16] In the current study, we also found that smaller cage size is a risk factor for PCM, consistent with previous reports[15] (Fig. 5). Intervertebral space collapse, facet joint degeneration, nerve root, and dural sac compression are main radiological manifestations in patients with degenerative lumbar disorders. The purpose of the surgical intervention for these patients is not only the nervous decompression but also the restoration of the intervertebral height, which can be achieved by both the pedicle screw-rod distraction and anterior reconstruction of cage. Postoperatively, the vertical transmission of gravity through functional spinal unit contains anterior vertebrae-cage-vertebrae and posterior pedicle screw-rod. Pedicle screw-rod compression should be prohibited due to the potential risk of iatrogenic foraminal stenosis and nerve root compression; if smaller cage is used, the posterior structures may bear more gravity force and anterior part may bear less, respectively. According to the Woff theory, the compressive stress may promote bone fusion, while the tensile stress inhibits bone fusion. As the endplate-cage-endplate stress transmission decrease could influence the intervertebral fusion, the formation of pseudarthrosis around the cage is inevitable, which may lead to PCM finally. Moreover, Kimura et al[16] recommend that undersized cages should not be used at all.
Figure 5.
(A–D) Preoperative anterioposterior X-ray and MRI show L4 spondylolisthesis, with compression of spinal cord. (E, F) Postoperatively, PLIF was performed at L4-L5. (G) Three months later, the lateral X-ray showed cage migration posteriorly with appropritate 16 mm.
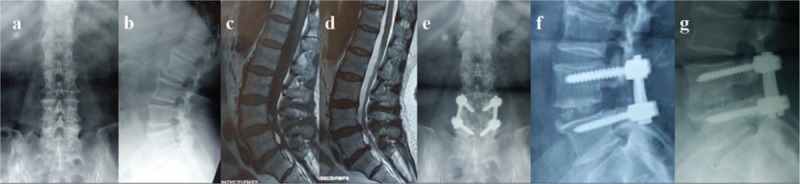
In the current study, lumbar spondylolisthesis was found to be an independent risk factor for PCM, which has never been addressed in the previous reports (Fig. 6). Spondylolisthesis differs from disc herniation in etiology, pathogenesis, imaging characteristics, clinical presentation, and treatment principle,[17] which may lead to the high incidence of cage migration. First, endplate sclerosis is common in spondylolisthesis, and is unfavorable to fusion between cage and vertebrae endplates due to the poor blood supply and osteoblast activity. Second, partial reduction, instead of complete reduction of the anterior dislocated vertebral body, is performed in most of the spondylolisthesis patients; this may decrease the interface between cage and endplates. Third, the decompression range for spondylolisthesis always includes laminar and bilateral articular process; both the exiting nerve roots and traversing nerve roots need to be exposed, especially for isthmic spondylolysis. Total facetectomy for decompression and cage insertion purposes always leads to destabilization, which might contribute to the probability of cage migration.[15] Fourth, decreased intervertebral height is another radiological feature in spondylolisthesis, and it is not easy to insert the cage into narrowed intervertebral space; although small size of cage may be preferred, this may increase the possibility of potential risk of cage migration as mentioned above. However, spondylolisthesis patients should not be excluded from the benefit of surgery, given that their postoperative neurological improvement and pain relief is encouraging. On the basis of above consideration, complete reduction of spondylolisthesis and use of large cage may be of some help to reduce the risk of postoperative cage migration, and should be strived as much as possible.
Figure 6.
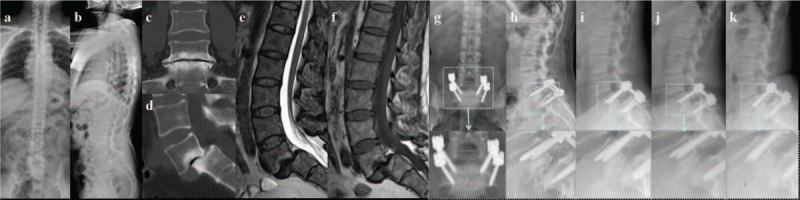
(A–F) Preoperative anterioposterior X-ray, CT, and MRI show L5 spondylolisthesis, with compression of spinal cord. (G, H) Postoperatively, PLIF was performed at L5-S1. (I) Three months later, the lateral X-ray showed cage migration posteriorly of 5 mm. (J) Six months later, the lateral X-ray showed cage migration posteriorly, without significant change when compared with postoperative condition. (K) Twelve months later, the lateral X-ray showed cage migration posteriorly, without significant change when compared with postoperative condition.
There are several potential limitations in this study. First, the number of patients is relatively small, and the study may be underpowered to detect the significance of some risk factors. Second, the study was conducted retrospectively by case selection, and was not randomized and controlled. Even with these issues in this study, we find that less than 3 years of surgeons experience, small cage size, and lumbar spondylolisthesis are risk factors for the PCM. For patients with lumbar spondylolisthesis, they should be fully informed about the risk of PCM before operation. While for spinal surgeons, large cage should be preferred, and careful manipulation should be adopted, especially for new learners with less than 3-year experience of fusion surgery.
Footnotes
Abbreviations: BMD = bone mineral density, BMI = body mass index, FIR = fatty infiltration rate, LDH = lumbar disc herniation, LL = lumbar lordosis, LSS = lumbar spinal stenosis, OR = odds ratio, PCM = posterior cage migration, PLIF = posterior lumbar interbody fusion, TLIF = transforaminal lumbar interbody fusion.
HL, HW, and YZ served as equal contributors.
The authors report no conflicts of interest.
References
- [1].Madera M, Brady J, Deily S, et al. The role of physical therapy and rehabilitation after lumbar fusion surgery for degenerative disease: a systematic review. J Neurosurg Spine 2017;10:1–1. [DOI] [PubMed] [Google Scholar]
- [2].Wang H, Ma L, Yang D, et al. Incidence and risk factors of adjacent segment disease following posterior decompression and instrumented fusion for degenerative lumbar disorders. Medicine (Baltimore) 2017;96:e6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deng QX, Ou YS, Zhu Y, et al. Clinical outcomes of two types of cages used in transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases: n-HA/PA66 cages versus PEEK cages. J Mater Sci Mater Med 2016;27:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhong HZ, Tian DS, Zhou Y, et al. Comparing the early efficacies of autologous bone grafting and interbody fusion cages for treating degenerative lumbar instability in patients of different ages. Int Orthop 2016;40:1211–8. [DOI] [PubMed] [Google Scholar]
- [5].Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine (Phila Pa 1976) 2005;30:2171–5. [DOI] [PubMed] [Google Scholar]
- [6].Corniola MV, Jägersberg M, Stienen MN, et al. Complete cage migration/subsidence into the adjacent vertebral body after posterior lumbar interbody fusion. J Clin Neurosci 2015;22:597–8. [DOI] [PubMed] [Google Scholar]
- [7].Xiao SW, Jiang H, Yang LJ, et al. Comparison of unilateral versus bilateral pedicle screw fixation with cage fusion in degenerative lumbar diseases: a meta-analysis. Eur Spine J 2015;24:764–74. [DOI] [PubMed] [Google Scholar]
- [8].Pan FM, Wang SJ, Yong ZY, et al. Risk factors for cage retropulsion after lumbar interbody fusion surgery: series of cases and literature review. Int J Surg 2016;30:56–62. [DOI] [PubMed] [Google Scholar]
- [9].Nikhil NJ, Lim JW, Yeo W, et al. Elderly patients achieving clinical and radiological outcomes comparable with those of younger patients following minimally invasive transforaminal lumbar interbody fusion. Asian Spine J 2017;11:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aoki Y, Yamagata M, Nakajima F, et al. Examining risk factors for posterior migration of fusion cages following transforaminal lumbar interbody fusion: a possible limitation of unilateral pedicle screw fixation. J Neurosurg Spine 2010;13:381–7. [DOI] [PubMed] [Google Scholar]
- [11].Kim MH, Kim SW, Kim SH, Kim HS. Extraforaminal lumbar interbody fusion for cage migration after screw removal: a case report. Korean J Spine 2013;10:104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aoki Y, Yamagata M, Nakajima F, et al. Posterior migration of fusion cages in degenerative lumbar disease treated with transforaminal lumbar interbody fusion: a report of three patients. Spine (Phila Pa 1976) 2009;34:E54–8. [DOI] [PubMed] [Google Scholar]
- [13].Zhao FD, Yang W, Shan Z, et al. Cage migration after transforaminal lumbar interbody fusion and factors related to it. Orthop Surg 2012;4:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim TH, Kwon H, Jeon CH, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine (Phila Pa 1976) 2001;26:951–6. [DOI] [PubMed] [Google Scholar]
- [15].Abbushi A, Cabraja M, Thomale UW, et al. The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J 2009;18:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kimura H, Shikata J, Odate S. Risk factors for cage retropulsion after posterior lumbar interbody fusion: analysis of 1,070 cases. Spine (Phila Pa 1976) 2012;37:1164–9. [DOI] [PubMed] [Google Scholar]
- [17].Zhong ZM, Deviren V, Tay B, et al. Adjacent segment disease after instrumented fusion for adult lumbar spondylolisthesis: incidence and risk factors. Clin Neurol Neurosurg 2017;156:29–34. [DOI] [PubMed] [Google Scholar]


