Abstract
The aim of this study was to evaluate prognostic values of ambulatory blood pressure (ABP) and clinic blood pressure (CBP) in diabetic patients with hypertension. A total of 450 diabetic hypertensive patients without established cardiovascular diseases were enrolled and 416 patients who had finished 12months’ follow-up were included in final analysis. Baseline data were collected and Cox proportional hazards regression analysis was used to evaluate prognostic value of ABP and CBP. Compared to those without study endpoints (n = 370), those experienced study endpoints (n = 46) were more elderly and more likely to be male, and had longer hypertension duration (7.0 ± 3.0 years vs. 6.4 ± 2.1 years, P < .05). No significant between-group differences in CBP indices were observed. However, those with study endpoints had significantly higher 24-hour systolic BP (SBP) (134 ± 10 mmHg vs. 128 ± 7 mmHg), nighttime SBP (130 ± 7 mmHg vs. 120 ± 5 mmHg), night/day SBP ratio (0.97 ± 0.09 vs. 0.94 ± 0.08), higher proportion of non-dipping BP pattern (39.1% vs. 31.4%) and higher 24-hour SBP variability. After extensively adjusted for traditional risk factors, nondipping BP pattern and 24-hourSBP, only 24-hour SBP and nighttime SBP remained independently related with cardiovascular outcomes, with hazard ratios and associated 95% confidence interval as 1.53 (1.28–2.03) and 1.50 (1.26–1.89), respectively. Although no independent relationship between BP pattern and cardiovascular outcomes was observed. In summary, in diabetic hypertensive patients without established cardiovascular diseases, baseline 24-hour SBP and nighttime SBP are useful markers for predicting short-term cardiovascular outcomes.
Keywords: ambulatory blood pressure, cardiovascular outcomes, diabetes, hypertension
1. Introduction
Hypertension is a major public health problem around the world owing to its high prevalence and association with a variety of vascular diseases such as heart failure, myocardial infarction, and ischemic stroke.[1,2] Most of previous studies used clinic blood pressure (CBP) to evaluate the association between BP and cardiovascular and renal outcomes.[3–6] In recent 2 decades, accumulating evidence has consistently shown that ambulatory blood pressure (ABP) is superior to CBP in relation to cardiovascular events.[7,8] The underlying mechanisms may be partly attributed to the more accuracy and comprehensiveness of BP measurement by 24-hour ambulatory blood pressure monitoring (24h-ABPM).[9]
Diabetes mellitus is highly prevalent in hypertensive populations compared with the normotensives.[10] In addition, diabetic hypertensive populations are at profoundly high risk for developing cardiovascular events in comparison to their hypertensive counterparts without diabetes.[10] Therefore, intuitively, diabetic hypertensive populations should have more strict BP control, that is, lower systolic/diastolic BP (SBP/DBP) target than their nondiabetic hypertensive counterparts. However, according to the JNC 8 guideline,[11] the BP goal of diabetic populations is defined as <140/90 mmHg, which is largely because of results of the ACCORD-BP trial and the grade of this recommendation is expert consensus.[3] Nevertheless, results from some major meta-analysis,[12,13] including many high-quality randomized controlled trials, indicated that SBP <130 mmHg was associated with less cardiovascular and renal benefits in diabetic patients. To our knowledge, these conflicting findings may be at least partly associated with less accuracy and high variability of CBP measurements. In contrast, as mentioned above, 24h-ABPM could overcome this limitation, which in turn may provide more accurate BP information and also may be better to predict cardiovascular events than CBP.
In light of the advantages of 24h-ABPM for BP evaluation, we conducted a prospective observational study to evaluate whether 24h-ABPM would be superior to CBP for predicting cardiovascular events in diabetic hypertensive populations.
2. Methods
2.1. Study participants
Diabetic hypertensive patients in outpatient clinic who were qualified to study inclusion criteria were enrolled after informed consent was obtained. Inclusion criteria were as follows: age 45 to 75 years and a documented diagnosis of type 2 diabetes mellitus and primary hypertension. Exclusion criteria were those who could not walk as usual when 24h-ABPM was performing, or who had established cardiovascular diseases (including myocardial infarction, ischemic/hemorrhagic stroke, heart failure, or revascularization either by percutaneous coronary intervention or coronary artery bridging grafting), or who were with severe liver function impairment (alanine transaminase [ALT] ≥3 times of upper normal limit) or moderate renal function impairment (estimated glomerular filtration rate [eGFR]≤60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease [MDRD] formula [14]) dysfunction, or who had other comorbidities such as autoimmune diseases (e.g., rheumatoid arthritis) or cancer with life expectancy <1 year. Our present study was approved by the Clinical Research Ethical Committee of Huizhou Municipal Central Hospital.
2.2. Blood pressure measurements
Clinic BP measurements were in line with the JNC 7 guideline,[15] and patients sit quietly for 5 minutes and appropriate cuff size was applied to non-dominant arm with bladder encircled at least 80% of the arm (HEM7200, Omron Healthcare, Tokyo, Japan). Patients’ backs were supported and arms were placed on the desk in parallel to the level of heart. Three times with 1-minute interval of each BP measurements were performed and the last two readings were averaged as CBP. 24h-ABPM was performed in accordance to the European Society Hypertension practice guideline for ABPM (The Spacelab's 90217, Spacelab's Inc, Redmond, WA).[9] Daytime and nighttime intervals were determined using sleep time reported by patients’ diary cards, and at least 20 valid awake and 7 valid asleep measurements should be recorded, and those with unqualified measurement were asked to take 24h-ABPM measurements again. Night/day ratio was calculated by mean nighttime BP value divided by daytime BP value, and night/day ratio <0.9 was defined as dipping pattern and ≥0.9 was as nondipping pattern.[16]
2.3. Clinical and biochemical data collection
Study participants were asked to finish structured questionnaire (including demographic and anthropometric indices) with the help of research investigator. Biochemical indices including lipid profiles, fasting plasma glucose, glycated hemoglobin (HbA1c), creatinine (Cr) and blood urine nitrogen, albumin (ALB), and ALT were measured using fasting venous blood. Morning first voided urine sample was used to evaluate ALB/Cr ratio by means of automatic dipstick analysis.[17]
2.4. Study endpoints
Study participants were followed-up every 3 months via telephone interview or in outpatient clinic and study endpoints including angina pectoris, myocardial infarction, ischemic/hemorrhagic stroke, hospitalization for heart failure, and cardiovascular death were reported by patients or their immediate relatives and were further confirmed by medical records or their charging physicians. The first occurred event was recorded and the duration of follow-up was 12 months.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation [SD] and between-group differences were analyzed using the independent Student t test. Categorical variables were expressed as number and frequency of cases, and between-group differences were analyzed using χ2 analysis or Fisher exact test. Cox proportional hazards regression analysis was used to evaluate prognostic value of baseline CBP and ABP. Analysis was restricted to the first event if patients who had experienced multiple events. The hazard ratio (HR) represents the risk associated with a 1-SD increment in BP. In multivariable Cox regression analysis, age, male sex, smoking, serum low-density lipoprotein-cholesterol (LDL-C) level, HbA1c, eGFR, urine ALB/Cr ratio, antidiabetic and antihypertensive medication, anti-platelet, and statin were adjusted. Statistical analysis was conducted in SPSS 17.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. General characteristics of study participants
Initially, 450 participants were enrolled from July of 2014 to July of 2015, and 34 were lost to follow-up. Among them, 28 moved to other cities and 6 refused to follow-up. Baseline characteristics of these 34 participants were comparable to the remaining participants. Generally, the mean age of 416 remaining participants was 59 years and 66.8% were male. The durations of hypertension and diabetes were 6.4 ± 2.4 years and 4.5 ± 2.5 years, respectively. The mean eGFR was 95.4 ± 9.3 mL/min/1.73 m2 and ALB/Cr ratio was 2.2 ± 0.5 mg/mmol. The mean numbers of anti-hypertensive and antidiabetic medications were 2.6 ± 0.4 and 2.3 ± 0.3, respectively.
3.2. Comparisons of baseline characteristics between participants with and without study end-points
After 12 months’ follow-up, 46 participants (11.1%) had study endpoints. Among them, 28 had angina pectoris, 5 myocardial infarction, 8 ischemic stroke, and 5 heart failure. As shown in Table 1, compared with participants without study endpoints, participants with study endpoints were more elderly and more likely to be male. The duration of hypertension was also longer (7.0 ± 3.0 years vs. 6.4 ± 2.1 years, P < .05). Proportion of participants with study endpoints used angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACRI/ARB) were significantly less compared to those without endpoints (54.3% vs. 61.9%, P < .05). No other significant between-group differences were observed.
Table 1.
Comparisons of baseline characteristics between participants without and with study endpoints.

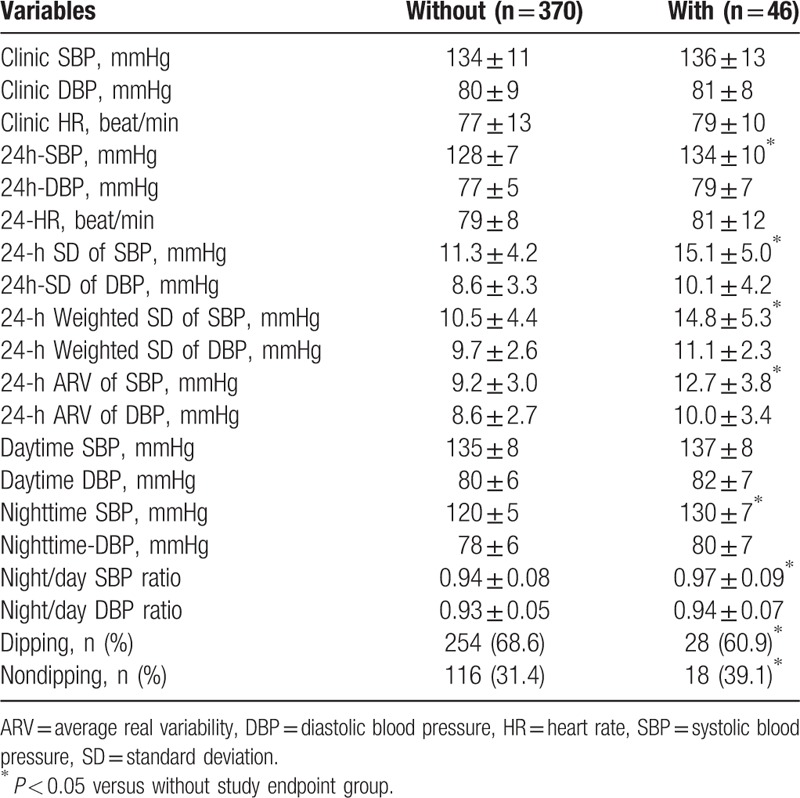
3.3. Comparisons of BP indices between participants with and without study endpoints
As shown in Table 2, there were no significant between-group differences in clinic BP indices. However, compared with participants without study endpoints, those with study endpoints had significantly higher 24h-SBP (134 ± 10 mmHg vs. 128 ± 7 mmHg, P < .05), nighttime-SBP (130 ± 7 mmHg vs. 120 ± 5 mmHg, P < .05) and night/day SBP ratio (0.97 ± 0.09 vs. 0.94 ± 0.08, P < .05), and 24h-SBP variability as indexed by 24-hour SD of SBP, 24-hour weighted SD of SBP, and 24-hour average real variability of SBP. Moreover, the proportion of participants with nondipping BP pattern was also significantly higher (39.1% vs. 31.4%, P < 0.05).
Table 2.
Comparisons of BP indices between participants with and without study endpoints.
3.4. Prognostic value of CBP and ABP
Prognostic values of CBP and ABP were separately evaluated using multivariable Cox regression analysis. As shown in Table 3, in SBP category, clinic SBP was not significantly and independently related with study endpoints after adjusted for nondipping BP pattern. While regarding ABP, after extensively adjusted for traditional risk factors, nondipping BP pattern and 24h-SBP, both 24h-SBP and nighttime-SBP remained independently related with cardiovascular outcomes, with HR and associated 95% confidence interval (CI) was 1.53 (1.28–2.03) and 1.50 (1.26–1.89), respectively. While in DBP category, after extensively adjusted for traditional risk factors, non-dipping BP pattern and 24h-DBP, neither DBP nor diastolic ABP indices were independently related with cardiovascular outcomes. Similarly, no significant and independent relationship between BP pattern and cardiovascular outcomes were observed.
Table 3.
Prognostic value of CBP and ABP.

4. Discussion
Results from our present study indicate that in diabetic hypertensive populations, baseline 24h-SBP and nighttime SBP are superior to clinic BP in predicting cardiovascular outcomes after 1-years follow-up. Combined with previous reports,[18–21] it is reasonable to conclude that in diabetic hypertensive populations, 24h-SBP or nighttime SBP should be better than CBP for cardiovascular risk prediction.
With respect to our study design, there are several issues which should be addressed. First of all, the mean durations of hypertension (6.4 years) and diabetes (4.5 years) in our study participants were <10 years, suggesting that the arterial system in our enrolled participants might be at a relative healthy condition compared to those with long-standing hypertension and diabetes, which in turn might have less impact on the accuracy of BP measurement by noninvasive approach. In contrast, patients with long-standing hypertension and diabetes, concurrent arterial stiffness, and arteriosclerosis might significantly influence the accuracy of BP measurement,[22] which in turn undermined the prognostic value of BP. Second, the follow-up was only 1 year and the long-term prognostic values of 24h-SBP and nighttime SBP should be further investigated. However, it appears that BP indices obtained by 24h-ABPM might be useful for short-term cardiovascular risk prediction. Third, among the 46 cardiovascular events, 28 (60.9%) were angina pectoris, which were relatively soft endpoints compared with others such as myocardial infarction and stroke. Long-term follow-up and larger sample size would help to determine whether baseline 24h-SBP and nighttime-SBP would be powered to predict hard outcomes in diabetic patients with hypertension. Fourth, we observed that compared to those without study endpoints, the proportion of ACEI/ARB usage in patients with study endpoints was less. Nevertheless, after adjusted for these potential covariates, 24h-SBP and nighttime SBP were still significantly related with cardiovascular outcomes, suggesting that the prognostic value of ABP was independent of antihypertensive treatment.
Other previous studies also showed that BP indices obtained from 24h-ABPM were better in prediction of vascular diseases. For example, in a prospective cohort study, Satrap et al[23] reported that after 9.2 years’ follow-up, nondipping BP pattern was independently associated with all-cause mortality in patients with diabetes. However, they did not report the association between ABP and specific cardiovascular outcomes. Moreover, they just enrolled 104 participants, which might prevent them evaluating the relationship between ABP and individual cardiovascular outcome. Sturrock et al[24] also reported that nondipping BP pattern predicted all-cause mortality in a mixed cohort of type 1 and type 2 diabetes mellitus patients. However, this was a retrospective study with a relative small sample size (75 participants), which undermined the generalizability of their findings. Nevertheless, in our present study, we did not observe independent and significant relationship between nondipping BP pattern and cardiovascular events after extensively adjusted for potential covariates as shown in Table 3. In a longitudinal observational study, Nakano et al[25] also reported that in type 2 diabetes subjects, ABP level rather than dipper/nondipper status predicted vascular events after a median follow-up of 86 months. The discrepancies between different studies might be related to the differences in study sample size, patients’ clinical characteristics, and study design.
In a prospective cohort study,[20] Salles et al reported that after a median 5.75 years’ follow-up, compared with clinic BP, ABPM provided more valuable information in cardiovascular risk stratification in type 2 diabetes mellitus populations. They also reported that ambulatory SBP was the strongest predictor. Consistent with their findings, our study also showed that ambulatory SBP (24h-SBP and nighttime SBP) was the most significant predictor for cardiovascular outcomes. However, the study of Salles et al was a mixed cohort of participants with and without established cardiovascular diseases at enrollment, whereas in our study, we did not recruit those with established cardiovascular diseases. In addition, in this study, we also evaluated the 24-hour variability of BP and as presented in Table 2 that, participants with study endpoints had higher SBP variability compared to those without endpoints, suggesting that parameters of BP variability assessed by 24h-ABPM might be also a useful marker to stratify cardiovascular risk.
There are several limitations of this study. First, 34 (7.5%) participants were lost to follow-up; however, no significant differences in baseline characteristics were observed. Second, although baseline medical treatments were adjusted, data on antihypertensive and antidiabetic medications during follow-up were not obtained and therefore it was impossible for us to adjust for the potential effect of treatment on cardiovascular outcomes. Third, CBP and ABP were collected only at baseline, and therefore the influence of changes in these BP indices on cardiovascular outcomes cannot be assessed. Finally, short-term duration of follow-up might not allow us to draw conclusion on long-term outcomes.
5. Conclusion
In summary, the principle findings of our study indicate that in diabetic hypertensive patients without established cardiovascular diseases, baseline 24h-SBP and nighttime SBP are useful markers for predicting short-term cardiovascular outcomes.
Acknowledgment
The authors appreciate very much the help Dr. Congou Huang provided.
Footnotes
Abbreviations: ABP = ambulatory blood pressure, ABPM = ambulatory blood pressure monitoring, ACRI/ARB = angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, ALB = albumin, ALT = alanine transaminase, BUN = blood urine nitrogen, CBP = clinic blood pressure, CI = confidence interval, Cr = creatinine, eGFR = estimated glomerular filtration rate, FPG = fasting plasma glucose, HbA1c = glycated hemoglobin, HR = hazard ratio, LDL-C = low-density lipoprotein-cholesterol, MDRD = modification of diet in renal disease, SBP/DBP = systolic/diastolic blood pressure.
J.S. and Z-M.L. are corresponding authors.
The study was supported by the Medical Scientific Research Foundation of Guangdong Province China (No.B2013401)
The authors report no conflicts of interest.
References
- [1].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2013;381:1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright JT, Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2016;374:2294. [DOI] [PubMed] [Google Scholar]
- [5].Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355:865–72. [DOI] [PubMed] [Google Scholar]
- [6].Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease: The HONEST Study. J Am Coll Cardiol 2016;67:1519–27. [DOI] [PubMed] [Google Scholar]
- [7].Drawz PE, Pajewski NM, Bates JT, et al. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure: results from the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension 2017;69:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in Europe trial investigators. JAMA 1999;282:539–46. [DOI] [PubMed] [Google Scholar]
- [9].Parati G, Stergiou G, O’Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014;32:1359–66. [DOI] [PubMed] [Google Scholar]
- [10].Hao Z, Li G, Sun Y, et al. Relationship and associated mechanisms between ambulatory blood pressure and clinic blood pressure with prevalent cardiovascular disease in diabetic hypertensive patients. Medicine (Baltimore) 2017;96:e6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [12].Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603–15. [DOI] [PubMed] [Google Scholar]
- [13].Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 suppl 1):S1–266. [PubMed] [Google Scholar]
- [15].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [16].Cai A, Zhong Q, Liu C, et al. Associations of systolic and diastolic blood pressure night-to-day ratios with atherosclerotic cardiovascular diseases. Hypertens Res 2016;39:874–8. [DOI] [PubMed] [Google Scholar]
- [17].Morris RK, Riley RD, Doug M, et al. Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre-eclampsia: systematic review and meta-analysis. BMJ 2012;345:e4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakano S, Konishi K, Furuya K, et al. A prognostic role of mean 24-h pulse pressure level for cardiovascular events in type 2 diabetic subjects under 60 years of age. Diabetes Care 2005;28:95–100. [DOI] [PubMed] [Google Scholar]
- [19].Laugesen E, Rossen NB, Poulsen PL, et al. Pulse pressure and systolic night-day ratio interact in prediction of macrovascular disease in patients with type 2 diabetes mellitus. J Hum Hypertens 2012;26:164–70. [DOI] [PubMed] [Google Scholar]
- [20].Salles GF, Leite NC, Pereira BB, et al. Prognostic impact of clinic and ambulatory blood pressure components in high-risk type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. J Hypertens 2013;31:2176–86. [DOI] [PubMed] [Google Scholar]
- [21].Roush GC, Fagard RH, Salles GF, et al. Prognostic impact from clinic, daytime, and night-time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens 2014;32:2332–40. discussion 2340. [DOI] [PubMed] [Google Scholar]
- [22].Xu L, Jiang CQ, Lam TH, et al. Impact of impaired fasting glucose and impaired glucose tolerance on arterial stiffness in an older Chinese population: the Guangzhou Biobank Cohort Study-CVD. Metabolism 2010;59:367–72. [DOI] [PubMed] [Google Scholar]
- [23].Satrap AS, Nielsen FS, Rossing P, et al. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens 2007;25:2479–85. [DOI] [PubMed] [Google Scholar]
- [24].Sturrock ND, George E, Pound N, et al. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 2000;17:360–4. [DOI] [PubMed] [Google Scholar]
- [25].Nakano S, Ito T, Furuya K, et al. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res 2004;27:647–56. [DOI] [PubMed] [Google Scholar]


