Abstract
Background:
The identification of pancreatic carcinoma (PC) patients with poor prognosis is a priority in clinical oncology because of their high 5-year mortality. However, the prognostic value of pretreatment 18F-fluorodeoxyglucose (18F-FDG)- positron emission tomography (PET)/computed tomography (CT) parameters in PC patients is controversial and no consensus exists as to its predictive capability. This meta-analysis was performed to comprehensively explore the prognostic significance of 18F-FDG-PET/CT parameters in patients with pancreatic carcinoma.
Methods:
Extensive literature searches of the PubMed, Embase, Web of Science, and Cochrane Library databases were conducted to identify literature published until March 5, 2017. Comparative analyses of the pooled hazard ratios (HRs) for event-free survival (EFS) and overall survival (OS) were performed to assess their correlations with pretreatment maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG). Either the fixed- or the random-effects model was adopted, depending on the heterogeneity observed across studies. Subgroup and sensitivity analyses were performed to assess the robustness of the results.
Results:
Sixteen studies including 1146 patients were identified. The pooled HRs for the probability of EFS were 1.90 (95% confidential interval (CI): 1.48–2.45) for SUVmax, 1.76 (95% CI: 1.20–2.58) for MTV, and 1.81 (95% CI: 1.27–2.58) for TLG. The pooled HRs for the probability of OS were 1.21 (95% CI: 1.12–1.31) for SUVmax, 1.56 (95% CI: 1.13–2.16) for MTV, and 1.70 (95% CI: 1.25–2.30) for TLG. A slight publication bias was detected using Begg test. After adjustment using the trim and fill procedure, the corrected HRs were not significantly different. The results of the subgroup analyses by SUVmax, MTV, and TLG showed that these factors may have similar prognostic significance.
Conclusion:
18F-FDG-PET/CT parameters, such as SUVmax, MTV, and TLG, may be significant prognostic factors in patients with pancreatic carcinoma. 18F-FDG-PET/CT imaging could be a promising tool to provide prognostic information for these patients.
Keywords: pancreatic carcinoma, PET, SUVmax, volume
1. Introduction
Pancreatic carcinoma (PC) is the fourth most common cause of cancer-related mortality in the United States, with a 5-year survival rate of less than 5%; additionally, the incidence of this cancer is steadily increasing in most countries.[1] At initial diagnosis, 30% of patients already have locally advanced pancreatic cancer (LAPC),[2] and less than 20% of cases will be considered for curative surgery;[3,4] furthermore, more than 50% of patients present with metastatic disease and may only be treated with palliative chemotherapy.[5] Among the patients who survive surgical resection, the 5-year survival rate remains low at approximately 15% to 40%.[6] Accurate predictors would be helpful and invaluable in stratifying patients for disease management, including predicting outcomes.
Glucose analogue 18F-fluorodeoxyglucose (18F-FDG) has become extensively used as a tracer of positron emission tomography/computed tomography (PET/CT) in clinical cancer imaging.[7] The most widely used PET-derived parameter designed to measure tracer accumulation in PET is the maximum standardized uptake value (SUVmax), which quantifies the rate of glucose metabolic uptake in tumor cells.[8,9] Several studies have shown 18F-FDG-PET/CT to play a significant role in the diagnosis, staging and restaging, planning of treatment, evaluation of response to treatment, and prognosis in PC.[10–12] Recent studies have supported the use of volumetric parameters, such as metabolic tumor volume (MTV) and FDG total lesion glycolysis (TLG), which have also proven to be beneficial as prognostic factors in PC.[13–15] While SUVmax, a single pixel value within a region of interest, is subject to considerable noise,[16] other FDG-PET parameters, such as MTV and TLG, lend support to its continued use as a summary of tumor FDG activity in PC that may be used in studies to predict disease progression. Although the impact of 18F-FDG-PET/CT parameters has been evaluated in patients with pancreatic carcinoma in previously published studies, there is little knowledge regarding the consistency of SUV and volumetric PET parameters in the prediction of PC prognosis.
Therefore, we conducted a systematic literature review and meta-analysis to identify, appraise, and synthesize results from all available studies and provide an unprecedented summary to address the prognostic value of pretreatment SUVmax, MTV, and TLG in patients with PC.
2. Materials and methods
2.1. Literature search
We searched the PubMed, EMBASE, Web of Science, and Cochrane Library databases for studies published in English language until March 5, 2017. The search strategy involved using on the following terms: (“pancreatic” or “pancreas” or “pancreatic ductal adenocarcinoma) AND (“neoplasm” or “tumor” or “cancer” or “carcinoma” or “adenocarcinoma”) AND (“positron emission tomography” OR “FDG” or “positron emission tomography-computed tomography” OR “positron emission tomography computed tomography” OR “PET” OR “PET-CT” OR “PET CT” OR “PET/CT” OR “fluorodeoxyglucose”) and (“prognostic” OR “prognosis” OR “predictive” OR “survival” OR “outcome”). Additionally, the reference lists of relevant studies were scrutinized to identify additional eligible studies. We performed all the analyses based on previously published studies, thus no ethical approval was required.
2.2. Selection of studies
The inclusion criteria included the following: patients were pathologically diagnosed with PC; case control or cohort study; at least 1 18F-FDG-PET/CT scan performed before and/or in treatment; at least 1 relevant prognosis factor was assessed, such as overall survival (OS), disease-free survival (DFS), event-free survival (EFS), progress-free survival (PFS), disease metastasis-free survival (DMFS) or EFS; hazard ratios (HRs) and 95% confidence intervals (CIs) were available or able to be calculated based on data from the original articles; and published in English. Articles meeting the following criteria were excluded: in vitro studies and animal experiments; reviews, comments, letters, case reports, or conference abstracts; insufficient data available to calculate the HRs and 95% CIs; research limited to investigating the role of PET-CT scans in diagnosis and tumor staging, and prognostic parameter data were not provided; and less than 10 patients were included. When eligible studies were published more than once, only data from the most complete or recent studies were included. Two authors (JC and HZ) independently evaluated the studies for eligibility. When data from the same patient population were published more than once, only the most recent or complete report was included in the review. Any discrepancies were resolved by consensus.
2.3. Data extraction and quality assessment
Data were independently extracted from each article by 2 reviewers and recorded on a standardized form. Univariate and multivariate HRs and their 95% CIs, P values for the log-rank test, and necessary statistics such as 95% CIs, the number of events, and the number included in each group assessed using Kaplan–Meier curves were recorded. Then, we used the methods suggested by Parmar et al and Williamson et al to convert these data into logHRs and SEs.[17,18] HR values were calculated by applying a spreadsheet and using the methods suggested by Tierney et al.[19] Relevant files or supplementary files included with the publications were also carefully scrutinized to identify available data.
The following data were also extracted from the publications: first author, year of publication, country of origin, study period, follow-up duration, age of patients, number of patients, and study design. The technical details of the 18F-FDG-PET/CT examinations, such as the PET/CT scanner used, duration of fasting before FDG injection, preinjection blood glucose level, radiation doses of FDG, and interval periods, were also extracted.
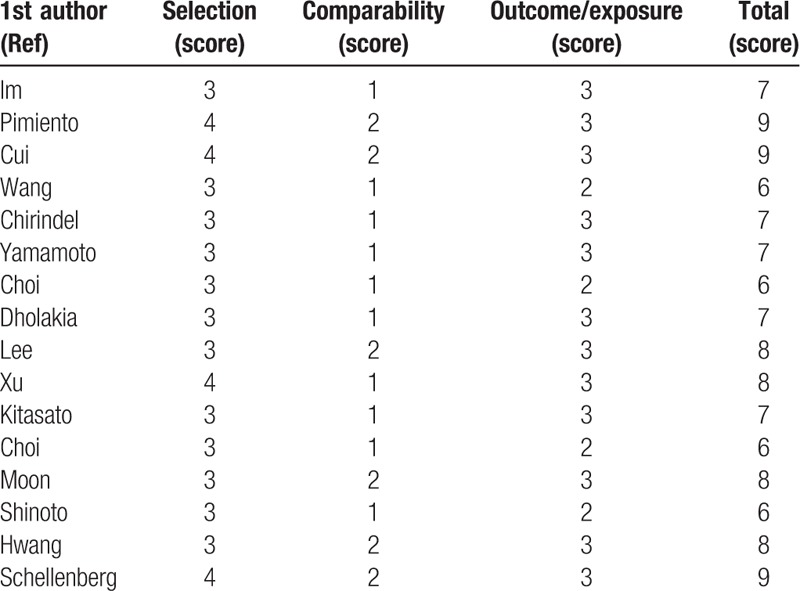
We assessed the quality of each included article using the Newcastle–Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/oxford.asp), a comprehensive, systematic reviewing tool that was designed for retrospective and prospective studies. Studies with scores ≥6 points on the NOS were considered high-quality studies and included in this meta-analysis. Discrepancies were resolved by consensus (Table 1).
Table 1.
Methodological quality for potentially included studies according to Newcastle–Ottawa scale in this meta-analysis.
2.4. Statistical analysis
In this meta-analysis, we followed the same methodology as used in the previous study.[20]The primary endpoint was EFS. DFS, PFS, DMFS in the included studies were obtained as primary outcomes and newly defined as EFS, which was measured from the date of initiation of therapy to the date of recurrence or metastasis.[21] The secondary endpoint was OS, which was measured from the date of initiation of therapy to the date of death from any cause. The effects of 18F-FDG-PET-derived parameters on survival outcomes were estimated by pooling the HR effect size and 95% CI data. An HR >1 indicated worse prognosis in patients with high parameter values, and an HR <1 indicated these patients to have better prognosis. The level of heterogeneity across studies was evaluated using the χ2 test and I2 statistic, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org). If the P value was >.1 and/the I2 value was < 50%, no or moderate heterogeneity was indicated, and the fixed-effects model was used; otherwise, the random-effects model was used when significant heterogeneity was observed. Begg funnel test and Egger test were performed to assess publication bias. The trim and fill method was applied adjust for asymmetry in the funnel plot.[22] The analyses described above were conducted using STATA version 12.0 (STATA Corp, College Station, TX). P values less than .05 were considered statistically significant.
3. Results
3.1. Study characteristics and qualitative assessment
Sixteen eligible articles remained after applying the inclusion and exclusion criteria (a total of 1146 patients), and these articles were included in the meta-analysis.[13,14,23–36] A flowchart of the literature review process is shown in Fig. 1. Six of the 16 studies had been conducted in South Korea,[13,23,28,30–32] 5 studies had been conducted in America,[14,24,26,33,36] 2 studies had been conducted in China,[25,34] and 3 studies had been conducted in Japan.[27,29,35] One study was prospective,[14] and the remaining studies were retrospective. Of these included studies, 16 provided the sample size data, and sample sizes ranged from 21 to 165 (median 72). The follow-up duration varied from 8.7 to 48.9 months (median 23.3 months). The principal characteristics of the 16 studies are listed in Table 2.
Figure 1.
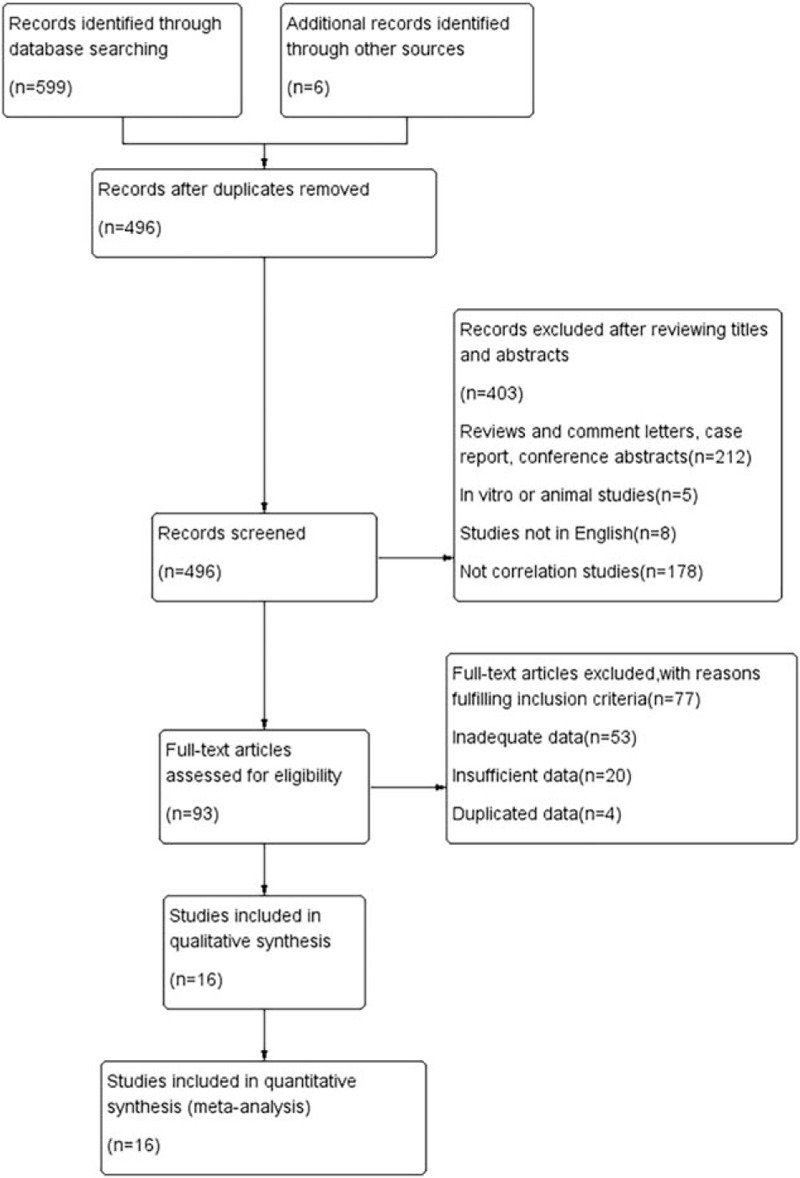
Flowchart for the identification of eligible studies.
Table 2.
Characteristics of eligible studies included in the meta-analysis.
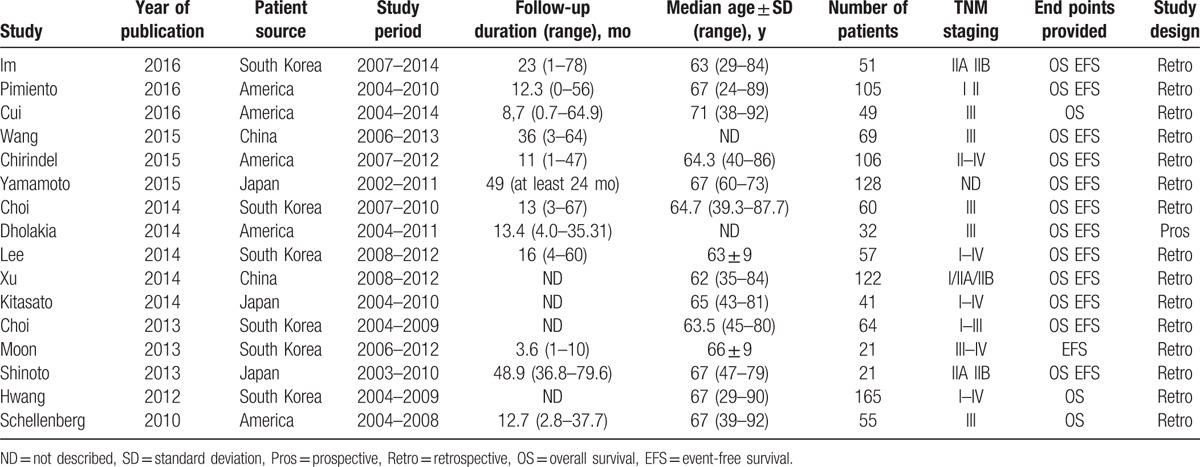
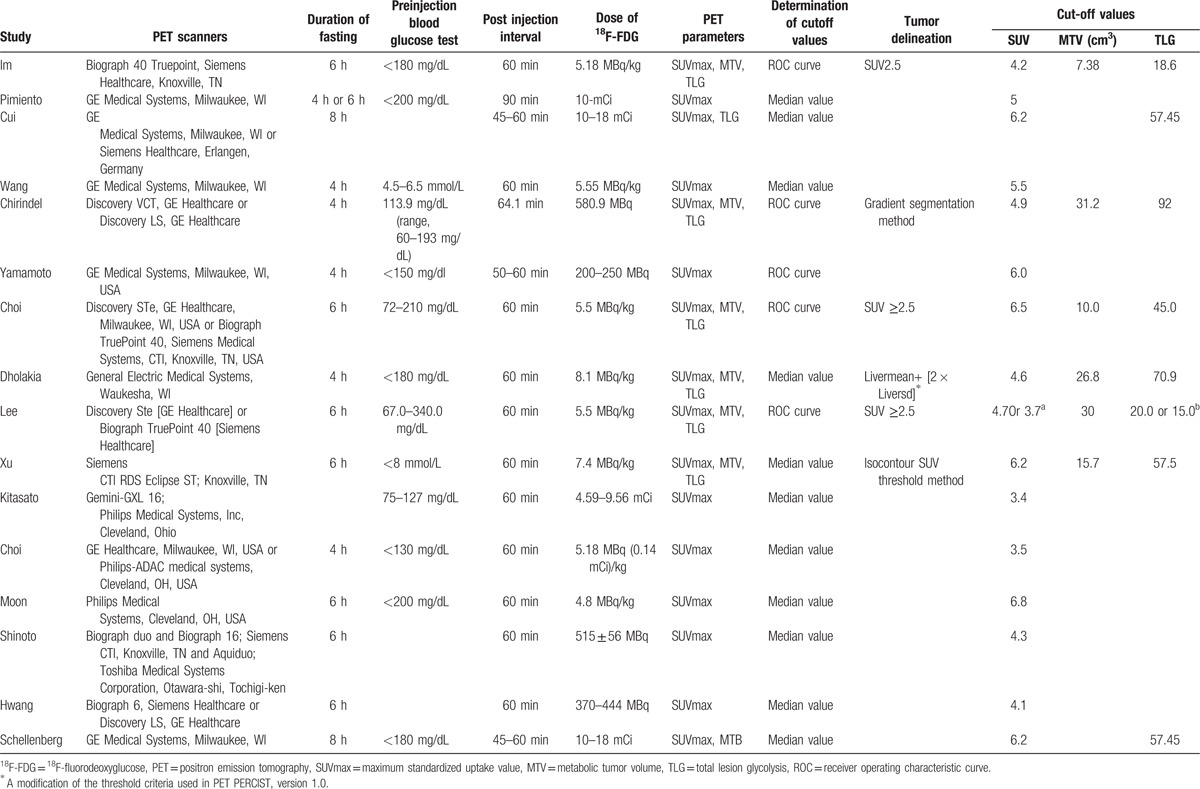
Table 3 shows the patterns identified in 18F-FDG-PET/CT scanning. SUVmax was measured in all 16 studies, and values standardized by body weight were provided. MTV was measured in 6 studies,[13,14,23,26,28,34] and TLG was measured in 8 studies.[13,14,23,26,28,33,34,36] Different scanners and various scanning protocols were used in each study. The duration of fasting varied from 4 hours to 8 hours, and this duration was not reported in 1 study. Preinjection serum blood glucose levels ranged from 67 to 340 mg/dL, and these data were not reported in 3 studies. The injected dose varied from 200 to 666 MBq, and the postinjection interval ranged from 45 to 90 minutes. Two threshold methods were used to calculate the cut-off values; receiver operating characteristics (ROCs) were used in 5 studies,[13,23,26–28] and median values were used in 11 study. Four threshold methods were applied for the measurement of MTV and TLG based on primary PC lesion volume. A fixed SUV of 2.5 was used in 4 articles;[13,23,28] the gradient segmentation method was used in 1 study,[26] and the isocontour method was used in 1 study.[34] In 1 study, a threshold was measured using the mean liver background SUV plus 2 standard deviations.[14] The median cut-off point was 5.1 (3.4–6.8) for SUVmax. The cut-off values for MTV ranged from 7.38 to 31.2 cm3, and TLG values were between 15.0 and 92. The NOS scores for the studies are shown in Table 1, and all the included studies had scores greater than 6.
Table 3.
Methods of 18F-FDG PET imaging of the included studies.
3.2. Outcome and publication bias
3.2.1. Primary outcome: EFS
Thirteen studies were included in the analysis of the association between SUVmax and EFS, and the pooled data revealed that high SUVmax values predicted poor EFS (HR = 1.90; 95% CI = 1.48–2.45, P = .000; I2 = 59.8%) (Fig. 2A). Additionally, we conducted a sensitively analysis to further estimate the impact of each study on the pooled HR. When the study conducted by Shinoto et al[29] was omitted from the analysis, an HR of 1.73 (1.50–2.00) and a decreased I2 value (49.3%) were identified using a fixed-model. Begg and Egger tests were performed to assess publication bias. The funnel plots illustrated the correlation between SUVmax and EFS (Fig. 3). Visual inspection of the Begg funnel plot and the results of the quantitative tests (Begg test = 0.077, Egger test = 0.191) indicated the presence of no significant publication bias.
Figure 2.
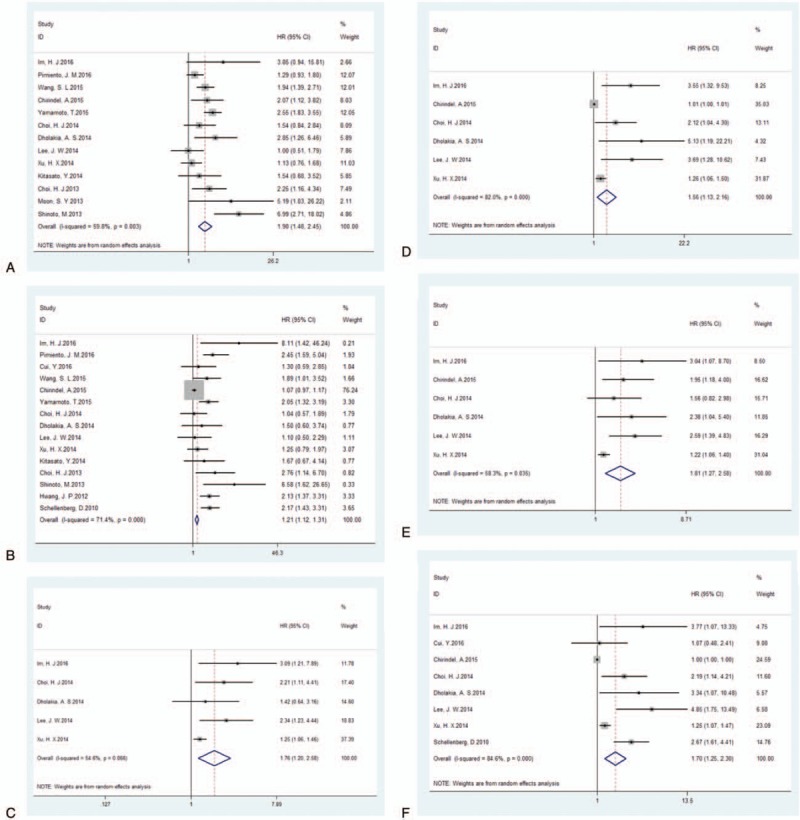
Forest plots of HR for EFS and OS with SUVmax (A, EFS; B, OS), MTV (C, EFS; D, OS) and TLG (E, EFS; F, OS). The χ2 test is a measurement of heterogeneity. P < .05 indicates significant heterogeneity. Squares = individual study point estimates. Horizontal lines = 95%CIs. Rhombus = summarized estimate and its 95% CI. Fixed = fixed effect model. Random = random effect model. CI = confidence interval, EFS = event-free survival, HR = hazard ratio, MTV = metabolic tumor volume, OS = overall survival, TLG = total lesion glycolysis, SUVmax = maximum standardized uptake value.
Figure 3.
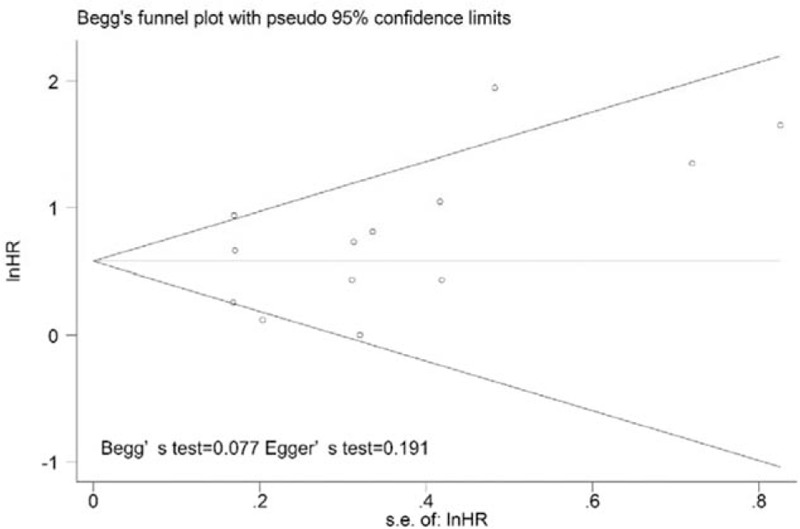
Publication bias test for the correlations of SUVmax with event-free survival. No significant publication bias was detected by Begg funnel plots (no apparent asymmetry was found) and estimation of P values. SUVmax = maximum standardized uptake value.
On the one hand, 5 studies were included in the analysis of the prognostic value of MTV for EFS. Since significant heterogeneity (χ2 = 8.81, P = .066; I2 = 54.6%) was observed across the included studies, the random-effects model was used. Using this model, the HR was 1.76 (95% CI = 1.20–2.58, P = .004) (Fig. 2C). When the study conducted Xu et al[34] was excluded, the observed heterogeneity decreased from 54.6% to 0% (P = .640), and the pooled HR reached 2.16 (95% CI = 1.49–3.13). The results of the quantitative tests (Begg test, z = 0.73, P = .462; Egger tests, t = 3.42, P = .042) indicated the possibility of publication bias, as illustrated by the statistically insignificant P value derived based on the Begg test. Therefore, we performed a trim and fill analysis to ensure the reliability of the pooled HR. A symmetrical funnel plot was observed after the trim and fill analysis was performed (Fig. 4). When potentially missing studies were added, the results (pooled HR = 1.30; 95% CI = 1.13–1.49, P = .033) of the sensitivity analysis still indicated that the correlation between MTV and EFS was significant.
Figure 4.
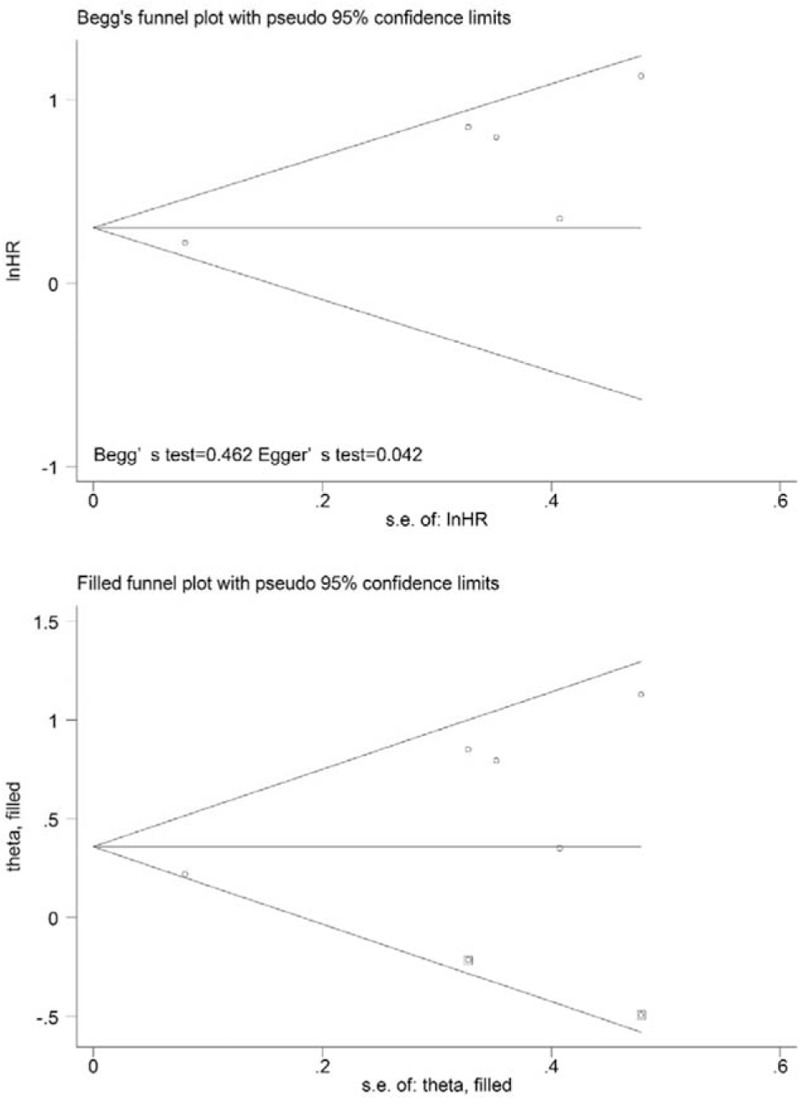
Funnel plots without (up column) and with (low column) trim and fill of MTV with event-free survival. MTV = metabolic tumor volume.
On the other hand, the results from 6 studies were pooled in the analysis of the prognostic value of TLG for EFS. Significant heterogeneity (χ2 = 11.99, P = .035; I2 = 58.3%) was observed across these studies; therefore, we used the random-effects model to calculate the HR (1.81, 95% CI = 1.27–2.58, P = .001) (Fig. 2E). When the study conducted by Xu et al[34] was excluded from the analysis, the observed heterogeneity decreased from 58.3% to 0% (P = .760), and the pooled HR reached 2.13 (95% CI = 1.56–2.92). Potential publication bias was evaluated using 2 statistical test methods (Begg test and Egger test). The results (Begg test, z = 1.13, P = .260; Egger tests, t = 5.63, P = .005) indicated the possibility of publication bias, as illustrated by the statistically insignificant P value derived based on the Begg test. The symmetrical funnel plot was demonstrated after the trim and fill analysis (Fig. 5). When potentially missing studies were added, the results (pooled HR = 1.40; 95% CI = 1.02–1.92, P = .012) of this sensitivity analysis still indicated that the correlation between TLG and EFS was significant.
Figure 5.
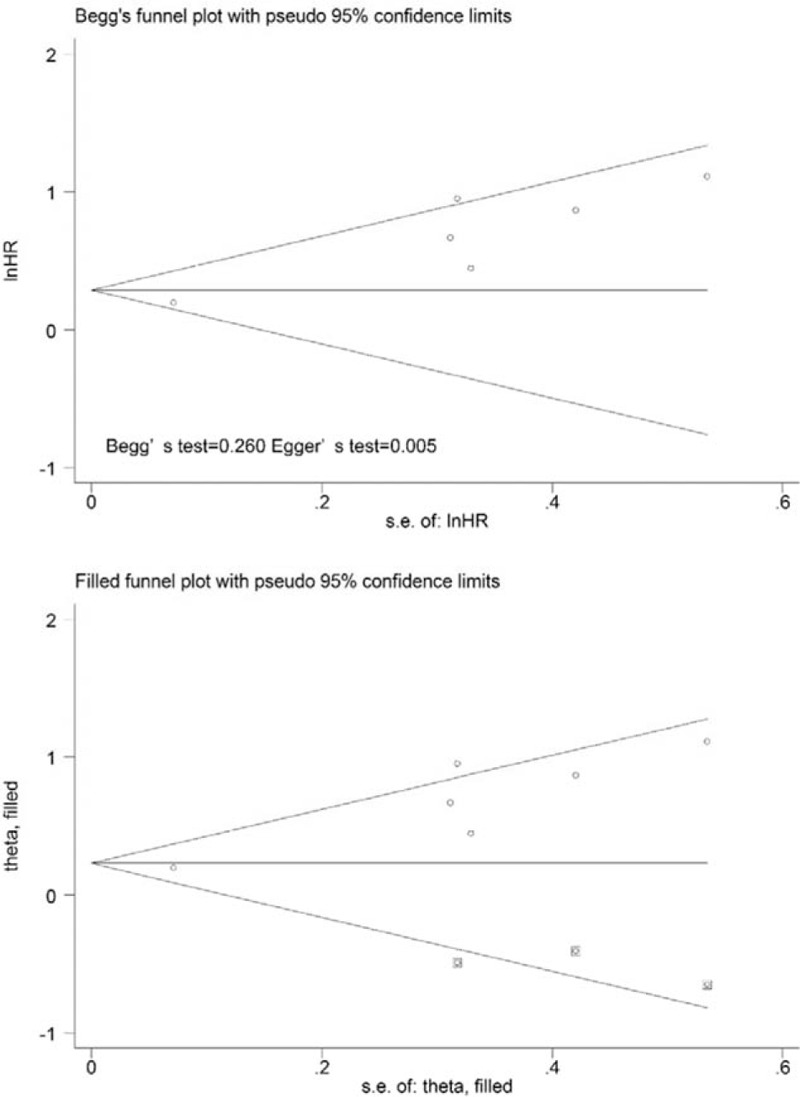
Funnel plots without (up column) and with (low column) trim and fill of TLG with event-free survival. TLG = total lesion glycolysis.
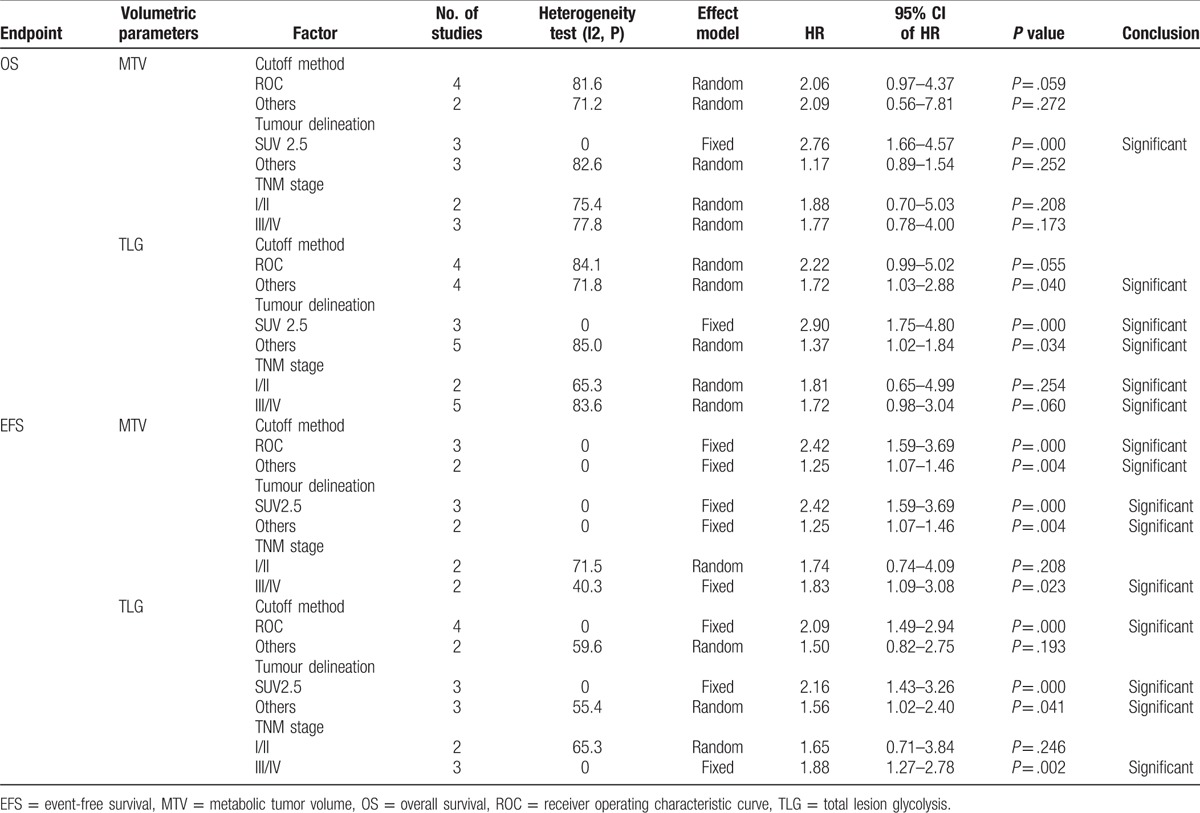
We conducted subgroup analyses by cut-off method, threshold, and analysis method. Among articles including data for SUVmax, the HR for studies that determined cut-off values using ROCs was 1.87 (95% CI: 1.26–2.77, P = .002), and the HR for studies using other methods was 1.96 (95% CI: 1.38–2.77, P = .000). Based on the median value of SUVmax, cut-off values groups were divided into the following 2 subgroups: high value (≥5.1) and low value (<5.1). The results of the subgroup meta-analyses indicated that the pooled HRs for SUVmax were 1.83 (95% CI: 1.28–2.64, P = .001) and 2.01 (95% CI = 1.36–2.98, P = .000) for high and low cut-off values, respectively. In the subgroup analysis by the analytic method, the HR for studies using univariate analyses was 2.17 (95% CI = 1.50–3.14, P = .000), and the HR for studies using multivariate analyses was 1.50 (95% CI = 1.18–1.91, P = .001). In the subgroup analysis by TNM staging method, the HR for studies using I/II staging was 2.03 (95% CI = 1.06–3.91, P = .034), and the HR for studies using III/IV staging was 1.99 (95% CI = 1.52–2.61, P = .000) (Table 4). We performed subgroup analyses stratified by cut-off method, tumor delineation, and disease stage to assess the impact of these factors on the associations between MTV and TLG and the outcomes of interest. One study[28] included their populations with stage I to IV tumors, and, thus, this study was not included in the additional subgroup meta-analysis of stage. The results of each subgroup analysis indicated the presence of a significant HR for death (Table 5).
Table 4.
Subgroup analyses of the associations between SUVmax and survival outcomes.
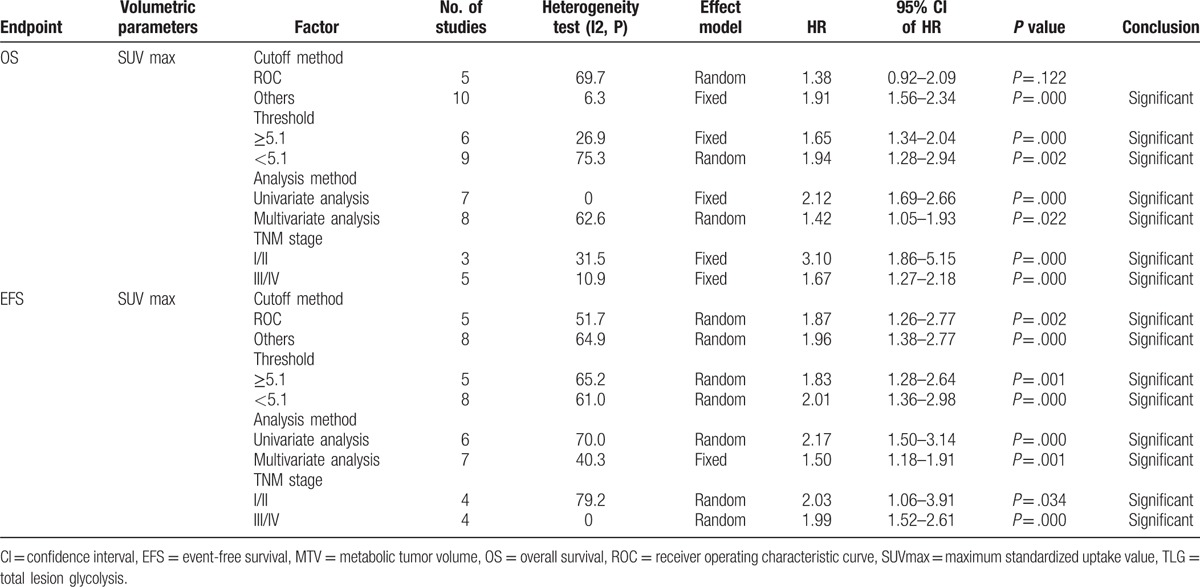
Table 5.
Subgroup analyses of the associations between MTV/TLG and survival outcomes.
3.2.2. Secondary outcome: OS
Fifteen studies were included in the assessment of the correlation between SUVmax and OS. Significant heterogeneity (P = .000, χ2 = 48.95; I2 = 71.4%) was observed across these studies; thus, the random-effects model was applied to calculate the pooled HR (1.21, 95% CI = 1.12–1.31; P = .000) (Fig. 2B). When the study conducted by Chirindel et al[26] was omitted from the analysis, an HR of 1.82 (1.54–2.14) and a decreased I2 of 27.7% were calculated using the fixed-effects model. The results of the quantitative tests (Begg test, z = 0.10, P = .921; Egger tests, t = 4.47, P = .001) indicated the possibility of publication bias, as represented by the statistically insignificant P value derived based on Begg test. A symmetrical funnel plot was generated after the trim and fill analysis performed (Fig. 6). When potentially missing studies were added, the results (pooled HR = 1.68; 95% CI = 1.30–2.17, P = .000) of this sensitivity analysis still indicated that the correlation between SUVmax and OS was significant.
Figure 6.
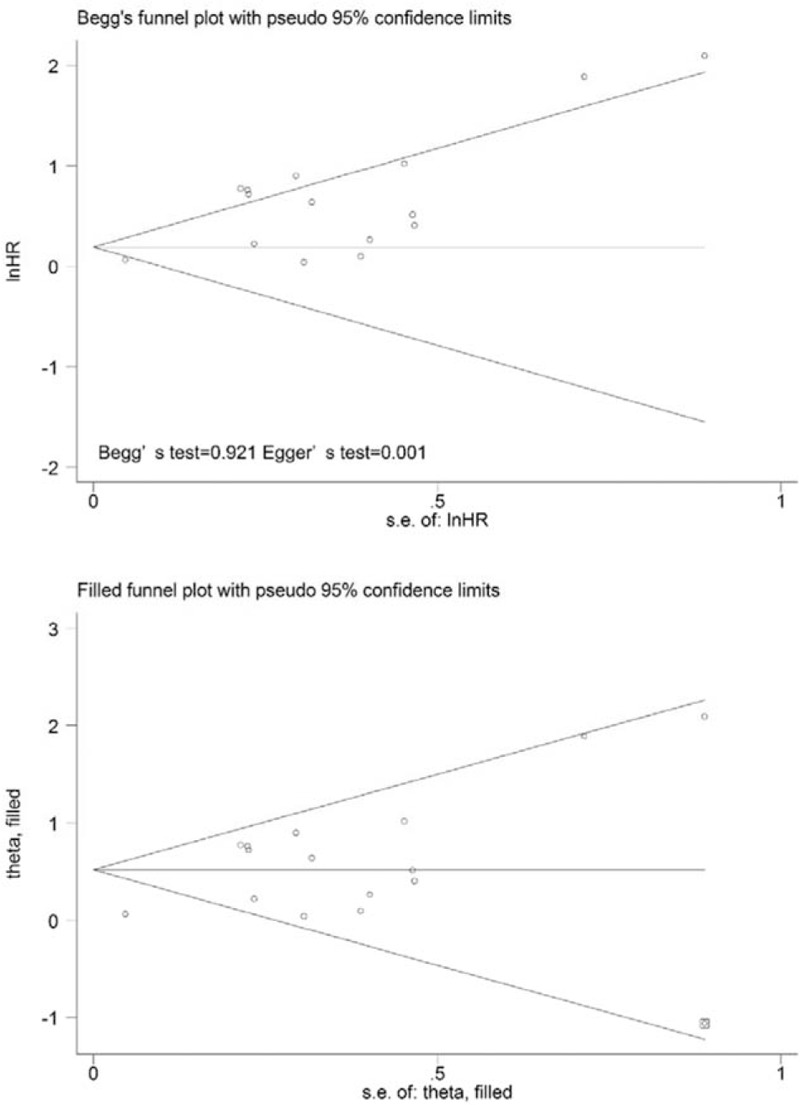
Funnel plots without (up column) and with (low column) trim and fill of SUVmax with overall survival. The pseudo 95% confidence interval (CI) is computed as part of the analysis that produced the funnel plot and corresponds to the expected 95% CI for a given standard error (SE). HR = hazard ratio, SUVmax = maximum standardized uptake value.
At the same time, 6 studies were included in the analysis of the association between MTV and OS. High MTV values were significant predictors of poor OS (HR = 1.56, 95% CI 1.13–2.16; P = .007), and significant heterogeneity was observed (χ2 = 27.78, P = .000; I2 = 82.0%) (Fig. 2D). When the study conducted by Chirindel et al[26] was excluded from the analysis, the observed heterogeneity decreased from 82.0% to 67.4% (P = .015), and the pooled HR reached 2.36 (95% CI = 1.31–4.25) using the random-effects model. The results of the quantitative tests (Begg test, z = 0.00, P = 1.000; Egger tests, t = 21.25, P = .000) indicated the possibility of publication bias, as illustrated by the statistically insignificant P value derived based on Begg test. A symmetrical funnel plot was observed after the trim and fill analysis was performed (Fig. 7). When the hypothesized literatures were added, the results (pooled HR = 1.006; 95% CI = 1.000–1.014, P = .000) of this sensitivity analysis still indicated that the correlation between MTV and OS was significant.
Figure 7.
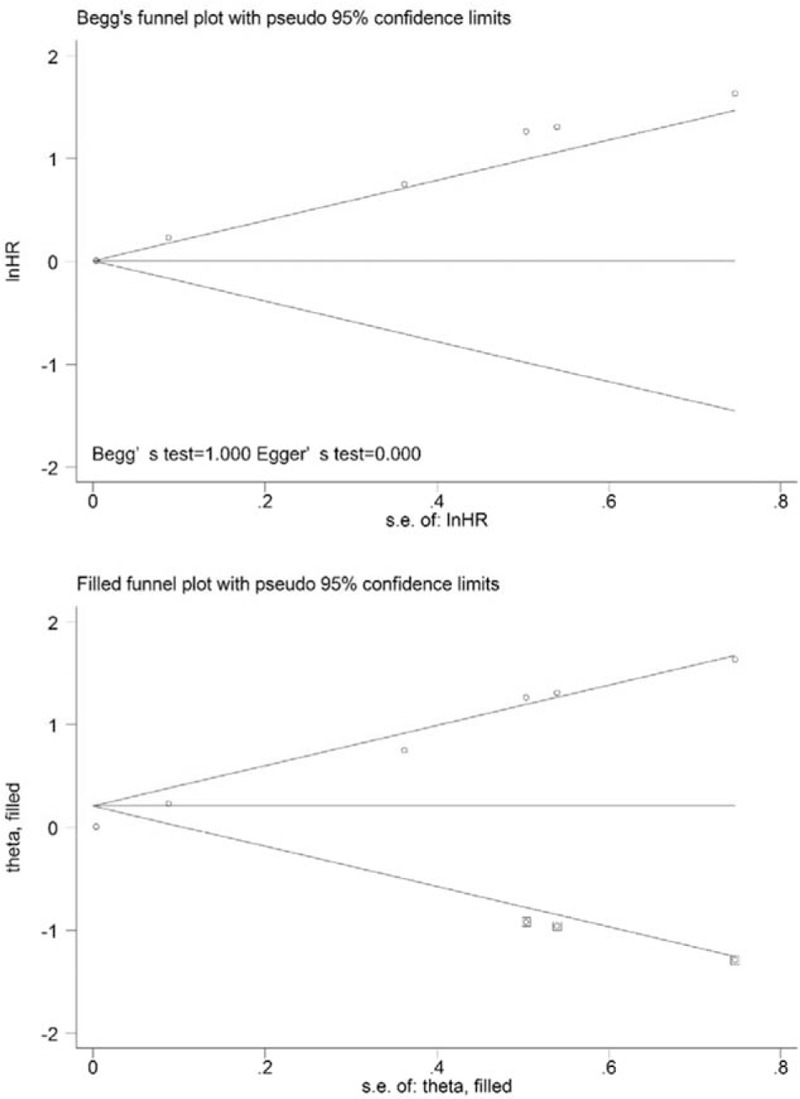
Funnel plots without (up column) and with (low column) trim and fill of MTV with overall survival. MTV = metabolic tumor volume.
Eight studies were included in the evaluation of the association between TLG and OS, and the results indicated that high TLG was a significant predictors of poor OS (HR = 1.70; 95% CI: 1.25–2.30; P = .01), and significant heterogeneity (χ2 = 45.49, P = .000; I2 = 84.6%) was observed across these studies (Fig. 2F). When the study by Chirindel et al[26] was excluded from the analysis, the level of heterogeneity decreased from 82.0% to 70.9% (P = .002), and the pooled HR reached 2.14 (95% CI = 1.37–3.35) using the random-effects model. The presence of publication bias was evaluated using 2 statistical tests (Begg test and Egger test). The results of the tests (Begg test, z = 1.71, P = .087; Egger tests, t = 2.61, P = .028) indicated the possibility of publication bias, as illustrated by the statistically insignificant P value derived based on the Begg test. Therefore, we performed a trim and fill analysis to ensure the reliability of the pooled HR. A symmetrical funnel plot was generated after the trim and fill analysis was performed (Fig. 8). When potentially missing studies were added, the results (pooled HR = 1.42; 95% CI = 1.07–1.88, P = .00) of this sensitivity analysis still indicated that the correlation between TLG and OS was significant.
Figure 8.
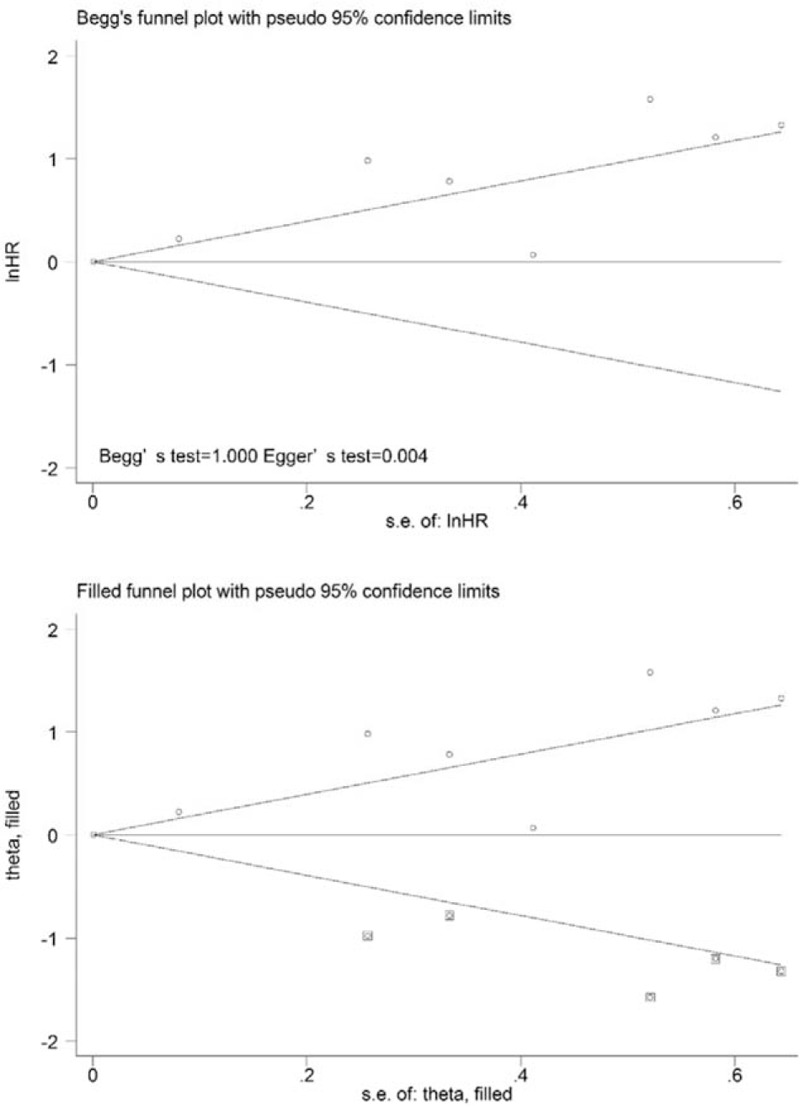
Funnel plots without (up column) and with (low column) trim and fill of TLG with overall survival. TLG = total lesion glycolysis.
The results of the subgroup meta-analyses were demonstrated as follows. Among the studies in which SUVmax was assessed, the HR for those identifying cut-off values using the ROC method was 1.38 (95% CI: 0.92–2.09, P = .122), and the HR for using other methods was 1.91 (95% CI: 1.56–2.34, P = .000); studies with high cut-off values had an HR of 1.65 (95% CI: 1.34–2.04, P = .000), and the HR for studies with low cut-off values was 1.94 (95% CI: 1.28–2.94, P = .002). Studies using univariate analyses had an HR of 2.12 (95% CI: 1.69–2.66, P = .000), and the HR for studies employing multivariate analyses was 1.42 (95% CI: 1.05–1.93, P = .022). For the subgroup analysis by TNM staging method, the HR for studies using I/II staging was 3.10 (95% CI = 1.86–5.15, P = .000), and the HR fur studies using III/IV staging was 1.67 (95% CI = 1.27–2.18, P = .000) (Table 4).
Subgroup meta-analyses stratified by cut-off method, tumor delineation, and disease stage were conducted. Each subgroup analysis indicated the presence of a significant HR for death (Table 5).
4. Discussion
Of late, 18F-FDG-PET/CT has been the imaging modality most commonly used for diagnosis, staging, evaluating response to treatment, and detecting postoperative recurrence and metastasis in PC.[37–41] Since the late 1980s, PET-derived quantitative SUV has been widely used, as this parameter is a robust indicator that can easily be calculated for the evaluation of PET data.[42] The potential role of FDG uptake values in the prediction of prognosis has been recently reported in several meta-analyses, high SUV values at diagnosis were more highly associated with poor survival than were low SUV values in a variety of cancers, such as head and neck cancer, hepatocellular carcinoma, and bone and soft tissue sarcoma.[43–45] MTV and TLG have been considered parameters for that are more reliable for predicting survival than SUVmax, as they reflect whole tumor burden.[46] Recent meta-analyses have also revealed that volumetric parameters, such as MTV and TLG, may serve as prognostic factors in non-small cell lung cancer and head and neck cancer.[20,47]
However, conflicting results have been published regarding the superiority or prognostic value of other quantitative methods derived from PET to SUVmax.[13,14,23,28,34] Although a previous meta-analysis reported that the identification of high SUVmax values based on PET imaging in PC was associated with increased risk of poor survival.[10] One of the main problems is that these studies only assessed a relatively small number of patients, resulting in limited statistical power. Therefore, we conducted a comprehensive meta-analysis to derive more robust estimates regarding the predictive performance of SUVmax. The results of our meta-analysis, which was conducted using the data from the largest number of patients with PC yet, indicated that SUVmax was a prognostic factor for the outcomes of interesting, suggesting that at diagnosis, 18F-FDG-PET/CT imaging may serve as an important imaging tool that plays a predictive role for patients with PC.
After pooling data from the available studies, we found that high SUVmax values were significantly correlated with poor prognosis, including poor EFS and OS. However, the association between SUVmax and survival outcomes may be affected by several confounding factors; therefore, a subgroup analysis was conducted by statistical analysis method to validate the parameters as independent prognostic factors. Multivariate analysis may also serve as an effective method when evaluating potential prognostic factors, and the Cox proportional hazards model or logistic regression model may be used to reduce biases resulting from major confounders.[48] In our study, SUVmax proved to be significantly associated with survival in both the univariate and multivariate analyses; therefore, one could presume that SUVmax might be an independent prognostic factor for survival outcomes.
To evaluate the effects of the methods utilized by each study (Table 1), we performed subgroup analyses by cut-off method, threshold, analysis method, and analysis method. For example, in the cut-off values analysis, we evaluate the methods used to determine cut-off values in the included studies, and specifically assessed the ROC curve and median value methods. In our meta-analysis, 5 articles used receiver operating characteristic curves to determine the optimal cut-off point,[13,23,26–28] which has been reported to be a more reasonable method for cut-off calculation. Therefore, subgroups stratified by the methods were created to evaluate the effect of using different cut-off values. The results of the subgroup analyses showed small and acceptable variations in the HRs for EFS associated with SUVmax (1.50–3.10) despite the wide range of SUVmax values observed (3.4–6.8). In the current meta-analysis, the cut-off value used for SUVmax varied in each study because SUV can be significantly influenced by measurement errors that are associated with both true biological changes and technological factors that cannot be entirely controlled, such as weight composition, diet, fasting state, scanner method, and reconstruction parameters.[49,50]
The question of whether traditional imaging technique can predict PC patient survival remains controversial because previous studies have focused on tumor size. While MTV and TLG, which are a combination of volumetric and metabolic parameters, may be utilized in metabolic analyses of radiotracer activity, reflecting both properties of the tumor tissues. Our findings confirm previous findings suggesting that high volumetric parameter values indicated poor EFS and OS, suggesting that 18F-FDG-PET/CT has vast prospects for predicting survival outcomes in PC patients. Although an SUVmax threshold of 2.5 was used tumor delineation in 3 of the 6 studies included in this meta-analysis, Abelson et al[51] found that an SUVmax threshold of 7 may be a better standard for volume of interest delineation in their patient population. Therefore, the identification of the cut-off values for MTV and TLG values most highly associated with worse OS and PFS should be the subject of further research, the methods used for SUV measurement and tumor delineation should be normalized and standardized, and controversies regarding the most appropriate segmentation method should be resolved. Currently, various commercially available tools for the measurement of volumetric parameters are being developed and disseminated, which may enable faster and easier tumor analysis.[48] Although the methods used for determining optimal cut-off MTV and TLG values or tumor delineation may have affected the MTV or TLG values reported in each study, high MTV and TLG values were associated with increased probability of disease progression and/or death. To assess the effects of the methods used in each included study, we also performed additional subgroup analyses by cut-off method, tumor delineation, and disease stage, the results of which showed there to be acceptable variations of the HRs for EFS and OS. Further prospective studies are in need to validate the findings.
Our meta-analysis had some limitations. Although all included studies were evaluated by NOS scores and considered high quality, we only included 1 prospective study, and most of the studies were retrospective in nature, which may have caused them to be more prone to potential biases. More prospective studies are needed to support and validate our meta-analysis results. Second, significant heterogeneity was observed across the included studies for both OS and EFS. Many possible factors could have caused the observed heterogeneity, such as differences in histology type, TNM stage, study region, treatment method, cut-off value, and HR estimation method. After the sensitivity analyses, the prognostic values of the parameters were not decreased. Second, we only included the English articles, and reviews, conference papers, and editorial materials were excluded; this may have resulted in a language bias and publication bias. However, the results of the Begg tests did not suggest clear evidence of bias. The results of the trim and fill sensitivity analysis further supported the prognostic role of 18F-FDG-PET/CT parameters in PC patients after potentially missing studies were included, which demonstrated that our analysis was reliable.
5. Conclusion
The results of this meta-analysis demonstrated that 18F-FDG-PET/CT parameters, such as SUVmax, MTV, or TLG, may serve as significant prognostic factors for predicting outcomes in PC patients. Despite the clinical and methodological heterogeneity observed across studies, the 18F-FDG-PET/CT parameters may be used to stratify patient risk in terms of disease control and survival and aid in the selection of appropriate treatment strategies for individual patients. Additional large multicenter studies are in need to validate our findings and explore the applicability of other prognostic variables associated with 18F-FDG-PET/CT in efforts to prolong survival of PC patients.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, DMFS = disease metastasis-free survival, EFS = event-free survival, HR = hazard ratio, LAPC = locally advanced pancreatic cancer, MTV = metabolic tumor volume, OS = overall survival, PC = pancreatic carcinoma, PFS = progress-free survival, ROC = receiver operating characteristic curve, SUVmax = maximum standardized uptake value, TLG = total lesion glycolysis, VOI = volume of interest.
DZ and LW contributed equally.
Dr ML had full access to all the data used in this study and is the guarantor of the paper and responsible for the integrity of the data and the accuracy of the data analysis.
Mr DZ and Miss LW contributed to the conception and design of the study; acquisition, analysis, and interpretation of data; and writing and approval of the manuscript.
Miss SB contributed to the design of the study, data analysis, and revision and approval of the manuscript.
Miss HZ contributed to the design study, provision of guidance for data collection, and revision and approval of the manuscript.
Miss JC contributed to the design of the study, data analysis, and approval of the manuscript.
Miss LW contributed to the design of the study, supervision of the field investigation and data collection, and revision and approval of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Can J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [2].Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Prob Cancer 2002;26:176–275. [DOI] [PubMed] [Google Scholar]
- [3].Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118–27. [DOI] [PubMed] [Google Scholar]
- [5].Hawes RH, Xiong Q, Waxman I, et al. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol 2000;95:17–31. [DOI] [PubMed] [Google Scholar]
- [6].Okano K, Suzuki Y. Strategies for early detection of resectable pancreatic cancer. World J Gastroenterol 2014;20:11230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology 2006;238:405–22. [DOI] [PubMed] [Google Scholar]
- [8].Westerterp M, Pruim J, Oyen W, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging 2007;34:392–404. [DOI] [PubMed] [Google Scholar]
- [9].Vriens D, Visser EP, de Geus-Oei LF, et al. Methodological considerations in quantification of oncological FDG PET studies. Eur J Nucl Med Mol Imaging 2010;37:1408–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Z, Chen JQ, Liu JL, et al. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol 2013;19:4808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grassetto G, Rubello D. Role of FDG-PET/CT in diagnosis, staging, response to treatment, and prognosis of pancreatic cancer. Am J Clin Oncol 2011;34:111–4. [DOI] [PubMed] [Google Scholar]
- [12].De Gaetano AM, Rufini V, Castaldi P, et al. Clinical applications of (18)F-FDG PET in the management of hepatobiliary and pancreatic tumors. Abdom Imaging 2012;37:983–1003. [DOI] [PubMed] [Google Scholar]
- [13].Go SI, Song HN, Kang JH, et al. The clinical impact of the sum of the maximum standardized uptake value on the pretreatment with F-FDG-PET/CT in small-cell lung cancer. Oncology 2014;86:1–9. [DOI] [PubMed] [Google Scholar]
- [14].Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schock SC, Edrissi H, Burger D, et al. Microparticles generated during chronic cerebral ischemia deliver proapoptotic signals to cultured endothelial cells. Biochem Biophys Res Commun 2014;450:912–7. [DOI] [PubMed] [Google Scholar]
- [16].Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50(suppl 1):122s–50s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statist Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [18].Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Statist Med 2002;21:3337–51. [DOI] [PubMed] [Google Scholar]
- [19].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014;55:884–90. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Q, Feng Y, Mao X, et al. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography or PET-computed tomography in cervical cancer: a meta-analysis. Int J Gynecol Cancer 2013;23:1184–90. [DOI] [PubMed] [Google Scholar]
- [22].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [23].Im HJ, Oo S, Jung W, et al. Prognostic value of metabolic and volumetric parameters of preoperative FDG-PET/CT in patients with resectable pancreatic cancer. Medicine 2016;95:e3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pimiento JM, Davis-Yadley AH, Kim RD, et al. Metabolic activity by 18F-FDG-PET/CT is prognostic for stage I and II pancreatic cancer. Clin Nucl Med 2016;41:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang SL, Cao S, Sun YN, et al. Standardized uptake value on positron emission tomography/computed tomography predicts prognosis in patients with locally advanced pancreatic cancer. Abdom Imaging 2015;40:3117–21. [DOI] [PubMed] [Google Scholar]
- [26].Chirindel A, Alluri KC, Chaudhry MA, et al. Prognostic value of FDG PET/CT-derived parameters in pancreatic adenocarcinoma at initial PET/CT staging. AJR A J Roentgenol 2015;204:1093–9. [DOI] [PubMed] [Google Scholar]
- [27].Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677–84. [DOI] [PubMed] [Google Scholar]
- [28].Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative (1)(8)F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014;55:898–904. [DOI] [PubMed] [Google Scholar]
- [29].Shinoto M, Yamada S, Yoshikawa K, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography as predictor of distant metastasis in preoperative carbon-ion radiotherapy for pancreatic cancer. Anticancer Res 2013;33:5579–84. [PubMed] [Google Scholar]
- [30].Choi HJ, Kang CM, Lee WJ, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J 2013;54:1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moon SY, Joo KR, So YR, et al. Predictive value of maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med 2013;38:778–83. [DOI] [PubMed] [Google Scholar]
- [32].Hwang JP, Lim I, Chang KJ, et al. Prognostic value of SUVmax measured by Fluorine-18 fluorodeoxyglucose positron emission tomography with computed tomography in patients with pancreatic cancer. Nucl Med Mol Imaging 2012;46:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schellenberg D, Quon A, Minn AY, et al. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2010;77:1420–5. [DOI] [PubMed] [Google Scholar]
- [34].Xu HX, Chen T, Wang WQ, et al. Metabolic tumour burden assessed by (1)(8)F-FDG PET/CT associated with serum CA19–9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging 2014;41:1093–102. [DOI] [PubMed] [Google Scholar]
- [35].Kitasato Y, Yasunaga M, Okuda K, et al. Maximum standardized uptake value on 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography and glucose transporter-1 expression correlates with survival in invasive ductal carcinoma of the pancreas. Pancreas 2014;43:1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cui Y, Song J, Pollom E, et al. Quantitative analysis of (18)F-Fluorodeoxyglucose positron emission tomography identifies novel prognostic imaging biomarkers in locally advanced pancreatic cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2016;96:102–9. [DOI] [PubMed] [Google Scholar]
- [37].Heinrich S, Goerres GW, Schafer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Asagi A, Ohta K, Nasu J, et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013;42:11–9. [DOI] [PubMed] [Google Scholar]
- [39].Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957–63. [DOI] [PubMed] [Google Scholar]
- [40].Okamoto K, Koyama I, Miyazawa M, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol 2011;16:39–44. [DOI] [PubMed] [Google Scholar]
- [41].Topkan E, Parlak C, Kotek A, et al. Predictive value of metabolic 18FDG-PET response on outcomes in patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy. BMC Gastroenterol 2011;11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–48. [PubMed] [Google Scholar]
- [43].Xie P, Li M, Zhao H, et al. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sun DW, An L, Wei F, et al. Prognostic significance of parameters from pretreatment (18)F-FDG PET in hepatocellular carcinoma: a meta-analysis. Abdom Radiol (NY) 2016;41:33–41. [DOI] [PubMed] [Google Scholar]
- [45].Li YJ, Dai YL, Cheng YS, et al. Positron emission tomography (18)F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: a meta-analysis. Eur J Surg Oncol 2016;42:1103–14. [DOI] [PubMed] [Google Scholar]
- [46].Malek E, Sendilnathan A, Yellu M, et al. Metabolic tumor volume on interim PET is a better predictor of outcome in diffuse large B-cell lymphoma than semiquantitative methods. Blood Cancer J 2015;5:e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging 2015;42:241–51. [DOI] [PubMed] [Google Scholar]
- [48].Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol 2013;14:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Adams MC, Turkington TG, Wilson JM, et al. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010;195:310–20. [DOI] [PubMed] [Google Scholar]
- [50].Bai B, Bading J, Conti PS. Tumor quantification in clinical positron emission tomography. Theranostics 2013;3:787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abelson JA, Murphy JD, Trakul N, et al. Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer 2012;78:219–24. [DOI] [PubMed] [Google Scholar]


