Abstract
Platelet, neutrophil, and lymphocyte ratio (PNLR) has its own unique role in influencing postoperative recurrence for patients with hepatocellular carcinoma (HCC). Surgical stress can change systemic inflammatory response of patients. Thus the aim of this study was to identify the prognostic value of changes of platelet times neutrophil to lymphocyte ratio in hepatitis B related HCC within Barcelona clinical liver cancer (BCLC) stage A.
Data of patients with HCC within BCLC stage A were reviewed. Pre-, intra- and postoperative variables were retrospectively and statistically analyzed. The postoperative variable was calculated based on the data obtained on the first postoperative month following liver resection.
A total of 556 patients were included in present study. During the follow-up period, 257 patients experienced recurrence and 150 patients died. Multivariate analyses suggested multiple tumors (hazard ratio [HR] = 2.409; 95% confidence interval [CI] = 1.649–3.518; P < .001), microvascular invasion (MVI) (HR = 1.585; 95% CI = 1.219–2.061; P = .001), and increased postoperative PNLR (HR = 1.900; 95% CI = 1.468–2.457; P < .001) independently associated with postoperative recurrence, whereas MVI (HR = 1.834; 95% CI = 1.324–2.542; P < .001), postoperative neutrophil to lymphocyte ratio (NLR) (HR = 1.151; 95% CI = 1.025–1.294; P = .018) and increased postoperative PNLR (HR = 2.433; 95% CI = 1.667–3.550; P < .001) contributed to postoperative mortality. The 5-year recurrence-free survival and overall survival rates of patients with increased postoperative PNLR (N = 285) versus those with decreased postoperative PNLR (N = 271) were 36.8% versus 61.5% and 47.6% versus 76.4% respectively (P < .001).
Changes of PNLR was a powerful prognostic indicator of poor outcomes in patients with HCC within BCLC stage A. PNLR should be monitored in our postoperative follow-up.
Keywords: hepatocellular carcinoma, liver resection, platelet times neutrophil to lymphocyte ratio, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent malignancy and the third most common cancer-related cause of death worldwide.[1] There are several risks that could contribute to the occurrence of HCC, which are infection of hepatitis B and C, alcoholic cirrhosis, nonalcoholic steatohepatitis, genetic hemochromatosis, primary biliary cirrhosis, among others.[2] Recently, researchers reviewed studies and summarized various risks from occupational exposure could also lead to HCC.[3,4] In China, hepatitis B infection is the most important etiologic agent of HCC. A seropidemiological survey which was performed in 2006 showed the hepatitis B surface antigen carrier rate was 7.18% in the overall Chinese population.[5] Owing to this high prevalence, China alone accounts for about 55% of the HCC cases in the world.[6] Liver resection is widely accepted as a curative treatment for patients with HCC. The Barcelona clinic liver cancer (BCLC) staging classification recommended liver resection for patients with BCLC stage A HCC (single tumor >2 cm; or up to 3 tumors, none of each >3 cm; without macrovascular invasion and extrahepatic metastasis; physical status 0–2; liver function is Child–Pugh A or B status).[7,8] However, the postoperative recurrence rate was still high, and was reported up to 50% to 70% after liver resection.[9]
The mechanism of postoperative recurrence is complex and due to multiple factors. Recently, systemic inflammatory response was considered as an important factor influencing HCC recurrence and/or overall survival for patients with HCC following liver resection, liver transplantation, radiofrequency ablation, and so on.[10–13] Some inflammation-based prognostic systems, such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), prognostic nutritional index (PNI), and so forth, have been developed by previous investigations to predictive postoperative recurrence and/or overall survival.[12–15] However, the prognostic power of these inflammation-based prognostic systems is still under debate.[16] Platelet, neutrophil, and lymphocyte have their own unique role in influencing postoperative recurrence for patients with HCC. Moreover, patient's systemic inflammatory response can be changed by surgical stress after liver resection. Hence, we suggested the changes of an inflammation-based prognostic system combined of platelet, neutrophil, and lymphocyte counts may be better able to mirror the status of host's immune and inflammatory response, and could strengthen the predictive ability. In this study, we would like to clarify whether the changes of platelet × neutrophil to lymphocyte ratio (PNLR) could predict the postoperative outcomes of patients with BCLC stage A hepatitis B-related HCC after liver resection.
2. Methods
2.1. Study group
Data on patients with BCLC stage A HBV-related HCC who underwent liver resection at our center between January 2007 and December 2013 were reviewed. All patients had Child A liver function and positive hepatitis B surface antigen. HCC was confirmed by postoperative pathology. Patients who fit any one of the following criteria were excluded from this study: co-infection with hepatitis C virus; experienced simultaneous splenectomy; ruptured HCC; infections during the perioperative period; re-resection; or received preoperative antitumor treatments. This study was approved by the ethics committee of West China Hospital.
2.2. Follow-up and definitions
All preoperative blood cell counts and differential counts were taken 2 days before liver resection. After operation, all patients were regularly followed up in the first postoperative month and then every 3 months in the subsequent years. Liver function, blood cell tests, serum alpha-fetoprotein (AFP), HBV-DNA, visceral ultrasonography or CT or MR imaging and chest radiography were monitored for all patients. Bone scintigraphy was performed whenever HCC recurrence was suspected. Entecavir or lamivudine was administrated to patients with positive HBV-DNA load. Postoperative recurrence was defined as positive imaging findings compared with preoperative examination values and newly rising tumor marker (AFP) values or confirmation by biopsy or resection. The NLR was defined as absolute neutrophil count divided by the lymphocyte count (109 cells/L).[16] The PLR was estimated as the absolute platelet count divided by the lymphocyte count (109 cells /L).[14] The PNI was calculated as following formula: serum albumin (gram per liter) + 5× lymphocyte count (109 cells/L).[12] PNLR was calculated as follows: platelet × neutrophil divided by lymphocyte count (109/L). Postoperative NLR, PLR, PNI, and PNLR were calculated based on the data obtained on the first postoperative month after operation. Change of PNLR was calculated by postoperative PNLR minus preoperative PNLR. If the postoperative PNLR minus preoperative PNLR was >0, the change of PNLR was considered as increased, if not, it was considered as decreased. An HBV-DNA >104 copies/mL was considered high.[17] An AFP >400 ng/mL was considered high AFP level.[18]
2.3. Statistical analysis
All statistical analyses were performed by SPSS 21.0 (SPSS Inc, Chicago, IL) for windows. All continuous variables are presented as the mean ± SD and compared by using t test for normal distributions and Mann-Whitney U test for abnormal distributions. Categorical variables were compared using the χ2 test or Fisher exact test. The independent risk factors for recurrence-free survival (RFS) and overall survival (OS) were identified by Cox regression. The Kaplan-Meier method was used to compare the postoperative RFS and OS for different groups. The difference of RFS and OS curves was compared using a log-rank test. A P value of <.05 was considered statistically significant.
3. Results
A total of 556 patients were involved in present study, including 95 females and 461 males. As shown in Table 1, the mean age was 50.3 ± 11.8 years. The mean tumor size was 5.6 ± 2.2 cm. A total of 48 patients had multiple tumors (tumor number ≥2). Microvascular invasion (MVI) was detected in 147 patients. One hundred eighty patients had high preoperative HBV-DNA load, whereas 219 patients suffered from high preoperative AFP level. Seventy-nine patients received intraoperative transfusion. The mean follow-up was 40.2 ± 20.0 months. After operation, the PNLR increased in 285 patients whereas decreased in 271 patients. During the follow-up period, 257 patients experienced recurrence, whereas 150 patients died. Among patients with postoperative recurrence, 17 patients received liver transplantation, 23 patients received radiofrequency ablation, 45 patients underwent reresection, and 172 patients underwent transcatheter arterial chemoembolization. The 1-, 3-, 5-year RFS rates were 83.3%, 55.1%, and 48.5% respectively, whereas the 1-, 3-, and 5-year OS rates were 97.5%, 78.7%, and 60.7%, respectively (Fig. 1A and B).
Table 1.
Demographic data of this study.
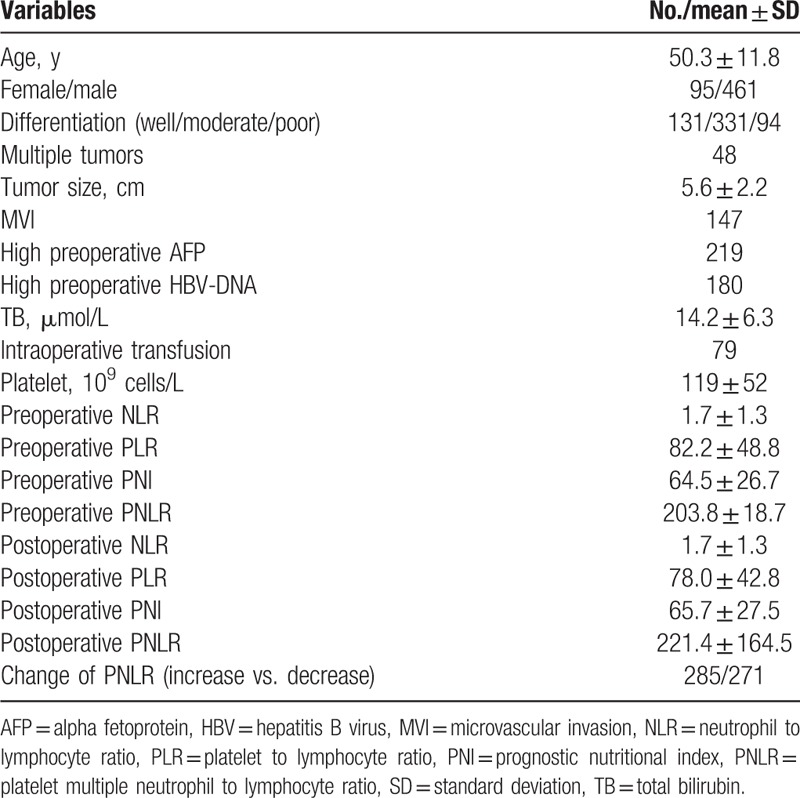
Figure 1.
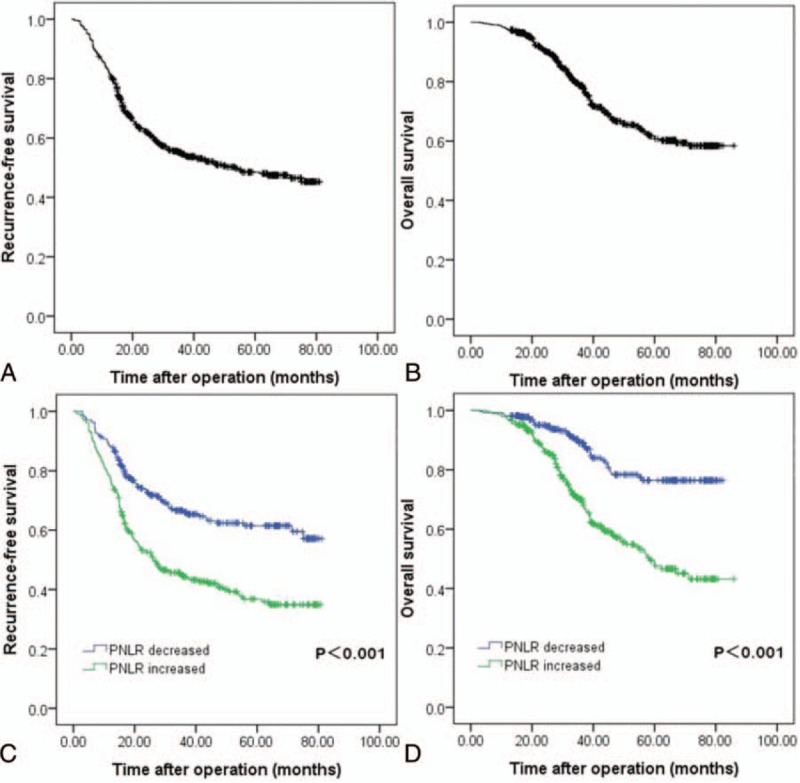
RFS (A) and OS (B) rates of HCC patients after liver resection. RFS (C) and OS (D) curves of patients with increased postoperative PNLR versus those with decreased postoperative PNLR. HCC = hepatocellular carcinoma, PNLR = platelet multiple neutrophil to lymphocyte ratio, RFS = recurrence-free survival, OS = overall survival.
3.1. Risk factors associated with postoperative recurrence
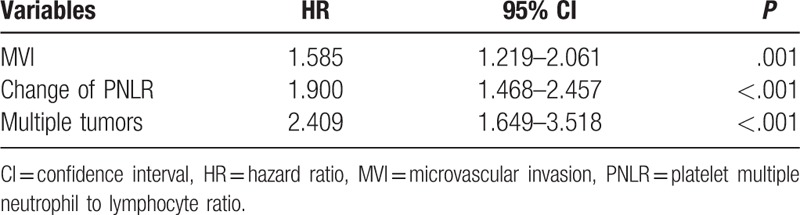
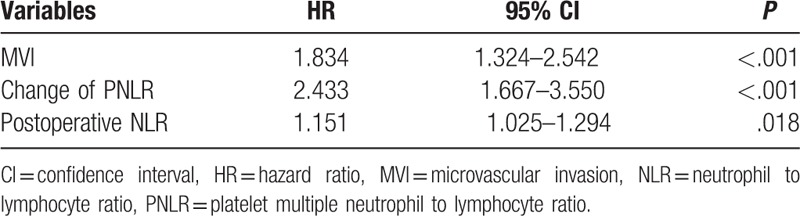
As shown in Table 2, univariate analyses suggested that multiple tumors, MVI, postoperative NLR, and change of PNLR were all potential impacts on RFS. However, only multiple tumors (hazard ratio [HR] = 2.409; 95% confidence interval [CI] = 1.649–3.518; P < .001), MVI (HR = 1.585; 95% CI = 1.219–2.061; P = .001), and change of PNLR (HR = 1.900; 95% CI = 1.468–2.457; P < .001) increased the incidence of postoperative recurrence in the multivariate analysis (Table 3). The 1-, 3-, and 5-year RFS rates of patients with increased PNLR were 77.5%, 44.3%, and 36.8%, respectively, whereas of patients with decreased postoperative PNLR were 88.2%, 66.1%%, and 61.5%, respectively (Fig. 1C, P < .001)
Table 2.
Factors associated with postoperative recurrence in the univariate analyses.
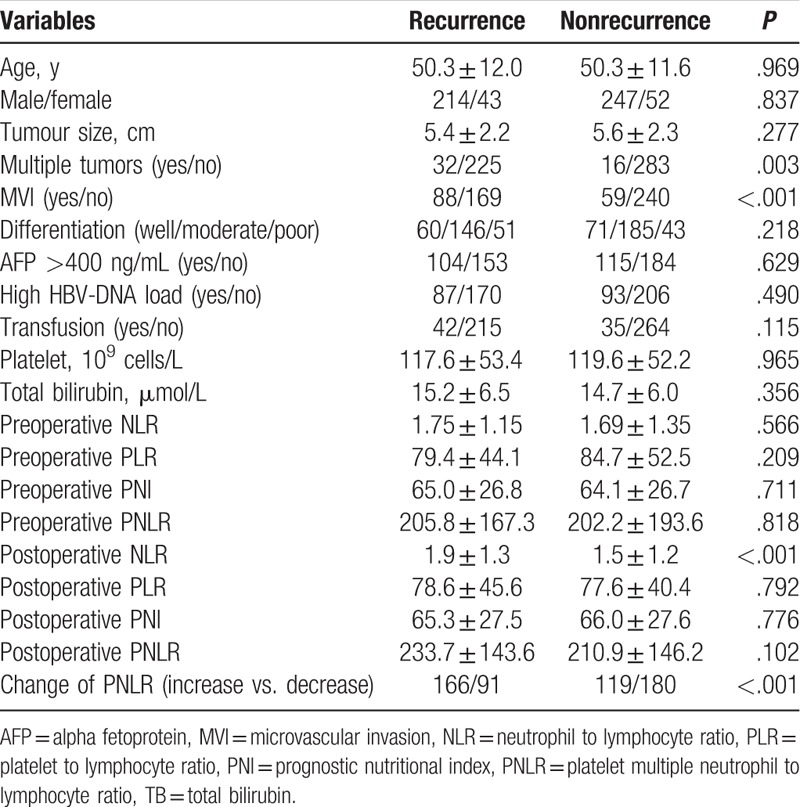
Table 3.
Factors associated with postoperative recurrence in the multivariate analyses.
3.2. Risk factors associated with overall survival
As shown in Table 4 and Table 5, additional analyses were also performed to identify risk factors associated with postoperative survival by univariate and multivariate Cox regression analyses. In the univariate analyses, multiple tumors, MVI, high preoperative AFP level, differentiation, intraoperative transfusion, tumor size, postoperative NLR, and change of PNLR were associated with mortality after liver resection for patients with HCC within the BCLC stage A. However, only MVI (HR = 1.834; 95% CI = 1.324–2.542; P < .001), postoperative NLR (HR = 1.151; 95% CI = 1.025–1.294; P = .018), and change of PNLR (HR = 2.433; 95% CI = 1.667–3.550; P < .001) were demonstrated as independent prognostic factors for poor OS in the multivariate Cox regression analysis. The OS rate of patients with increased PNLR was significantly poor than those with decreased postoperative PNLR. The 1-, 3-, 5-year OS rates were 97.2%, 70.1%, 47.6%, respectively, in patients with increased postoperative PNLR, and 98.2%, 89.0%, 76.4%, respectively, in patients with decreased postoperative PNLR (Fig. 1D, P < .001).
Table 4.
Factors associated with postoperative survival in the univariate analyses.
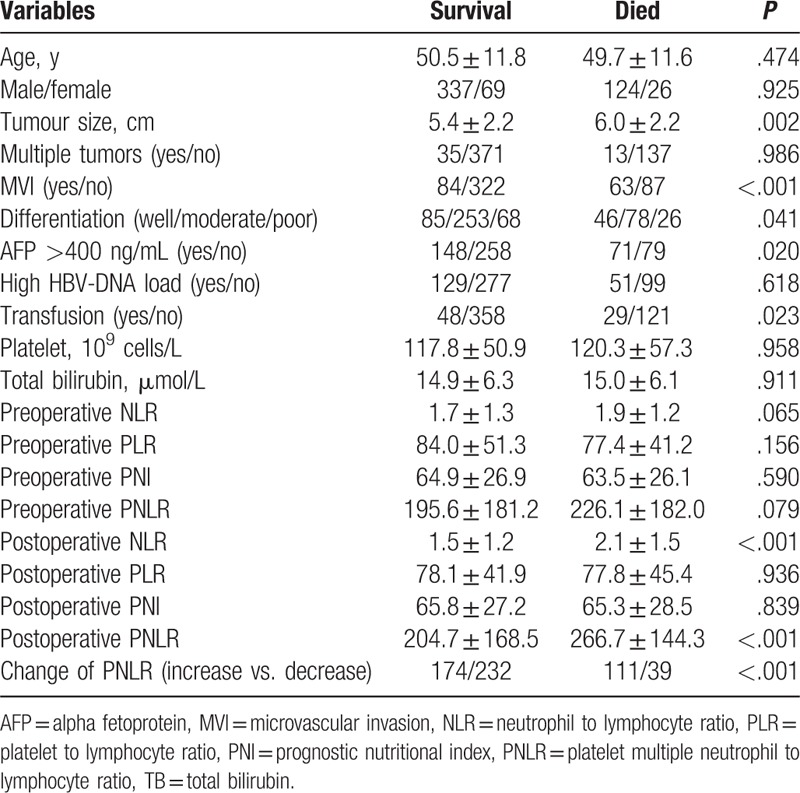
Table 5.
Factors associated with postoperative survival in the multivariate analyses.
4. Discussion
Liver resection is one of the curative managements for patients with HCC. However, postoperative recurrence remains the predominant obstacle to long-term survival for patients with HCC following liver resection. Researchers in this field have established several prediction model to help clinical practice.[19–22] In the present study, we determined increased postoperative PNLR was an independent and useful prognostic predictor of postoperative prognosis for patients with BCLC stage A HCC after liver resection. Both RFS and OS in increased postoperative PNLR group were significantly lower compared with the rates in decreased postoperative PNLR group.
Inflammation plays an important role in the development and progression of malignancy.[23] Some inflammation-based prognostic systems, such as NLR and PLR, were confirmed to influence the prognosis of HCC after liver resection by previous investigations.[11,14,24] However, the mechanisms of these inflammation-based prognostic systems in predicting postoperative recurrence were not identical. Previous studies suggested platelet can shield tumor cells from immune responses by providing a procoagulant surface facilitating amplification of cancer-related coagulation, thus facilitating HCC growth and metastasis.[25,26] Experimental study also confirmed platelet could enhance the progress and invasion of several HCC cell lines.[27] Moreover, platelets contain many angiogenesis-regulating proteins. More than 80% vascular endothelial growth factor (a proangiogenic protein) was compromised in the platelet pool.[28] Sitia et al's experimental study even suggested antiplatelet therapy could prevent HCC and improve survival in a mouse model of chronic hepatitis B.[29] A large cohort study which was performed by Wu et al[30] confirmed aspirin use contributes to a reduced risk of HCC recurrence after liver resection. Different to platelet, Wang et al's study[31] showed the intratumoral expression of PD-L1, which is expressed in HCC and associated with local tumor antigen-specific tolerance for HCC, correlated with NLR, but not PLR and PNI. Motomura et al[32] reported that higher IL-17 levels and higher CD163-expressing tumor-associated macrophages were found in the peritumoral regions for patients with high NLR for patients with HCC after liver transplantation. Different to platelet and neutrophil, lymphocyte did not promote HCC progression and invasion, but play a key role in overcoming HCC recurrence. Accordingly, different inflammation-related variables have different role in influencing HCC recurrence after liver resection. Some investigators also suggested combination of inflammation-based prognostic systems may predict the outcomes of patients with other malignancies.[33] Recently, Wu et al[34] confirmed combination of PLR and NLR was a useful prognostic factor in advanced non-small cell lung cancer patients. Cummings et al[35] also reported PLR and NLR predict endometrial cancer survival. Combination of PLR and NLR can be better to patient's stratification.[35]
A number of investigations suggested preoperative inflammation-based prognostic system could indicate the outcomes of HCC.[12,13] However, liver resection is a stress to patients. We believe the stress which was proposed by operation may influence the balance of immune and inflammation for patients with HCC. Accordingly, we thought change of inflammation-based prognostic system after liver resection may be better than preoperative inflammation-based prognostic system. Our study also confirmed the prognostic power of change of PNLR was better than NLR, PLR, and PNI. Dan et al[10] also suggested the NLR change at the first month after radiofrequency ablation could predict the OS of patients with small HCC.
There are some limitations in the present study. Firstly, this is a single center's respective study. Secondly, our study only involved patients with hepatitis B virus infection. In China, up to 80% HCC patients had a history of hepatitis B virus infection.[36] Whether this conclusion is suitable to other causes related HCC needs a further study.
5. Conclusion
In conclusion, our study suggested change of postoperative PNLR, a simple and easily calculated inflammatory marker, can independently predict postoperative OS and RFS for patients with BCLC A stage HCC after liver resection. Change of postoperative PNLR helps us to identify patients at high risk of postoperative recurrence, should be clinical monitored in our postoperative follow-up.
Footnotes
Abbreviations: BCLC = Barcelona Clinical Liver Cancer, HCC = hepatocellular carcinoma, MVI = microvascular invasion, NLR = neutrophil to lymphocyte ratio, PLR = platelet to lymphocyte ratio, PNI = prognostic nutritional index, PNLR = platelet multiple neutrophil to lymphocyte ratio.
Funding: This study was supported by grants from Scientific and Technological Support Project of Sichuan Province, China (2015SZ0049 and 2016SZ0025).
Author contributions: CJ, CL, WP, and TFW designed the research; CJ, CL, and WP collected the data followed up; CJ analyzed the data; CJ, and CL, and TFW drafted the manuscript; TFW, LNY, BL, WTW, JYY, and MQX contributed to operations and discussion; all listed authors read and approved the final manuscript.
The authors report no conflicts of interest.
References
- [1].Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335–52. [DOI] [PubMed] [Google Scholar]
- [2].Bertino G, Demma S, Ardiri A, et al. Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future therapies. Biomed Res Int 2014;2014:203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [3].Uccello M, Malaguarnera G, Corriere T, et al. Risk of hepatocellular carcinoma in workers exposed to chemicals. Hepat Mon 2012;12:e5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rapisarda V, Loreto C, Malaguarnera M, et al. Hepatocellular carcinoma and the risk of occupational exposure. World J Hepatol 2016;8:573–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 2009;122:98–102. [PubMed] [Google Scholar]
- [6].Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [7].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [8].Maida M, Orlando E, Camma C, et al. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol 2014;20:4141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tateishi R, Shiina S, Akahane M, et al. Frequency, risk factors and survival associated with an intrasubsegmental recurrence after radiofrequency ablation for hepatocellular carcinoma. PLoS One 2013;8:e59040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One 2013;8:e58184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32–41. [DOI] [PubMed] [Google Scholar]
- [12].Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol 2015;22:4138–48. [DOI] [PubMed] [Google Scholar]
- [13].Okamura Y, Ashida R, Ito T, et al. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg 2015;39:1501–9. [DOI] [PubMed] [Google Scholar]
- [14].Chen Q, Dai Z, Yin D, et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore) 2015;94:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu JT, Liao CK, Le PH, et al. Prognostic value of the preoperative neutrophil to lymphocyte ratio in resectable gastric cancer. Medicine (Baltimore) 2015;94:e1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou JY, Zhang L, Li L, et al. High hepatitis B virus load is associated with hepatocellular carcinomas development in Chinese chronic hepatitis B patients: a case control study. Virol J 2012;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726–32. [DOI] [PubMed] [Google Scholar]
- [19].Toro A, Ardiri A, Mannino M, et al. Effect of pre- and post-treatment alpha-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: a retrospective study. BMC Surg 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zou Q, Li J, Wu D, et al. Nomograms for pre-operative and post-operative prediction of long-term survival of patients who underwent repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg Oncol 2016;23:2618–26. [DOI] [PubMed] [Google Scholar]
- [21].Shen J, He L, Li C, et al. Prognostic nomograms for patients with resectable hepatocelluar carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget 2016;7:80783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mehta N, Heimbach J, Harnois DM, et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lu H. Inflammation, a key event in cancer development. Mol Cancer Res 2006;4:221–33. [DOI] [PubMed] [Google Scholar]
- [24].Yamamura K, Sugimoto H, Kanda M, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 2014;21:682–8. [DOI] [PubMed] [Google Scholar]
- [25].Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237–49. [DOI] [PubMed] [Google Scholar]
- [26].Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015;126:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carr BI, Cavallini A, D’Alessandro R, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peterson JE, Zurakowski D, Italiano JE, Jr, et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol 2010;85:487–93. [DOI] [PubMed] [Google Scholar]
- [29].Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012;109:E2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–14. [DOI] [PubMed] [Google Scholar]
- [31].Wang Q, Blank S, Fiel MI, et al. The severity of liver fibrosis influences the prognostic value of inflammation-based scores in hepatitis b-associated hepatocellular carcinoma. Ann Surg Oncol 2015;22(suppl 3):S1125–32. [DOI] [PubMed] [Google Scholar]
- [32].Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58–64. [DOI] [PubMed] [Google Scholar]
- [33].Arigami T, Okumura H, Matsumoto M, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma: a promising blood marker of tumor progression and prognosis. Medicine (Baltimore) 2015;94:e1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu G, Yao Y, Bai C, et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer 2015;6:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cummings M, Merone L, Keeble C, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer 2015;113:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–73. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


