Supplemental Digital Content is available in the text
Keywords: age factors, epidemiology, health surveys, oral health, periodontitis
Abstract
The purpose of this study is to determine an appropriate age threshold at which to recommend the evaluation of moderate and severe periodontitis among Korean adults.
This study involved a cross-sectional analysis using data from the Korean National Health and Nutrition Examination Survey from 2012 to 2014. Incidence rates of periodontitis with the 95% confidence interval (CI) were evaluated. The predictive accuracy of age for periodontitis was determined by calculating the area under curve (AUC) on the basis of the receiver operating characteristic (ROC) curve.
The cutoff value of age was 43 years in men having periodontitis with an AUC of 0.70 with 95% CI of 0.69 to 0.72. The AUC was 0.72 (95% CI: 0.70–0.73), and the cutoff value of age (49 years) was identified for the moderate periodontitis in women. The cutoff values for age with AUCs and 95% CI for individuals with periodontitis were 46 years (0.72 [0.71–0.73]), 43 years (0.73 [0.72, 0.74]), 45 years (0.71 [0.70,0.72]), 43 years (0.73 [0.72, 0.74]), and 45 years (0.74 [0.72, 0.75]) for no obesity, no abdominal obesity, no diabetes mellitus, no hypertension, and no metabolic syndrome groups, respectively.
This study proposed the guideline for the appropriate age threshold at which to recommend the evaluation of moderate and severe periodontitis for the general population and additionally added the guideline for the individuals without systemic disease including diabetes mellitus, hypertension, metabolic syndrome, and obesity. This study suggests that the participants with certain age may be recommended for the regular periodontal evaluation.
1. Introduction
Periodontitis is reported to be prevalent in the general adult population.[1,2] Previous reports evaluated the rate of periodontal destruction before 40 years of age and found out that without interference the periodontal lesion progresses at a relatively even pace and that the progress is continuous.[3] Another report evaluated the rate of progress of periodontal disease in a population never exposed to any programs regarding dental diseases and it was shown that tooth mortality started after 30 years of age and increased throughout the decade in a moderate progression group.[4] It was also reported that older individuals have experienced more generalized severe loss and 50% to 75% of older adults experienced loss of attachment over relatively short periods of time.[5] Event though, detailed epidemiological data on periodontal conditions in elderly people are limited,[6] higher proportions of aged, man, and African–American individuals had established periodontitis, and it was suggested that these groups were at a higher risk for periodontitis.[7] However, in another report evaluating the periodontal conditions in 35- to 44-year-old and 65 to 74-year-old participants showed that a similar percentage of 17% of the younger and 15% of the older cohort scored a Community Periodontal Index of Treatment Needs code 4.[8]
Recommendations for evaluation and treatment of periodontitis with systemic diseases including diabetes mellitus and hypertension is generally accepted,[9,10] and the general recommendation for individuals without systemic diseases is not well established yet. It can be suggested that guidelines when patients would receive the most benefits from treatment of periodontitis considering the individual's general health, since age is an important independent predictor of periodontitis.[11] The purpose of this study is to determine an appropriate age threshold at which to recommend the evaluation of moderate and severe periodontitis among Korean adults.
2. Methods
2.1. Survey and subjects
This study used data from the Korean National Health and Nutrition Examination Survey (KNHANES), which was performed between 2012 and 2014 under the Korean Centers for Disease Control and Prevention and the Korean Ministry of Health and Welfare, Sejong, Korea.[12,13] A total of 18,382 individuals were candidates for the KNHANES. The analysis in this study was confined to a total of 18,382 respondents over 19 years of age. Individuals without oral health values were excluded, reducing the sample to 15,747. The number of individuals was reduced to 15,170 due to insufficient data regarding the fasting time. Finally, 13,162 individuals without missing values for the outcome variables were analyzed. All participants in the survey signed an informed consent form prior to participation. This study was conducted according to the Helsinki Declaration-based Ethical Principles for Medical Research Involving Human Subjects. This study was approved by an institutional review board of the Korean Center for Disease Control and Prevention (2012-01EXP-01-2C, 2013-07CON-03-4C, and 2013-12EXP-03-5C).
2.2. Sociodemographic and lifestyle variables
Trained interviewers from the KNHANES performed the standardized health examination and questionnaire. Current smokers were defined as the participants who smoked currently and had smoked more than 100 cigarettes in their life time. Participants were also categorized, based on the quantity of alcohol consumed per day for the month prior to the interview and the individuals were considered heavy drinkers if they had consumed of >30 g per day. Participants who performed moderate exercise at least 5 times per week for 30 minutes or more per session, or who performed vigorous exercise at least 3 times per week for 20 minutes or more per session were considered regular exercisers. In this study, education levels were categorized as university graduate or higher. If the monthly income of a participant's household was less than $1092.40, the individuals were categorized as the lowest quartile in income.
2.3. Anthropometric and biochemical measurements
Trained staff members from the KNHANES performed the anthropometric measurements. Body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Waist circumference (WC) was measured in a standing position at the narrowest point between the lower border of the rib cage and the iliac crest. A BMI ≥25 kg/m2 was defined as general obesity,[14] and abdominal obesity was defined as a WC ≥90 cm in men or ≥85 cm in women.[15]
Systolic blood pressure and diastolic blood pressure were measured twice at 5-minute intervals using a standard mercury sphygmomanometer (Baumanometer; W.A. Baum Co., Inc., Copiague, NY), and the average values were used for the analysis. A blood sample was collected from the antecubital vein of each individual after fasting for more than 8 hours to measure the concentrations of serum fasting plasma glucose, total cholesterol, triglycerides, and high-density lipoprotein cholesterol.
The individuals were diagnosed as having diabetes if the individual's fasting plasma sugar was ≥126 mg/dL, if hemoglobin A1c was 6.5% or greater, if they were currently using antidiabetic medications or if they had physician-diagnosed diabetes.[16] The individuals were considered to have hypertension if the participant's systolic blood pressure was 140 mm Hg or greater, if the individual's diastolic blood pressure was 90 mm Hg or greater or if the individuals were under the current use of antihypertensive medication.[17] The individuals were considered to have metabolic syndrome if the individuals sufficed 3 or more of the following criteria: waist circumference of 90 cm or greater in men and 80 cm or greater in women; fasting triglycerides ≥150 mg/dL or use of lipid-lowering medication; high-density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women or use of medication; blood pressure ≥130/85 mm Hg or use of antihypertensive medication in a patient with a history of hypertension; and fasting blood glucose ≥100 mg/dL or current use of antidiabetic medication.[18]
2.4. Oral health behaviors, periodontitis, and number of natural teeth
The community periodontal index (CPI) developed by the World Health Organization was used to assess periodontal condition.[19] Participants were considered to have moderate periodontitis if their CPI was ≥3. If the participants’ CPI was ≥4, the individuals were considered to have severe periodontitis.
Tooth brushing frequency and use of secondary oral products were used for the evaluation of oral health behaviors. The frequency of tooth brushing was calculated by the total number of tooth brushing per day. Secondary oral products included dental floss, mouthwash, interdental brushes, electric toothbrushes, irrigation devices, tongue cleaners, end-tufted brushes, and special devices for dentures. Presence of tooth pain, experience of orthodontic treatment, and dental checkup within a year were obtained. Self-reported oral state, chewing ability, and speech ability were categorized into favorable, average, and problematic.
2.5. Statistical analysis
Results are presented as percentages (standard error) for categorical variables. Differences between nominal variables were compared using a chi-square test. Incidence rates of periodontitis were calculated by dividing the number of events by person-time at risk, with the 95% CI estimated by exact binomial probabilities. The predictive accuracy of age for periodontitis was calculated by calculating the c-index on the basis of the receiver operating characteristic (ROC) curve. The cutoff age value in the prediction of periodontitis was defined as the cutoff point having the highest Youden index (sensitivity + specificity − 1). Statistical significance was set at a P value of <.05.
3. Results
Table 1 shows the baseline characteristics of the studied individuals according to their age groups. Moderate and severe periodontitis seemed to increase with age. The percentage of waist circumference ≥90 cm in men and ≥80 cm in women increased with age. The percentage of man in whole population decreased with increasing age. The percentage of current drinking decreased with increasing age. The percentage of diabetes mellitus, hypertension, and metabolic syndrome increased with age. Interestingly, the percentage of diabetes mellitus increased >3 times in the 45 to 49 age group when compared with the 30 to 44 age group. Similarly, the percentage of hypertension increased 2.6 times in the 45 to 49 age group when compared with the 30 to 44 age group. The percentage of metabolic syndrome increased 1.9 times in the 45 to 49 age group when compared with the 30 to 44 age group.
Table 1.
The baseline characteristics of the studied individuals according to the age groups.
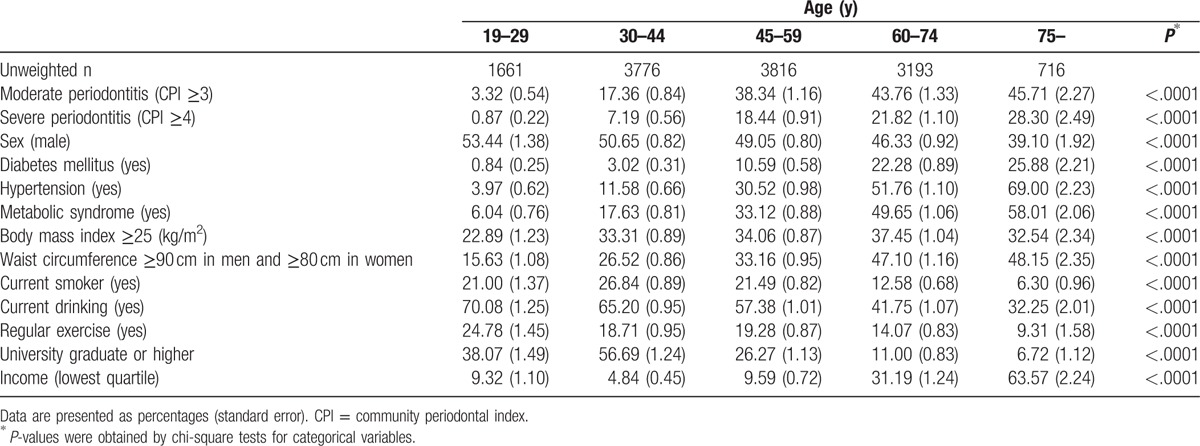
The prevalence of moderate and severe periodontitis is shown in Figure 1. The percentage of periodontitis (moderate periodontitis + severe periodontitis) increased from 24.2% (age group of 40–44 years) to 33.7% (age group of 45–49 years). Figure 2 shows the ROC curve on the basis of age for the prediction of moderated and severe periodontitis. AUC for age was 0.71 (95% confidence of interval [CI]: 0.70–0.72), and the cutoff value for age (46 years) was identified for the moderate periodontitis on the basis of the highest Youden index. The c-index for age was 0.70 (95% CI: 0.69–0.71), and the cutoff value for age (45 years) was identified for the severe periodontitis on the basis of the highest Youden index. The sensitivity, specificity, and Youden index in predicting for moderated and severe periodontitis for different cutoff values of age are shown in Supplementary Table 1.
Figure 1.
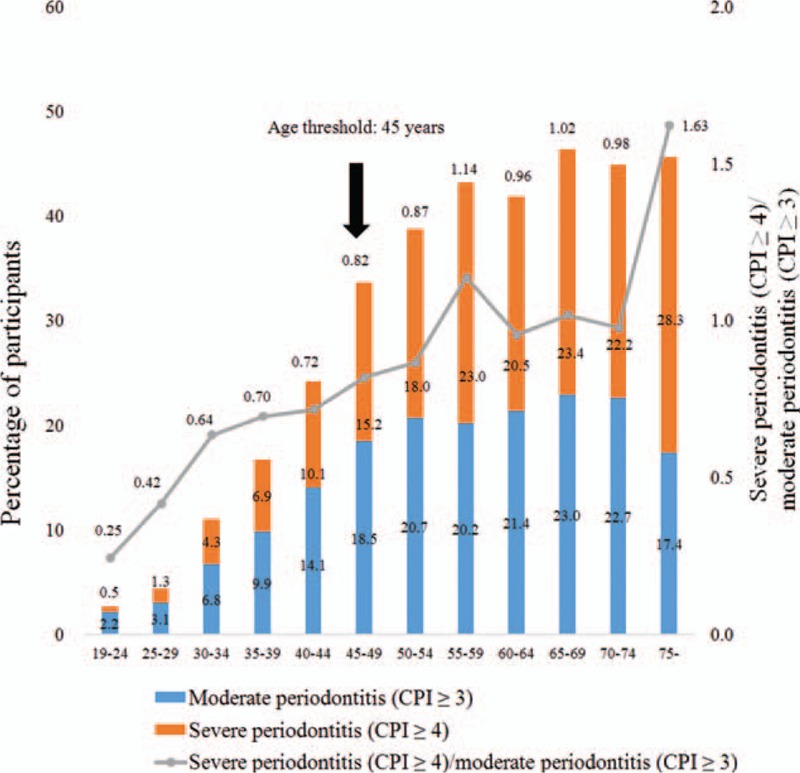
The prevalence of moderate and severe periodontitis.
Figure 2.
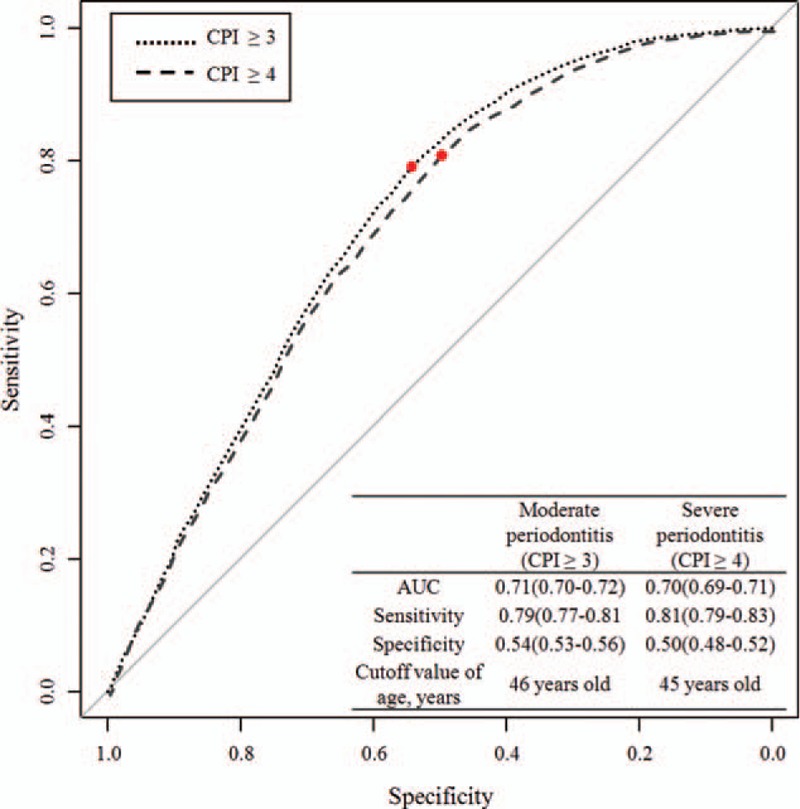
ROC curve of age in predicting moderate and severe periodontitis. The AUC on the basis of the AUC for age in predicting moderate periodontitis and severe periodontitis are 0.71 and 0.70, respectively. The cutoff values of ages (46 years for moderate periodontitis and 45 for severe periodontitis) were identified on the basis of the highest Youden indices. AUC = area under the curve, ROC = receiver operating characteristic. ∗Youden index = [Sensitivity + Specificity − 1].
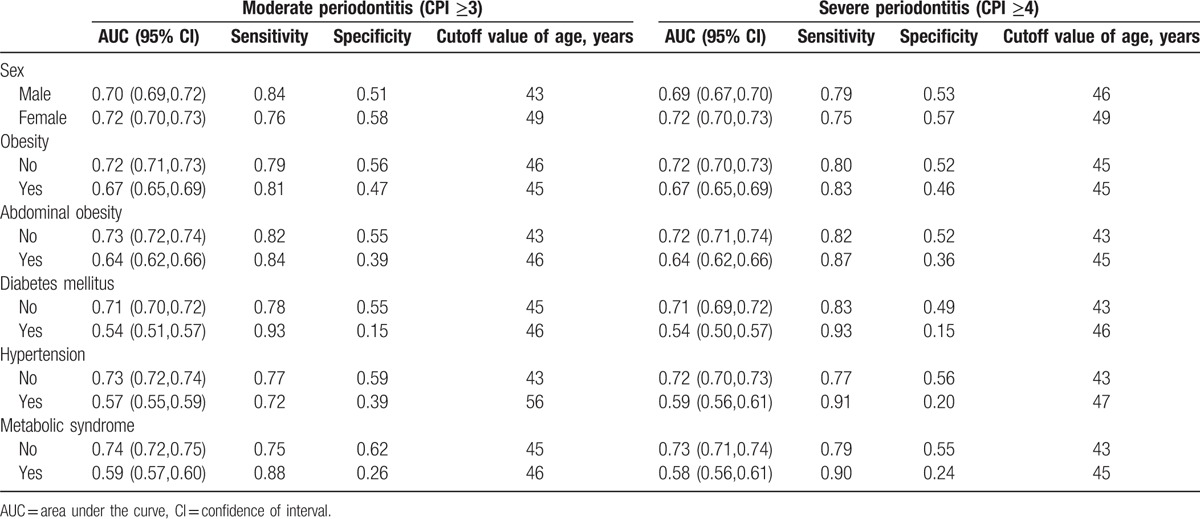
Table 2 shows the AUC, sensitivity, and specificity of the studied individuals categorized by sex, presence of obesity, and the presence of systemic diseases. The cutoff value of age was 43 years in men having periodontitis with an AUC of 0.70 with 95% CI of 0.69 to 0.72. The AUC was 0.72 (95% CI: 0.70–0.73), and the cutoff value for age (49 years) was identified for the moderate periodontitis in women. The AUC of the individuals with systemic diseases for periodontitis was 0.67 for obesity, 0.64 for abdominal obesity, 0.54 for diabetes mellitus, 0.57 for hypertension, and 0.59 for metabolic syndrome. The cutoff values for age with AUCs and 95% CI for individuals with periodontitis were 46 years (0.72 [0.71–0.73]), 43 years (0.73 [0.72, 0.74]), 45 years (0.71 [0.70, 0.72]), 43 years (0.73 [0.72, 0.74]), and 45 years (0.74 [0.72, 0.75]) for no obesity, no abdominal obesity, no diabetes mellitus, no hypertension, and no metabolic syndrome groups, respectively.
Table 2.
Area under the curve, sensitivity, and specificity of the studied individuals categorized by sex, presence of obesity, and the presence of systemic diseases.
The oral health characteristics of the studied individuals according to the presence of periodontitis are shown in Supplementary Table 2. Individuals with moderate periodontitis experienced more tooth pain, chewing problems, and speech problems. Participants with periodontitis had less dental checkups (P < .05). The participants with frequent tooth brushing habits made up a smaller portion of participants with periodontitis. The individuals with periodontitis flossed less and performed less interdental brushing (P < .05).
Supplementary Table 3, shows the oral health characteristics of the studied individuals according to age groups. The percentage of favorable chewing decreased from 73.97% in age 30 to 44 group to 59.87% age 45 to 59 group. The percentage of individuals with 28 teeth dropped from 63.58% in the age 30 to 44 group to 38.34% in the age 45 to 59 group. The percentage of individuals with floss use dropped from 30.21% in the age 30 to 44 group to 18.59% in the age 45 to 59 group.
4. Discussion
This study suggests that for general population, the evaluation of periodontitis should be recommended for all men and women above the ages of 43 and 49, respectively. For subgroup analysis, the evaluation of periodontitis should be routinely be considered at the age of 45 or above for the individuals without diabetes mellitus, 43 or above for individuals without hypertension, 45 or above for individuals without metabolic syndrome, and 46 or above for individuals without obesity.
It should be addressed that continuous destruction may be noted if optimal treatment is not performed at appropriate time point.[20] Predictors of destructive periodontal disease incidence and progression were evaluated in Chinese adults, and it was suggested that age was 1 factor in predicting for pressing disease.[21] In a previous report from a province in Europe, the prevalence of periodontitis increased significantly with age and remained constant after the age of 50 to 59.[22] This study proposed 45 years to be the cutoff value for the evaluation of the need of treatment of periodontitis. This report can be of great importance for making the strategy for the general population as well as individuals without systemic diseases.
Many definitions of periodontitis have been used in the previous researches for population-based studies.[23] The clinical diagnosis of periodontitis is based on measurements of periodontal probing depth, clinical attachment level, presence of gingival inflammation, radiographic evaluation, and loss of alveolar bone.[24] Some authors suggested that a combination of moderate and severe periodontitis may not be sufficient to determine the total prevalence of periodontitis in a population, and they proposed a definition for mild periodontitis.[25] However, this study used partial mouth recording protocols of CPI for the definition of moderate periodontitis as CPI ≥3 and severe periodontitis as CPI ≥4, because there were limited resources including funding, the number of dental practitioners, and time.[26] It must also be noted that there is limitation of possible underestimation of the prevalence of periodontitis with partial mouth recordings.[19,27]
In spite of these limitations, this study had several strengths. The KNHANES is composed of nationally representative samples and the data were obtained using a systematic sampling method that was adjusted for the number of members in households while accounting for administration district, region, and type of residence in the Republic of Korea.[28] The protocol of sampling uses a stratified, multistage, probability-cluster survey of the non-institutionalized civilian population in the Republic of Korea.[29] Trained interviewers visited the subjects in their homes and performed the standardized health examination and questionnaire.[30,31] Moreover, this study assessed the effects of systemic diseases by subgroup analysis. Diagnostic accuracy was evaluated by the AUC in the ROC curve in this study, and it was shown that the diagnostic accuracy was good if the AUC was 0.70 to 0.80, poor for 0.60 to 0.70, and a failure for 0.50 to 0.60.[32,33] In this study, the AUC of subgroups without systemic disease was >0.70, indicating good diagnostic accuracy.
Conclusively, this study proposed the guideline for the appropriate age threshold at which to recommend the evaluation of moderate and severe periodontitis for the general population and additionally added the guideline for the individuals without systemic disease including diabetes mellitus, hypertension, metabolic syndrome, and obesity. This study suggests that the participants with certain age may be recommended for the regular periodontal evaluation.
Supplementary Material
Acknowledgments
The authors thank the Korea Centers for Disease Control and Prevention for providing the data.
Footnotes
Abbreviations: AUC = area under curve, BMI = body mass index, CI = confidence interval, CPI = community periodontal index, KNHANES = Korean National Health and Nutrition Examination Survey, ROC = receiver operating characteristic.
Funding: This research was partly supported by Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea and partly supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technology & Future Planning (NRF-2017R1A1A1A05001307).
Competing interests: The authors declare that no competing interests exist.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999;70:13–29. [DOI] [PubMed] [Google Scholar]
- [2].Fox CH. New considerations in the prevalence of periodontal disease. Curr Opin Dent 1992;2:5–11. [PubMed] [Google Scholar]
- [3].Loe H, Anerud A, Boysen H, et al. The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol 1978;49:607–20. [DOI] [PubMed] [Google Scholar]
- [4].Loe H, Anerud A, Boysen H, et al. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol 1986;13:431–45. [DOI] [PubMed] [Google Scholar]
- [5].Locker D, Slade GD, Murray H. Epidemiology of periodontal disease among older adults: a review. Periodontol 2000 1998;16:16–33. [DOI] [PubMed] [Google Scholar]
- [6].Hirotomi T, Yoshihara A, Yano M, et al. Longitudinal study on periodontal conditions in healthy elderly people in Japan. Community Dent Oral Epidemiol 2002;30:409–17. [DOI] [PubMed] [Google Scholar]
- [7].Machtei EE, Christersson LA, Grossi SG, et al. Clinical criteria for the definition of “established periodontitis”. J Periodontol 1992;63:206–14. [DOI] [PubMed] [Google Scholar]
- [8].Holmgren CJ, Corbet EF, Lim LP. Periodontal conditions among the middle-aged and the elderly in Hong Kong. Community Dent Oral Epidemiol 1994;22:396–402. [DOI] [PubMed] [Google Scholar]
- [9].Linden G, Patterson C, Evans A, et al. Obesity and periodontitis in 60-70-year-old men. J Clin Periodontol 2007;34:461–6. [DOI] [PubMed] [Google Scholar]
- [10].Martin-Cabezas R, Seelam N, Petit C, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J 2016;180:98–112. [DOI] [PubMed] [Google Scholar]
- [11].Abdellatif HM, Burt BA. An epidemiological investigation into the relative importance of age and oral hygiene status as determinants of periodontitis. J Dent Res 1987;66:13–8. [DOI] [PubMed] [Google Scholar]
- [12].Lim SG, Han K, Kim HA, et al. Association between insulin resistance and periodontitis in Korean adults. J Clin Periodontol 2014;41:121–30. [DOI] [PubMed] [Google Scholar]
- [13].Ko SH, Kwon HS, Kim DJ, et al. Higher prevalence and awareness, but lower control rate of hypertension in patients with diabetes than general population: the fifth korean national health and nutrition examination survey in 2011. Diabetes Metab J 2014;38:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oh SW, Shin SA, Yun YH, et al. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obes Res 2004;12:2031–40. [DOI] [PubMed] [Google Scholar]
- [15].Lee S, Park HS, Kim SM, et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J Obes 2006;15:1–9. [Google Scholar]
- [16].Jeon JY, Ko SH, Kwon HS, et al. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J 2013;37:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lenfant C, Chobanian AV, Jones DW, et al. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 2003;41:1178–9. [DOI] [PubMed] [Google Scholar]
- [18].Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [19].Park JB, Han K, Park YG, et al. Association between alcohol consumption and periodontal disease: the 2008 to 2010 Korea National Health and Nutrition Examination Survey. J Periodontol 2014;85:1521–8. [DOI] [PubMed] [Google Scholar]
- [20].Onabolu O, Donos N, Tu YK, et al. Periodontal progression based on radiographic records: an observational study in chronic and aggressive periodontitis. J Dent 2015;43:673–82. [DOI] [PubMed] [Google Scholar]
- [21].Baelum V, Luan WM, Chen X, et al. Predictors of destructive periodontal disease incidence and progression in adult and elderly Chinese. Community Dent Oral Epidemiol 1997;25:265–72. [DOI] [PubMed] [Google Scholar]
- [22].Holtfreter B, Schwahn C, Biffar R, et al. Epidemiology of periodontal diseases in the study of health in Pomerania. J Clin Periodontol 2009;36:114–23. [DOI] [PubMed] [Google Scholar]
- [23].Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78:1387–99. [DOI] [PubMed] [Google Scholar]
- [24].Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J 2009;54(Suppl):S11–26. [DOI] [PubMed] [Google Scholar]
- [25].Eke PI, Page RC, Wei L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 2012;83:1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol 2008;35:659–67. [DOI] [PubMed] [Google Scholar]
- [27].Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal disease. J Periodontol 2005;76:262–7. [DOI] [PubMed] [Google Scholar]
- [28].Lee M, Choi YH, Sagong J, et al. The interactive association of smoking and drinking levels with presence of periodontitis in South Korean adults. BMC Oral Health 2016;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hong E. Comparison of quality of life according to community walking in stroke patients. J Phys Ther Sci 2015;27:2391–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han K, Ko Y, Park YG, et al. Associations between the number of natural teeth in postmenopausal women and duration of lactation: The 2010–2012 Korea National Health and Nutrition Examination Survey. Maturitas 2016;85:73–8. [DOI] [PubMed] [Google Scholar]
- [32].Musiitwa PC, Galukande M, Bugeza S, et al. Emergency ultrasound predicting the need for therapeutic laparotomy among blunt abdominal trauma patients in a Sub-Saharan African hospital. Emerg Med Int 2014;2014:793437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


