Supplemental Digital Content is available in the text
Keywords: breast cancer, endocrine therapy, first-line treatment, network meta-analysis
Abstract
Background:
Endocrine therapy was recommended as the preferred first-line treatment for hormone receptor-positive (HR+, i.e., ER+ and/or PgR+), human epidermal growth factor receptor-2-negative (HER2−) postmenopausal advanced breast cancer (ABC), but which endocrine monotherapy is optimal lacks consensus. We aimed to identify the optimal endocrine monotherapy with a network meta-analysis.
Methods:
We performed a network meta-analysis for a comprehensive analysis of 6 first-line endocrine monotherapies (letrozole, anastrozole, exemestane, tamoxifen, fulvestrant 250 mg and 500 mg) for HR+ HER2− metastatic or locally advanced breast cancer in postmenopausal patients. The main outcomes were objective response rate (ORR), time to progression (TTP), and progression-free survival (PFS). Secondary outcomes were adverse events.
Results:
We identified 27 articles of 8 randomized controlled trials including 3492 patients in the network meta-analysis. For ORR, the treatments ranked in descending order of effectiveness were letrozole > exemestane > anastrozole > fulvestrant 500 mg > tamoxifen > fulvestrant 250 mg. For TTP/PFS, the order was fulvestrant 500 mg > letrozole > anastrozole > exemestane > tamoxifen > fulvestrant 250 mg. We directly compared adverse events and found that tamoxifen produced more hot flash events than fulvestrant 250 mg.
Conclusions:
Fulvestrant 500 mg and letrozole might be optimal first-line endocrine monotherapy choices for HR+ HER2− ABC because of efficacious ORR and TTP/PFS, with a favorable tolerability profile. However, direct comparisons among endocrine monotherapies in the first-line therapy setting are still required to robustly demonstrate any differences among these endocrine agents. Clinical choices should also depend on the specific disease situation and duration of endocrine therapy.
1. Introduction
Breast cancer is the most common cancer among women in the world; nearly 1.67 million new cancer cases were diagnosed (25% of all cancers) in 2012.[1] Advanced breast cancer (ABC) is a treatable but still generally incurable disease; most patients are postmenopausal women with hormone receptor-positive and human epidermal growth factor receptor-2-negative (HR+ HER2−) ABC. Current goals of therapy are to both optimize survival time and palliate symptoms to maintain quality of life. Endocrine therapy was recommended as the preferred first-line treatment to achieve these goals because of proven efficacy and generally favorable tolerability profile.[2–4]
Tamoxifen, a selective estrogen receptor modulator, was the most widely used first-line endocrine therapy for postmenopausal patients with HR+ HER2− locally advanced or metastatic breast cancer for many years.[5] In recent years, third-generation aromatase inhibitors (AIs) including anastrozole, letrozole, and exemestane have largely replaced tamoxifen as first-line endocrine therapy because of greater efficacy and tolerability.[6,7] Fulvestrant is an estrogen receptor (ER) downregulator distinct from other endocrine agents, and fulvestrant, 500 mg (high dose), has efficacy superior to fulvestrant 250 mg (low dose) for treating ER-positive ABC with progression after previous endocrine therapy.[8–10] A clinical trial found that high-dose fulvestrant was at least as effective as anastrozole in clinical benefit rate and ORR and was associated with significantly longer TTP in first-line ABC therapy.[11] So far, no study has directly compared first-line treatment with letrozole and the 2 other AIs or high-dose and low-dose fulvestrant. We have insufficient evidence from head-to-head clinical trials in the first-line treatment setting. The clinical significance and difference of these different endocrine therapies remain uncertain.
Network meta-analysis can combine direct and indirect evidence from different studies simultaneously and compare all therapeutic methods to assess the relative efficacy of each treatment based on randomization,[12,13] so it can assess the relative effects of different endocrine therapies better than traditional head-to-head meta-analysis. Thus, we performed a network meta-analysis for a comprehensive analysis of 6 first-line endocrine monotherapies (letrozole, anastrozole, exemestane, tamoxifen, fulvestrant 250 and 500 mg) for HR+ HER2− ABC in postmenopausal patients.
2. Materials and methods
The reporting of this study adhered to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.[14] Two investigators (JZ and YH) independently performed the literature search, study selection, and data extraction. Discrepancies were resolved by discussion with a third investigator (KW). This study was performed with the approval of the institutional review board of Shantou University Medical College.
2.1. Literature search
ABC comprises both inoperable locally ABC (LABC) and metastatic breast cancer (MBC) or stage IV.[3] We identified randomized controlled trials (RCTs) of endocrine therapy for human HR+ HER2− ABC by searching MEDLINE via PubMed for articles published through May 2015 with the following MeSH terms and free text words: breast neoplasm, breast, mammary, cancer, carcinoma, neoplasm, tumor; advanced, metastatic; aromatase inhibitors, anastrozole, arimidex, letrozole, femara, exemestane, aromasin, tamoxifen, nolvadex, fulvestrant, selective estrogen receptor down regulator. In addition, reference lists of retrieved articles and the websites of American Society of Clinical Oncology, San Antonio Breast Cancer Symposium, and ClinicalTrials.gov were checked to identify further studies.
2.2. Study selection
Eligible studies were RCTs, blinded or not, assessing the efficacy and safety of anastrozole, letrozole, exemestane, tamoxifen, fulvestrant 250 and 500 mg, for first-line monotherapy of HR+ (ER+ and/or PgR+) postmenopausal women with metastatic or LABC who had no endocrine or cytotoxic chemotherapy for advanced disease, or had received no adjuvant endocrine therapy within 12 months before entry into the trials. We excluded studies that did not report the outcomes of interest, polyendocrine therapy studies, studies of endocrine monotherapy used as neoadjuvant treatment, abstracts from scientific meetings, and publications not in English or Chinese.
2.3. Data extraction and quality assessments
Two investigators independently extracted the first author, publication year, study location, study design, type of blinding, patient characteristics, and outcome measures from reports. We used the Cochrane Collaboration Risk of Bias tool to assess study quality, including the following potential biases: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias.[15] Any disagreements during extraction were resolved by consensus.
The primary outcome was efficacy, including objective response rate (ORR), time to progression (TTP), and progression-free survival (PFS). Secondary outcomes were adverse events. Because adverse events were inconsistently reported across studies, we selected the most frequently reported events (hot flashes, weight gain, nausea, and bone pain). For ORR and adverse events, dichotomous data were extracted as the number of patients with the outcome of interest and the total number of patients in the treatment groups. For TTP and PFS, survival data were extracted as the hazard ratio (HR) and 95% confidence interval (95% CI). Because the survival time in ABC is short, most deaths were assumed to be disease-specific. Each analyzed study included disease-specific death events as an endpoint; therefore, TTP and PFS were assumed to be similar.
2.4. Statistical analysis
The analysis of patients was based on intent-to-treat and that of efficacy on total number of randomly assigned patients. For ORR and adverse events, if only percentages were reported, the nearest whole number of events was estimated instead of the actual number.
For direct comparison of different treatments, we conducted pair-wise meta-analysis to synthesize studies comparing the same pair of treatments.[16] Odds ratios (ORs) and 95% CIs were calculated for dichotomous outcomes. Statistical heterogeneity among studies was assessed with the inconsistency statistic (I2). I2 < 25% was considered low heterogeneity and I2 > 50% high heterogeneity.[17] Calculations involved use of STATA 12.0 (StataCorp, College Station, TX).
For the primary analysis, we conducted Bayesian network meta-analysis to synthesize direct and indirect treatment comparisons to assess the treatment effect between all interventions and rank the treatments graphically.[18–21] Analysis based on noninformative priors for effect sizes and precision involved the Markov chain Monte Carlo method with 10,000 initial iterations to burn in and the next 55,000 iterations for estimations.[20,22] We compared outcome variables with a fixed-effects model. The consistency between direct and indirect evidence is one important assumption of the network meta-analysis. We checked this assumption by the Bucher method to determine whether it was similar enough to combine the direct and indirect evidence.[23–25] That is, we calculated the difference between direct and indirect evidence in closed loops in the network. Inconsistent loops were identified with a 95% CI excluding 0, which could confirm the disagreement between direct and indirect evidence.[26] We also performed a sensitivity analysis repeating the main computations with a random-effects model. Deviance information criteria (DIC) was used to compare the fit of the fixed-effects and random-effects models .[23] Calculations involved use of R (http://www.R-project.org, the R Foundation for Statistical Computing, Vienna, Austria) and WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK).
3. Results
3.1. Characteristics of included trials
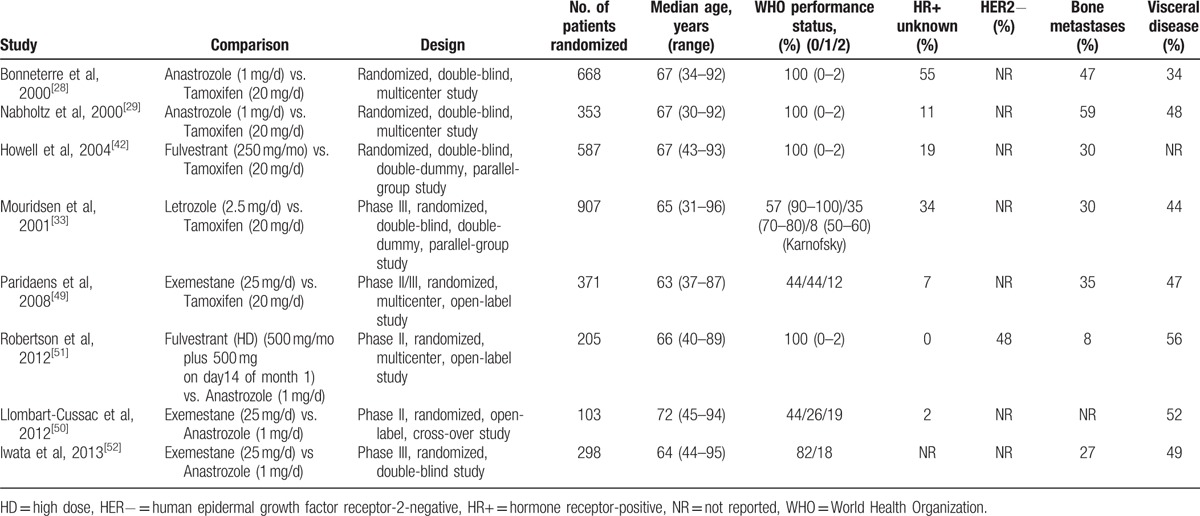
The literature search yielded 2006 records; 59 records remained after screening titles and abstracts. We added another 2 articles from reference lists, for 61 full-text articles assessed for eligibility; 34 articles were excluded. Finally, 27 articles of 8 RCTs were included (Fig. 1). All were 2-arm trials [11,27–52] with 3492 patients with ABC randomly assigned to receive 1 of the 6 first-line monotherapies: anastrozole, letrozole, exemestane, tamoxifen, fulvestrant, 250 and 500 mg. The main characteristics of the studies are in Table 1. The median ages of patients ranged from 63 to 72 years. The methodological quality of 5 double-blind studies was high and that of 3 other open-label studies [11,49,50] was moderate (Supplemental Data, S1 Fig, S2 Fig). All studies were considered to have no selective reporting bias or other bias, but most did not report the techniques for concealment.
Figure 1.
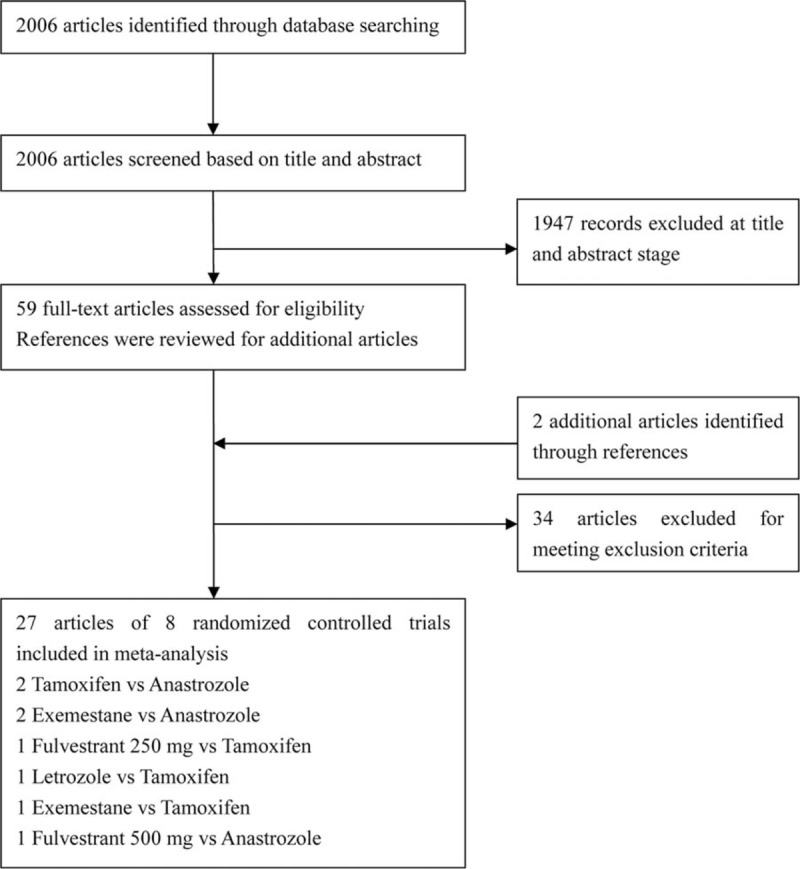
Flowchart for study selection.
Table 1.
Characteristics of included studies.
3.2. Direct comparisons
For direct comparison of different treatments (Supplemental Data, S1 Table), the results suggested that letrozole was more efficacious for both ORR and TTP/PFS than tamoxifen; exemestane was more efficacious for ORR than tamoxifen; and fulvestrant 500 mg was more efficacious for TTP/PFS than anastrozole. In side-effect analysis, fulvestrant 250 mg produced fewer hot flash events than tamoxifen, with no difference between other adverse event types.
3.3. Network meta-analysis
The full network of comparisons is illustrated in Fig. 2. We found one closed loop of comparisons connecting anastrozole, exemestane, and tamoxifen. We assessed the difference between direct and indirect estimates for this loop by inconsistency factors (IFs) with corresponding 95% CIs. IFs were compatible with zero (ORR, IF = 0.61, 95% CI −0.17 to 1.39; TTP/PFS, IF = 0.18, 95% CI −0.21 to 0.58), which indicated that the loops were consistent.
Figure 2.
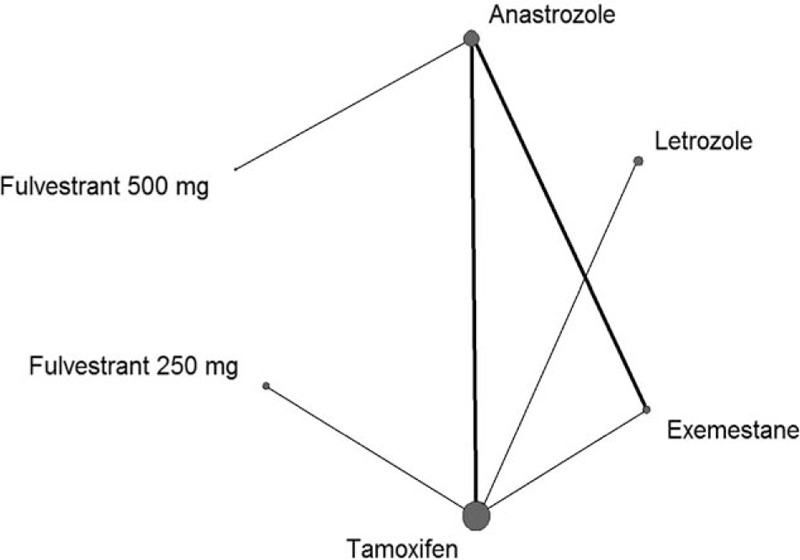
Network of eligible comparisons for the network meta-analysis for efficacy. The size of the nodes is proportional to the number of randomized participants (sample size), and the width of the lines is proportional to the number of trials comparing each pair of treatments.
The network meta-analysis results were based on a fixed-effects model because of better goodness of fit than random-effect models. Overall, the model fit was relatively robust. The efficacy of the 6 first-line monotherapies in the network meta-analysis is presented in Table 2. For ORR, letrozole was more efficacious than tamoxifen and fulvestrant 250 mg (OR = 0.59, 95% CI 0.43–0.80 and OR = 0.54, 95% CI 0.34–0.85, respectively) and exemestane was more efficacious than tamoxifen and fulvestrant 250 mg (OR = 0.67, 95% CI 0.48–0.91 and OR = 0.61, 95% CI 0.37–0.97, respectively). Most studies reported TTP; only 1 study reported PFS.[49] For TTP/PFS, anastrozole and letrozole were more efficacious than tamoxifen (HR = 0.84, 95% CI 0.72–0.99 and HR = 0.70, 95% CI 0.60–0.81, respectively); anastrozole, letrozole, and exemestane were more efficacious than fulvestrant 250 mg (HR = 0.72, 95% CI 0.56–0.93; HR = 0.60, 95% CI 0.46–0.76; HR = 0.75, 95% CI 0.57–0.97, respectively); and fulvestrant 500 mg was more efficacious than the other treatments except letrozole (HR = 1.54, 95% CI 1.09–2.11; HR = 1.61, 95% CI 1.08–2.25; HR = 1.84, 95% CI 1.24–2.55; HR = 2.17, 95% CI 1.35–3.11 for anastrozole, exemestane, tamoxifen, and fulvestrant 250 mg, respectively).
Table 2.
Network meta-analysis comparison of the efficacy of 6 first-line endocrine monotherapies for ORR and TTP/PFS.
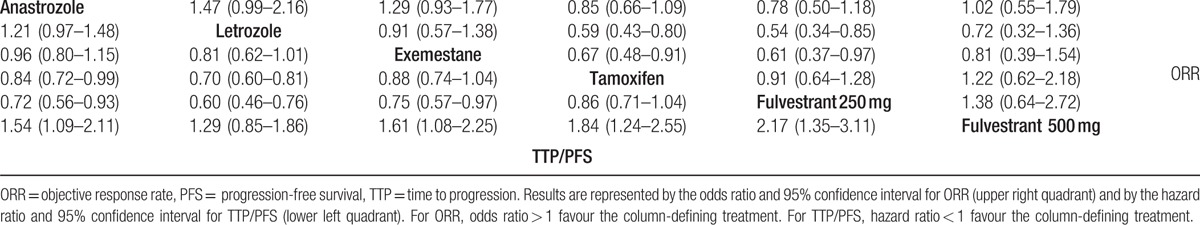
According to the Bayesian framework, we ranked treatments and estimated the cumulative probabilities of being the best treatment (Supplemental Data, S2 Table). For TTP/PFS, the order was fulvestrant 500 mg > letrozole > anastrozole > exemestane > tamoxifen > fulvestrant 250 mg. For ORR, the treatments ranked in descending order of effectiveness were letrozole > exemestane > anastrozole > fulvestrant 500 mg > tamoxifen > fulvestrant 250 mg.
A sensitivity analysis of efficacy with random-effects model revealed no significant difference among the 6 endocrine therapies (Supplemental Data, S3 Table), but the rank orders are consistent with the fixed-effects model.
4. Discussion
Our network meta-analysis of the efficacy of 6 first-line endocrine monotherapies for HR+ HER2− postmenopausal women with ABC was based on 8 studies including 3492 patients randomly assigned to receive 6 endocrine therapies. TTP/PFS was significantly longer with fulvestrant 500 mg, versus the other endocrine therapies except letrozole; for ORR, fulvestrant 500 mg was not differed from the other therapies. Over all, fulvestrant 500 mg may be the best option for first-line treatment of HR+ HER2− ABC to prolong TTP/PFS. We found no significant difference among the 3 AIs for ORR or TTP/PFS. However, we identified a class effect for the 3 AIs because they were generally more efficacious than fulvestrant 250 mg and tamoxifen. Among AIs, letrozole may be preferred because it was significantly more efficacious than tamoxifen and fulvestrant 250 mg for both ORR and TTP/PFS. In addition, for TTP/PFS, it was the only therapy with no significant difference from fulvestrant 500 mg, and letrozole was ranked higher in efficacy than the 2 other AIs.
All studies indicated that the 2 monotherapies they compared were well tolerated. Direct comparisons revealed no significant difference among the 6 regimens except that tamoxifen produced more hot-flash events than fulvestrant 250 mg.[42] However, fulvestrant 250 mg was used more as second-line treatment for MBC with progression after antiestrogen therapy because it was approved by the US Food and Drug Administration for this purpose.[53]
Ferretti et al[54] conducted a traditional meta-analysis to evaluate the effectiveness and safety of AIs compared with tamoxifen as first-line endocrine therapy in postmenopausal MBC women. AIs were significantly better than tamoxifen for ORR, TTP, and clinical benefit in a fixed-effects but not random-effects model. In terms of safety, tamoxifen was associated with more thromboembolic and vaginal bleeding events when compared with AIs. Rob et al[55] conducted a systematic review of 3 first-line AIs for hormone-sensitive ABC. The authors indirectly compared 3 AIs in a network meta-analysis and found that letrozole and exemestane were better than anastrozole for ORR. Our study included 2 RCTs that directly compared exemestane and anastrozole [50,52] and found no significant difference between the AIs. However, the study by Rob et al found no significant difference with the more clinically relevant outcome of TTP/PFS, which was consistent with our findings. Mustafa et al[56] conducted a traditional meta-analysis to copare the relative efficacy and safety of fulvestrant to other endocrine therapy options (including anastrozole, exemestane, tamoxifen) in ABC and found that first-line monotherapy with fulvestrant 500 mg may delay progression when compared with AIs, which was also consistent with our findings.
To our knowledge, this is the first comparison of 6 endocrine monotherapies for first-line treatment of HR+ HER2− ABC that incorporated both direct and indirect evidence in a network meta-analysis. To compare trials with similar clinical features, we included only first-line treatment studies to avoid potential confounders from prior treatments and also excluded studies of different doses.[57] Our findings are consistent with the suggestions of prior published reviews,[54–56,58] indicating that some of the endocrine monotherapies differed both statistically and clinically. Thus, our results confirmed previous conclusions.
Our study has some limitations. First, we used published data rather than individual patient information, which contains more detailed appraisal of outcomes. Second, some included studies did not report randomization and allocation concealment adequately, which might undermine the validity of the overall findings.
5. Conclusion
In conclusion, our study found that fulvestrant 500 mg and letrozole might be the preferred first-line endocrine monotherapy choices for HR+ HER2− postmenopausal women with ABC because of their more efficacious ORR and TTP/PFS with favorable tolerability profiles. However, direct comparisons among first-line endocrine monotherapies are still required to robustly demonstrate the possible differences among these endocrine agents, especially fulvestrant 500 mg and letrozole. Clinical choices should also depend on the specific disease situation and duration of endocrine therapy.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ABC = advanced breast cancer, AIs = aromatase inhibitors, DIC = Deviance information criteria, ER = estrogen receptor, HER2− = human epidermal growth factor receptor-2-negative, HR = hazard ratio, HR+ = hormone receptor-positive, I2 = inconsistency statistic, IFs = inconsistency factors, LABC = locally advanced breast cancer, MBC = metastatic breast cancer, ORR = objective response rate, PFS = progression-free survival, PgR = progesterone receptor, PRISMA = Systematic Reviews and Meta Analyses, RCTs = randomized controlled trials, TTP = time to progression.
This study was funded by Natural Science Foundation of Guangdong Province, China (Grant No. 2014A030313474).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Migliaccio I, Malorni L, Hart CD, et al. Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med 2015;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol 2014;25:1871–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].NCCN clinical practice guidelines in oncology: Breast Cancer Version 3. National Comprehensive Cancer Network. 2015. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 20, 2015. [Google Scholar]
- [5].Jaiyesimi IA, Buzdar AU, Decker DA, et al. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol 1995;13:513–29. [DOI] [PubMed] [Google Scholar]
- [6].Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev 2009;4: Cd003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong ZW, Ellis MJ. First-line endocrine treatment of breast cancer: aromatase inhibitor or antioestrogen? Br J Cancer 2004;90:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010;28:4594–600. [DOI] [PubMed] [Google Scholar]
- [9].Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014;106: djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2). Breast Cancer Res Treat 2010;123:453–61. [DOI] [PubMed] [Google Scholar]
- [11].Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 2009;27:4530–5. [DOI] [PubMed] [Google Scholar]
- [12].Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- [13].Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liberati A1, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. [DOI] [PubMed] [Google Scholar]
- [19].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [20].Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics 2006;24:1–9. [DOI] [PubMed] [Google Scholar]
- [21].Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 2010;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009;373:746–58. [DOI] [PubMed] [Google Scholar]
- [23].Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. Royal Statist Soc B 2002;64:583–639. [Google Scholar]
- [24].Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. J Clin Epidemiol 2010;63:875–82. [DOI] [PubMed] [Google Scholar]
- [25].Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91. [DOI] [PubMed] [Google Scholar]
- [26].Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol 2009;62:857–64. [DOI] [PubMed] [Google Scholar]
- [27].Nabholtz J, Bonneterre J, Buzdar A, et al. Preliminary results of two multi-center trials comparing the efficacy and tolerability of arimidex (anastrozole) and tamoxifen (TAM) in postmenopausal (PM) women with advanced breast cancer (ABC). Breast Cancer Res Treat 1999;57:31. [Google Scholar]
- [28].Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000;18:3748–57. [DOI] [PubMed] [Google Scholar]
- [29].Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol 2000;18:3758–67. [DOI] [PubMed] [Google Scholar]
- [30].Paridaens R, Dirix L, Beex L, et al. Promising results with exemestane in the first-line treatment of metastatic breast cancer: a randomized phase II EORTC trial with a tamoxifen control. Clin Breast Cancer 2000;1(suppl 1):S19–21. [DOI] [PubMed] [Google Scholar]
- [31].Vergote I, Bonneterre J, Thürlimann B, et al. Randomised study of anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women. Eur J Cancer 2000;36(Suppl 4):S84–5. [DOI] [PubMed] [Google Scholar]
- [32].Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 2001;92:2247–58. [DOI] [PubMed] [Google Scholar]
- [33].Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001;19:2596–606. [DOI] [PubMed] [Google Scholar]
- [34].Vergole I, Thürlimann B. First line endocrine therapy in postmenopausal patients with advanced breast cancer and visceral metastases: anastrozole (Arimidex) versus tamoxifen. Eur J Cancer 2001;37(suppl 6):S191. [Google Scholar]
- [35].Lipton A, Ali SM, Leitzel K, et al. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J Clin Oncol 2003;21:1967–72. [DOI] [PubMed] [Google Scholar]
- [36].Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003;21:2101–9. [DOI] [PubMed] [Google Scholar]
- [37].Nabholtz JM. Advanced breast cancer updates on anastrozole versus tamoxifen. J Steroid Biochem Mol Biol 2003;86:321–5. [DOI] [PubMed] [Google Scholar]
- [38].Nabholtz JM, Bonneterre J, Buzdar A, et al. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur J Cancer 2003;39:1684–9. [DOI] [PubMed] [Google Scholar]
- [39].Paridaens R, Dirix L, Lohrisch C, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 2003;14:1391–8. [DOI] [PubMed] [Google Scholar]
- [40].Smith IE. Letrozole versus tamoxifen in the treatment of advanced breast cancer and as neoadjuvant therapy. J Steroid Biochem Mol Biol 2003;86:289–93. [DOI] [PubMed] [Google Scholar]
- [41].Atalay G, Dirix L, Biganzoli L, et al. The effect of exemestane on serum lipid profile in postmenopausal women with metastatic breast cancer: a companion study to EORTC Trial 10951, ’Randomized phase II study in first line hormonal treatment for metastatic breast cancer with exemestane or tamoxifen in postmenopausal patients’. Ann Oncol 2004;15:211–7. [DOI] [PubMed] [Google Scholar]
- [42].Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 2004;22:1605–13. [DOI] [PubMed] [Google Scholar]
- [43].Mouridsen H, Chaudri-Ross HA. Efficacy of first-line letrozole versus tamoxifen as a function of age in postmenopausal women with advanced breast cancer. Oncologist 2004;9:497–506. [DOI] [PubMed] [Google Scholar]
- [44].Mouridsen H, Sun Y, Gershanovich M, et al. Superiority of letrozole to tamoxifen in the first-line treatment of advanced breast cancer: evidence from metastatic subgroups and a test of functional ability. Oncologist 2004;9:489–96. [DOI] [PubMed] [Google Scholar]
- [45].Thürlimann B, Hess D, Köberle D, et al. Anastrozole (’Arimidex’) versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95--a sub-study of the TARGET (Tamoxifen or ’Arimidex’ Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat 2004;85:247–54. [DOI] [PubMed] [Google Scholar]
- [46].Irish W, Sherrill B, Cole B, et al. Quality-adjusted survival in a crossover trial of letrozole versus tamoxifen in postmenopausal women with advanced breast cancer. Ann Oncol 2005;16:1458–62. [DOI] [PubMed] [Google Scholar]
- [47].Mouridsen HT. Letrozole in advanced breast cancer: the PO25 trial. Breast Cancer Res Treat 2007;105(suppl 1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lipton A, Leitzel K, Chaudri-Ross HA, et al. Serum TIMP-1 and response to the aromatase inhibitor letrozole versus tamoxifen in metastatic breast cancer. J Clin Oncol 2008;26:2653–8. [DOI] [PubMed] [Google Scholar]
- [49].Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 2008;26:4883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Llombart-Cussac A, Ruiz A, Antón A, et al. Exemestane versus anastrozole as front-line endocrine therapy in postmenopausal patients with hormone receptor-positive, advanced breast cancer: final results from the Spanish Breast Cancer Group 2001-03 phase 2 randomized trial. Cancer 2012;118:241–7. [DOI] [PubMed] [Google Scholar]
- [51].Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ’FIRST’ study. Breast Cancer Res Treat 2012;136:503–11. [DOI] [PubMed] [Google Scholar]
- [52].Iwata H, Masuda N, Ohno S, et al. A randomized, double-blind, controlled study of exemestane versus anastrozole for the first-line treatment of postmenopausal Japanese women with hormone-receptor-positive advanced breast cancer. Breast Cancer Res Treat 2013;139:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].FASLODEX (fulvestrant) injection. US FDA-approved manufacturer's package insert. 2010. Available at: http: //www. accessdata. fda. gov/drugsatfda docs/label/2010/021344s007s 012lbl.pdf. Accessed October 20, 2015. [Google Scholar]
- [54].Ferretti G, Bria E, Giannarelli D, et al. Second- and third-generation aromatase inhibitors as first-line endocrine therapy in postmenopausal metastatic breast cancer patients: a pooled analysis of the randomised trials. Br J Cancer 2006;94:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Riemsma R, Forbes CA, Kessels A, et al. Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer. Breast Cancer Res Treat 2010;123:9–24. [DOI] [PubMed] [Google Scholar]
- [56].Al-Mubarak M, Sacher AG, Ocana A, et al. Fulvestrant for advanced breast cancer: a meta-analysis. Cancer Treat Rev 2013;39:753–8. [DOI] [PubMed] [Google Scholar]
- [57].Milla-Santos A, Milla L, Portella J, et al. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: a prospective, randomized, phase III study. Am J Clin Oncol 2003;26:317–22. [DOI] [PubMed] [Google Scholar]
- [58].Berry J. Are all aromatase inhibitors the same? A review of controlled clinical trials in breast cancer. Clin Ther 2005;27:1671–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


