Abstract
The aim of this study was to analyze the impact of disease duration on carotid to femoral pulse wave velocity (cfPWV) in rheumatoid arthritis (RA) patients without either known traditional cardiovascular risk factors or previous comorbidities.
Patients with RA diagnosis attending the rheumatology outpatient clinic of Hospital Civil Juan I. Menchaca, Guadalajara, Mexico, were analyzed. A total of 106 RA patients without known traditional cardiovascular risk factors were selected. All subjects were evaluated for RA disease duration, RA disease activity score on 28 joints (DAS28), serum lipids, rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Arterial stiffness was measured as cfPWV by noninvasive tonometry. A multivariate regression model was used to analyze the contribution of RA disease duration and age on cfPWV. cfPWV was positively correlated with age (r = 0.450, P < .001), RA disease duration (r = 0.340, P < .001), total cholesterol (r = 0.312, P = .002), and low density lipoprotein (LDL-c) cholesterol (r = 0.268, P = .012). Patients with a RA disease duration ≥10 years exhibited significantly increased cfPWV compared with patients with disease duration <2 years (8.4 ± 1.8 vs 7.0 ± 0.8) and ≥2 to <10 years (8.4 ± 1.8 vs 7.8 ± 1.3), respectively. Age, RA disease duration, and triglycerides were predictors of cfPWV in multivariate analyses. According to the β-coefficients, each year of disease duration (β = 0.072) had a greater impact on cfPWV than age (β = 0.054).
Each year of life with RA contributes to a higher rate of vascular aging or stiffening than a year of life without RA. The cumulative damage provided by RA was most pronounced in patients with disease duration ≥10 years.
Keywords: arterial stiffness, carotid to femoral pulse wave velocity, rheumatoid arthritis, subclinical cardiovascular disease, vascular aging
1. Introduction
Ischemic heart disease and stroke are the leading causes of death in rheumatoid arthritis (RA) patients.[1,2] In addition, atherosclerosis can be found in early stages of RA with faster progression rate compared with subjects without RA. Epidemiological studies have suggested that the elevated cardiovascular risk occurred only after RA symptom onset,[3] suggesting that disease activity may be closely related to cardiovascular disease (CVD) in subjects with RA.
Early vascular aging in RA patients may be a consequence of chronic inflammation. The measurement of carotid-femoral pulse wave velocity (cfPWV) is the gold standard to evaluate arterial stiffness.[4] Vascular aging is a result of changes in the biomechanical properties of the vascular wall. This process can be accelerated by the accumulated damage of high mechanical stress (high blood pressure), chronic inflammation, and comorbidities such as smoking, diabetes mellitus, and dyslipidemia.[5]
The increased vascular stiffness in RA patients compared with age-matched healthy controls has been well-established.[6–8] However, although these studies underline the importance of vascular aging in RA patients, the effects in relation to RA symptom onset and disease duration have not previously been examined. Because of this knowledge gap, there is no consensus on the timing to establish preventive measures and treating early vascular aging in RA patients.
The aim of this study was to establish the impact of RA disease duration on arterial stiffness in patients without known traditional cardiovascular risk factors and prevalent CVD.
2. Methods
We performed a cross-sectional study to establish the main determinants of cfPWV in RA patients. Only patients without traditional cardiovascular risk factors and/or prevalent CVD were included to allow assessment of the influence of RA on arterial stiffness without the confounding factor of prevalent CVD. This report is part of a larger project whose main objective is to assess predictors and evolution of CVD in RA patients.[9,10]
2.1. Patients
We included patients classified as having RA per American College of Rheumatology (ACR) 1987 criteria[11] attending the rheumatology outpatient clinic at OPD Hospital Civil “Juan I. Menchaca,” Guadalajara, Jalisco, Mexico, from January to December 2015. The inclusion criteria were >18-years old without known history of CVD, diabetes mellitus, high blood pressure, thyroid, renal, or hepatic disease. Exclusion criteria were current pregnancy and use of corticosteroids such as >10 mg of prednisone or equivalent.
2.2. Clinical assessment
Each patient was interviewed using a structured questionnaire to gather demographic and clinical variables, including disease duration and treatment. Clinical evaluation was performed by a rheumatologist and RA disease activity was calculated with the disease activity score on 28 joints (DAS28).[12]
2.3. Laboratory measurements
Venous blood samples were collected at the moment of clinical assessment. Serum was obtained by centrifugation of whole blood at 2000 rpm for 15 minutes, and aliquots were stored at −70 °C until used. Cumulative erythrocyte sedimentation rate (ESR) was measured using Wintrobe's method.[13] C-reactive protein (CRP) and rheumatoid factor (RF) were measured by nephelometry. Total cholesterol (TC), triglycerides (Tg), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were measured by standard techniques. Anti-CCP antibody levels were determined by enzyme-linked immunosorbent assay (ELISA, Axis-Shield). Patients were considered as anti-CCP positive at antibody levels ≥5 U/mL.
2.4. Arterial stiffness measurement
Arterial stiffness was determined by cfPWV using tonometry. This is the recommended method to assess arterial stiffness,[14] and was performed using the Pulse Pen device (DiaTecne s.r.l., Milan, Italy), a technique previously validated for its efficacy to measure cfPWV.[15] The cfPWV was determined at 2 intervals, first positioning the tonometer at the common carotid artery while simultaneously performing an electrocardiogram (ECG), and then repeating the procedure at the femoral artery. The time delay from the rise of the proximal and distal pulse waves to the R-wave of the ECG qRs complex were recorded. The cfPWV was calculated as the distance between the two arterial segments evaluated divided by the time delay between the detected pulses in meters/second (m/s).
2.5. Ethics
All subjects gave their written consent before enrollment in the study. This protocol was approved by the Institutional Review Board committee with the register 1068/10 of Hospital Civil “Dr. Juan I. Menchaca” of the Universidad de Guadalajara. Research was conducted following Helsinki criteria according to its last update in 2013, Fortaleza, Brazil.
2.6. Statistical analysis
A minimum sample size of 42 was calculated (using the software G∗Power 3.1.9.2) to be needed for linear multiple regression expecting at least three predictors and a minimum effect on R2 of 0.25, considering α = 0.05, and 1-β = 95%. Data were analyzed with the statistical software SPSS v22 (IBM Inc., Chicago, IL), and GraphPad Prism v6.01 (GraphPad Software Inc, La Jolla, CA). Results are given as mean ± standard deviation (SD) or percentages, as appropriate. Normal distribution was tested with Kolmogorov-Smirnov's test. For comparisons, subjects were grouped according to age (<40, 40–50, and >50 years), and disease duration (<2, 2 to <10, and ≥10 years). The age stratification per decades was chosen to allow for age-dependent changes in cfPWV to be taken into account based on previous reference studies in this context.[16] The disease duration stratification was arbitrary.
Patients with missing cfPWV values were excluded from the analysis. One-way analysis of variance (ANOVA) and Dunnet's t tests were used for multiple comparisons. Pearson's correlation coefficients were calculated between cfPWV and independent variables. Subsequently, we performed analysis of co-variance (ANCOVA) to assess their contribution to cfPWV. Variables with a P < .2 in univariate analyses were considered for inclusion in ANCOVA. A 2-tailed P > .05 was considered significant for both univariate and multivariate analysis.
3. Results
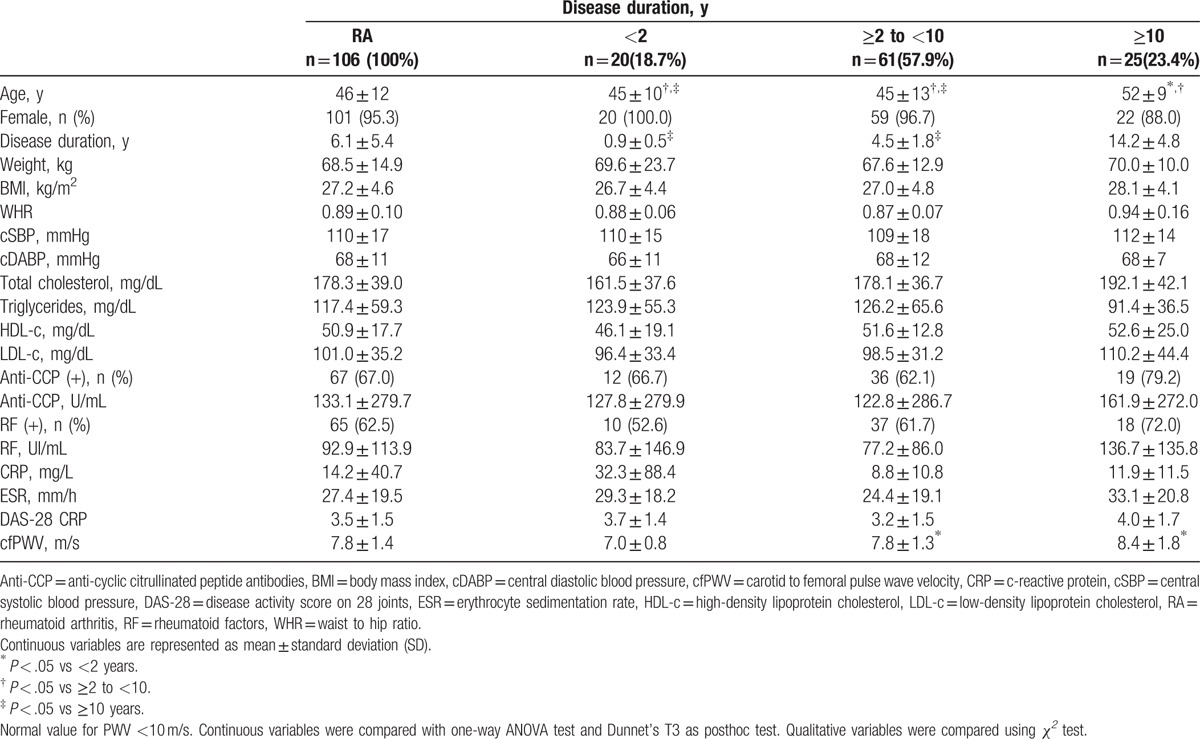
We recruited a total of 115 RA patients, of which 9 were excluded because of missing cfPWV values. Consequently, 106 RA patients were included in the final analysis. The mean age of the final group was 46 ± 12 years and 101 were female (95.3%). Demographic, clinical characteristics, and arterial stiffness assessment are shown in Table 1. The whole group's cfPWV mean was within normal ranges (mean <10 m/s). However, patients with RA disease duration ≥10 years were significantly older and exhibited significantly increased cfPWV.
Table 1.
Demographic, clinical characteristics, and arterial stiffness assessment of RA patients.
3.1. RA disease duration correlated with cfPWV
cfPWV exhibited significant positive correlation with age (r = 0.450, P < .001) and disease duration (r = 0.340, P < .001) (Fig. 1). In addition, significant correlations were observed with total cholesterol (r = 0.312, P = .002) and LDL-c (r = 0.268, P = .012).
Figure 1.
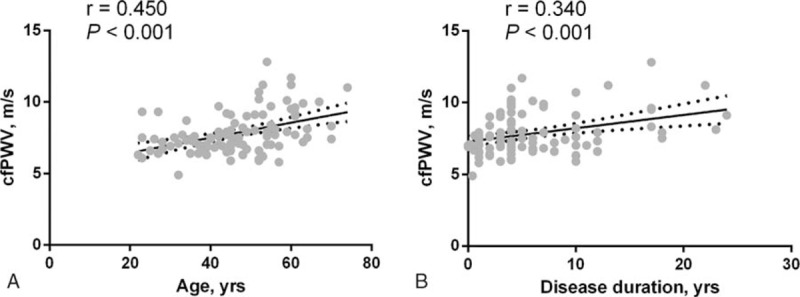
Correlation between (A) age and (B) RA disease duration with cfPWV. cfPWV = carotid to femoral pulse wave velocity, RA = rheumatoid arthritis.
In the stratified analysis, cfPWV was significantly increased in patients >50 years old (8.6 ± 1.7 m/s) compared with patients in the 40 to 50-year old (7.5 ± 0.9 m/s) and <40-years old (7.1 ± 0.9 m/s) groups (Fig. 2A). Patients with >10 years (8.4 ± 1.8 m/s) and 2 to 10 years (7.8 ± 1.3 m/s) disease duration exhibited significantly increased cfPWV compared with those having a recent diagnosis <2 years (6.9 ± 0.8 m/s) (Fig. 2B).
Figure 2.
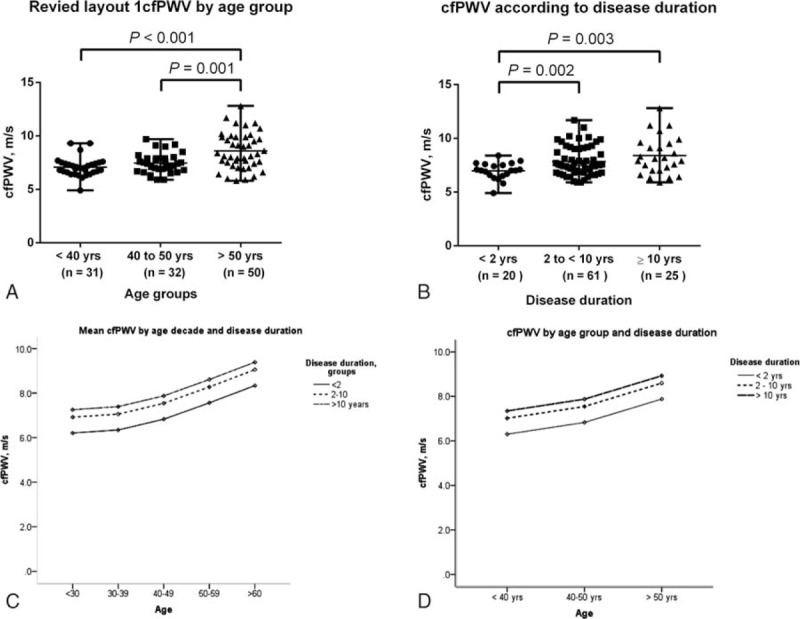
Comparison of cfPWV according to (A) age group and (B) RA disease duration and predicted mean cfPWV according to (C) age decade and (D) age group. Means were compared with ANOVA and Dunnet's t test. Predicted means were analyzed by ANCOVA. cfPWV = carotid to femoral pulse wave velocity, RA = rheumatoid arthritis.
The impact of age and disease duration on cfPWV in RA patients are shown in Fig. 2C and D. RA patients with ≥10 years of disease duration exhibited signs of early vascular aging demonstrated by increase cfPWV. In other words, RA patients <40 years old with disease duration ≥10 years had a similar arterial stiffness as subjects >50 years old with disease duration <2 years (Fig. 2C and D).
3.2. Predictive value of disease duration and age on cfPWV in RA patients
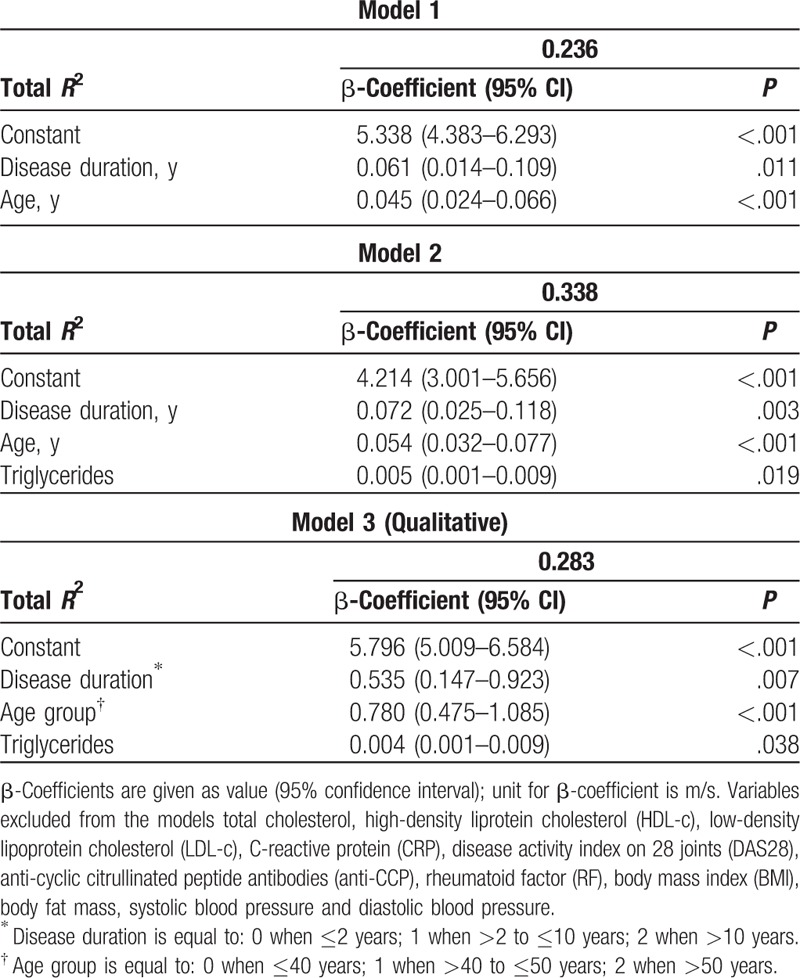
To further characterize the respective impact of age and disease duration on cfPWV, we analyzed these variables by ANCOVA (Table 2). In Model 1, age and disease duration were predictors of 23.6% cfPWV variance. In Model 2, we included markers of inflammation and metabolic disease (body fat mass and lipids) in addition to RA disease duration and age. Among the serum lipid measures, only triglycerides were predictors of cfPWV. Nonetheless, according to the β-coefficients, each year of disease duration had greater impact on cfPWV than each year of life without RA. In Model 3, disease duration and age were included as qualitative variables to highlight their relevance in cfPWV.
Table 2.
Multiple linear regression analysis for carotid to femoral pulse wave velocity (m/s).
4. Discussion
The current study points to disease duration as a predictor of vascular stiffness in RA patients. Arterial stiffness resulting from vascular remodeling and loss of arterial elasticity is now increasingly recognized as a surrogate marker of CVD.[17,18] Hence, our results support the notion of RA as a causal factor for CVD,[9] and suggest that monitoring vascular stiffness may be an additional means to facilitate cardiovascular risk stratification of RA patients.
Previous studies have shown that RA is associated with increased arterial stiffness compared with age-matched healthy controls. Age, CRP, ESR, and disease activity are associated with increased cfPWV in RA patients.[6–8] The current study extends those findings by revealing RA disease duration as a predictor of cfPWV. The cfPWV values of our RA patients were within the ranges previously reported as normal in European[16] and Hispanic[19] populations when considered the cohort as a whole, without taking disease duration into account. This, however, may not be unexpected as the included patients were not only free of prevalent CVD but were also normotensive. The elevated cfPWV observed in the examined patients showed longer time after RA diagnosis, suggesting that vascular stiffness may occur after a certain disease duration time.
Certainly, aging induces vascular remodeling in terms of changes in the collagen and elastin balance in the vascular wall, leading to arterial stiffening. This process is mainly modulated by the accumulated damage from mechanical stress (eg, high blood pressure), chronic inflammation and traditional CVD risk factors.[5] However, taking age and lipid levels into consideration in our cohort of RA patients without cardiovascular comorbidities did not obscure the association with RA disease duration, further reinforcing the importance of our findings.
Subclinical atherosclerosis can indeed be detected in RA patients without comorbidities and in early disease.[9] Several lines of evidence suggest that the inflammatory activity in RA may directly contribute to accelerated atherosclerosis mediated by vascular activation and endothelial dysfunction.[20,21] In addition, several studies have shown premature vascular aging in RA patients with comorbidities, suggesting that evaluation of arterial stiffness is a useful measure of accumulated vascular damage. In the general population and in subjects with chronic diseases like hypertension, chronic kidney disease, and diabetes mellitus, cfPWV has shown predictive value for CVD events.[17,18]
Possible limitations of our study include the female predominance in the cohort, low number of cases with early RA, and lack of control group of age- and sex-matched individuals.
In summary, the current study highlights the contribution of disease duration to arterial stiffness in RA patients without traditional CVD risk factors. Our main finding indicates that each year of life with RA contributes to a higher rate of vascular aging or stiffening than age. The cumulative damage provided by RA was more pronounced in patients with disease duration ≥10 years, as indicated by the higher cfPWV in this group compared with those having a shorter time since diagnosis. This means that vascular remodeling might occur in silence in early RA when the disease may still be in subclinical stage. In conclusion, the current study suggests that deleterious vascular remodeling may accumulate after RA onset, likely contributing to increase CVD in these patients.
Acknowledgments
The authors would like to thank Dr. Carlos A. Casiano from Loma Linda University, for his support for reviewing this article as a native English speaker.
Footnotes
Abbreviations: ACR = American College of Rheumatology, ANCOVA = analysis of covariance, ANOVA = analysis of variance, Anti-CCP = anticyclic citrullinated peptide antibodies, cfPWV = carotid to femoral pulse wave velocity, CRP = C-reactive protein, CVD = cardiovascular disease, DAS28 = disease activity index on 28 joints, ECG = electrocardiogram, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, HDL-c = high-density lipoprotein cholesterol, LDL-c = low density lipoprotein cholesterol, RA = rheumatoid arthritis, RF = rheumatoid factor, SD = standard deviation, TC = total cholesterol, Tg = tryglycerides.
This study received funding from PRODEP 2016 “Reconocimiento y/o apoyo a profesor con perfil deseable” grant awarded to MV-DM and Programa de incorporación y permanencia del posgrado en el PNPC, PROINPEP 2016.
The authors declare no conflicts of interest.
References
- [1].Kramer HR, Giles JT. Cardiovascular disease risk in rheumatoid arthritis: progress, debate, and opportunity. Arthritis Care Res (Hoboken) 2011;63:484–99. [DOI] [PubMed] [Google Scholar]
- [2].Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303–7. [DOI] [PubMed] [Google Scholar]
- [3].Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA 2012;308:1350–6. [DOI] [PubMed] [Google Scholar]
- [4].Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–8. [DOI] [PubMed] [Google Scholar]
- [5].Kozakova M, Morizzo C, Guarino D, et al. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J Hypertens 2015;33:1446–51. [DOI] [PubMed] [Google Scholar]
- [6].Mathieu S, Couderc M, Glace B, et al. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics 2013;7:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crilly MA, Kumar V, Clark HJ, et al. Arterial stiffness and cumulative inflammatory burden in rheumatoid arthritis: a dose-response relationship independent of established cardiovascular risk factors. Rheumatology 2009;48:1606–12. [DOI] [PubMed] [Google Scholar]
- [8].Maki-Petaja KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006;114:1185–92. [DOI] [PubMed] [Google Scholar]
- [9].Vazquez-Del Mercado M, Nunez-Atahualpa L, Figueroa-Sanchez M, et al. Serum levels of anticyclic citrullinated peptide antibodies, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein are associated with increased carotid intima-media thickness: a cross-sectional analysis of a cohort of rheumatoid arthritis patients without cardiovascular risk factors. BioMed Res Int 2015;2015:342649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomez-Banuelos E, Martin-Marquez BT, Martinez-Garcia EA, et al. Low levels of CD36 in peripheral blood monocytes in subclinical atherosclerosis in rheumatoid arthritis: a cross-sectional study in a Mexican population. BioMed Res Int 2014;2014:736786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [12].Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [13].Wintrobe MM, Landsberg JW. A standardized technique for the blood sedimentation test, 1935. Am J Med Sci 2013;346:148–53. [DOI] [PubMed] [Google Scholar]
- [14].Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Salvi P, Lio G, Labat C, et al. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens 2004;22:2285–93. [DOI] [PubMed] [Google Scholar]
- [16].Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ’establishing normal and reference values’. Eur Heart J 2010;31:2338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002;39:10–5. [DOI] [PubMed] [Google Scholar]
- [19].Diaz A, Galli C, Tringler M, et al. Reference values of pulse wave velocity in healthy people from an urban and rural argentinean population. Int J Hypertens 2014;2014:653239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Capria A, De Nardo D, Baffetti FR, et al. Long-term anti-TNF-alpha treatments reverse the endothelial dysfunction in rheumatoid arthritis: the biological coherence between synovial and endothelial inflammation. Int J Immunopathol Pharmacol 2010;23:255–62. [DOI] [PubMed] [Google Scholar]
- [21].Del Porto F, Lagana B, Lai S, et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology 2007;46:1111–5. [DOI] [PubMed] [Google Scholar]


