Abstract
Rationale:
Inflammatory myofibroblastic tumor (IMT) is an uncommon neoplastic entity with a tendency of local recurrence and a low risk of distant metastasis. Involvement of trachea is extremely rare.
Patient concerns:
A 34-week pregnant woman previously diagnosed with asthma for 2 months was admitted with persistent wheezing and hemoptysis. A computed tomography scan and bronchoscopy revealed a gigantic polyp in the trachea.
Diagnoses:
Tracheal inflammatory myofibroblastic tumor.
Interventions:
The mass was removed with an electrocautery snare and identified histologically as an IMT. Further immunochemical staining showed strong positive staining for smooth muscle actin and platelet-derived growth factor receptor α (PDGFRA), weak positive staining for caldesmon, and negative staining for anaplastic lymphoma kinase (ALK)1, desmin, S-100, and CD34. The tracheal IMT strongly expressed estrogen receptor-α (ER-α), which indicated that the development of this rare IMT might have been associated with hormone fluctuations that occurred during the pregnancy.
Outcomes:
Follow-up and histological analyses revealed no evidence of recurrence and metastasis.
Lessons:
This report describes an extremely rare case of a tracheal IMT that presented a diagnostic dilemma for the clinician and the pathologist. Tracheal IMT is a challenge for the clinician in diagnosis due to the nonspecific clinical presentation. Histology and immunohistochemistry are required to reach an accurate diagnosis of IMT.
Keywords: asthma, hormone, inflammatory myofibroblastic tumor, pregnant woman
1. Introduction
Inflammatory myofibroblastic tumor (IMT) is an indolent mesenchymal tumor; first described in lung in 1939.[1] IMT has been referred as plasma cell granuloma, inflammatory pseudotumor, fibrous histiocytoma, pseudosarcomatous fibromyxoid tumor, and xanthomatous pseudotumor with an understanding of the reactive benign nature. Growing knowledge of the defining immunohistochemical and genetic features of tumor has facilitated recognition of IMT, which is deemed as a neoplastic process. Anaplastic lymphoma kinase (ALK) immunostaining is detected in approximately 50% of IMT. The activation and overexpression of ALK are caused by ALK gene rearrangements. Several ALK fusion partners are identified and involve TPM3/4, CLTC, CARS, SEC31L1, and RANBP2.[2] ROS1 and PDGFRβ fusions have been recently reported in ALK-negative IMT and 85% of IMTs are revealed to harbor kinase fusions.[3]
IMT virtually involves any anatomic sites including thorax, bone, genitourinary tract, and central nervous system, but preferentially occurs in the lung, soft tissues in abdominopelvic region, and retroperitoneum. This report presented a rare case of a tracheal IMT with ALK-negative in a pregnant woman who was misdiagnosed with asthma.
2. Case presentation
A 30-year-old woman in her 34th week of pregnancy was admitted to the emergency department. She had developed progressive and persistent wheezing, coughing, and sputum for 2 months, and was diagnosed with asthma in her community hospital. She had been inhaling fluticasone propionate/salmeterol (50/250 μg) twice daily with no noticeable effect in terms of symptom relief. The patient was a nonsmoker with a history of allergic rhinitis triggered by exposure to some types of pollen. Two days before admission, an acute onset of wheezing and hemoptysis occurred. The patient was treated with humidified oxygen and intravenous corticosteroid in the emergency department. However, her symptoms did not improve.
On arrival at the respiratory department, a physical examination revealed an obese and anxious female in moderate respiratory distress with rhonchi and biphasic stridor that was more obvious in the expiratory phase. Her peripheral white blood count was 7.47 × 109 L−1 (normal range: 3.50–9.50 × 109 L−1) and eosinophil count was 0.04 × 109 L−1 (normal range: 0.02–0.52 × 109 L−1). The total serum IgE level was slightly elevated at 205.56 IU/mL (normal range: 1.31–165.30 IU/mL). Her kidney and liver functions, protein levels in the blood, erythrocyte sedimentation rate, and an arterial blood gas analysis were normal. The results of a sputum acid-fast bacillus smear and a T-SPOT were negative. The patient rejected radiological examinations and bronchoscopy due to the pregnancy after being informed of the necessities and risks of these examinations. The available treatments for asthma, including systemic methylprednisolone (80 mg/d) and bronchodilators, had no significant effect on her dyspnea. After we repeatedly discussed the likely benefits and risks with a gynecologist and the patient, the patient consented to an emergent cesarean section and subsequent chest radiography 6 days after admission.
A computed tomography scan of chest revealed soft-tissue opacity within the tracheal wall (Fig. 1A, arrow). The mass obstructed approximately 85% of the tracheal lumen. Immediate bronchoscopy was performed and revealed a single gigantic smooth pedunculated mass in the lower trachea (Fig. 1B). The mass was removed with an electrocautery snare (Fig. 1C). The dyspnea was immediately relieved following the mass removal. The patient was discharged 3 days later, and no bronchodilators or inhaled steroids were needed.
Figure 1.
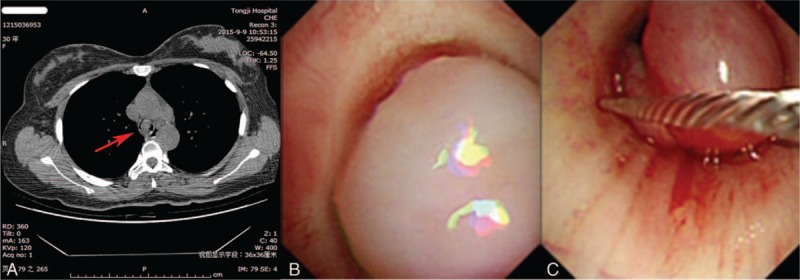
(A) Chest computed tomography with a soft tissue opacity within the tracheal wall at the level of the thoracic inlet, obstructing approximately 85% of tracheal lumen. Internal scale bar = 1 cm. The red arrow indicates a large polyp in the trachea. Bronchoscopic image showing a single gigantic smooth pedunculated mass in the trachea (B) and bronchoscopic view of the electrocautery snare resection (C).
Histopathologic diagnosis of the resected mass was consistent with IMT of approximately 2 × 3 × 4 cm. The IMT was covered with squamous epithelium and composed of spindle cells in a highly vascular background with an edematous and mucinous matrix with capillary vessels that were infiltrated by inflammatory cells (Fig. 2A). Immunohistochemistry revealed that the spindle cells were diffusely positive for smooth muscle actin (SMA) and platelet-derived growth factor receptor α (PDGFRA), focally positive for caldesmon (Fig. 2B), and negative for desmin, CD117, DOG1, S-100, ALK1, PCK, EMA, CK8/18, Ki-67, and CD34 (not shown). The patient only experienced symptoms after becoming pregnant, thus we investigated the hormone receptors in the IMT tissue, including estrogen receptor-α (ER-α) and progestin receptor. The results revealed moderate to strong expressions of ER-α in the spindle cells (Fig. 2B). No progestin receptor-positive cells were detected.
Figure 2.
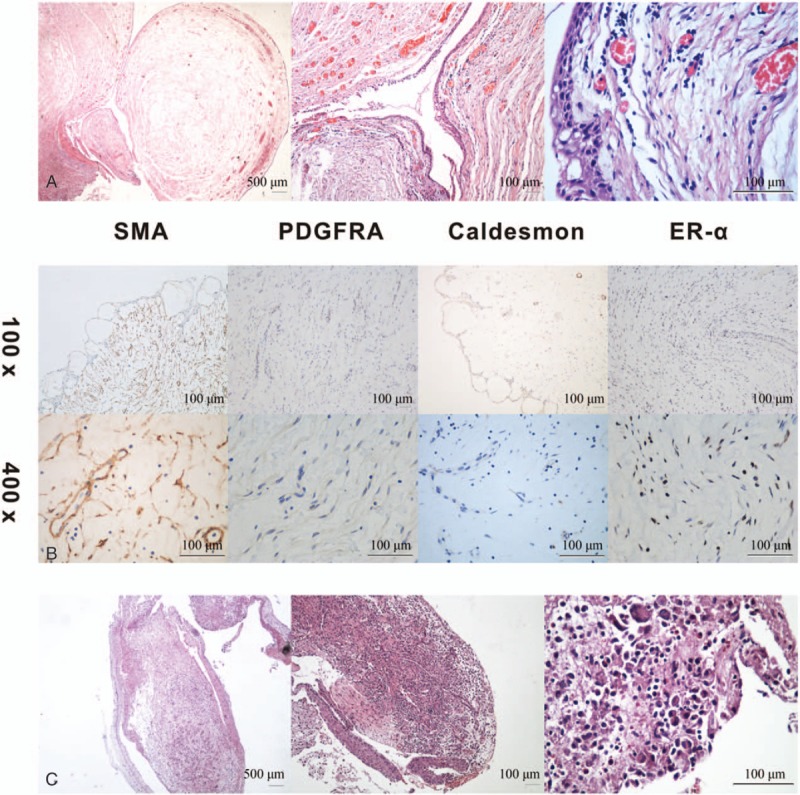
(A) Hematoxylin-eosin staining of the mass covered with squamous epithelium and composed of spindle cells in a highly vascular background with an edematous and mucinous matrix with capillary vessels that were infiltrated by inflammatory cells at the original magnification ×20, ×100, and ×400. (B) Immunochemistry staining revealed moderate to strong expressions of SMA, PDGFRA, caldesmon, and ER-α in the spindle cells at magnifications of ×100 and ×400. (C) At one-month follow-up, the mucosal protrusion at the original area for hematoxylin-eosin staining of the inflammatory granulation with eosinophil and mononuclear cell infiltrations at the original magnification ×20, ×100, and ×400. Histological stains of the tissue revealed inflammatory granulation with eosinophil and mononuclear cell infiltrations. SMA = smooth muscle actin, PDGFRA = platelet-derived growth factor receptor α, ER-α = estrogen receptor-α.
The patient was followed up at 1 month after the tumor removal. A bronchoscopy revealed mucosal protrusion in the original area. After tissue biopsy, cryotherapy was applied to prevent the granulation tissue hyperplasia following an electrocautery resection. Histological stains of the tissue revealed inflammatory granulation with eosinophil and mononuclear cell infiltrations (Fig. 2C). A follow-up bronchoscopy 12 months later was negative for local recurrence and metastasis.
Previously, the patient had not performed a pulmonary function test due to the pregnancy. At 1 month after delivery and corticosteroid withdrawal, a pulmonary function test was prescribed, and the results revealed normal lung function and a negative provocation test.
3. Discussion
IMT is an uncommon mesenchymal neoplasm of intermediate biological potential with a global prevalence of 0.04% to 0.7%. IMT has a predilection for children, adolescents, and young adults. Pulmonary IMT can invade tracheal, bronchus, chest wall, mediastinal structures, and the diaphragm with a predilection for the lower lobes.[4] Tracheal IMT is extremely rare. The clinical symptom of IMT is nonspecific and varies depending on the location. Patients are usually asymptomatic in the early stage of tracheal IMT and may present with hemoptysis, wheezing, and dyspnea until the tumor occludes the lumen by 50% to 75%. Therefore, IMT is commonly misdiagnosed and underdiagnosed.
The diagnosis of IMT is usually confirmed following resection and depends on the pathologic and immunohistochemical analyses. The current World Health Organization classification for this rare tumor entity is the proliferating myofibroblastic spindle cells mixed with variable amounts of lymphoplasmacytic inflammatory infiltrate in a myxoid background.[5] Immunohistochemistry is needed to make a definitive diagnosis of IMT. In general, IMT shows positive for ALK, SMA, desmin, keratin, cytokeratin, and calponin, and negative for S-100, CD117, CD23, CD34, and c-kit. ALK reactivity ranged from 36% to 71% in previous studies. Histologic analyses of 59 IMT cases show that ALK-negative IMTs have greater nuclear pleomorphism and atypical mitoses.[6] Several clinical and pathological features should be considered before a diagnosis is confirmed. IMT diagnosis should be cautious in middle-age or older patients. IMT should be considered a diagnosis of exclusion in the skin and superficial somatic soft tissues, lymph node, spleen, or bladder. The presence of intratumoral necrosis, hemorrhage, and calcification are uncommon in IMT.[2]
The patient in this case was a pregnant woman with a history of allergic rhinitis, which increased the likelihood of a misdiagnosis. Neither pulmonary function test nor chest radiography was preformed because of her pregnancy and her refusal to undergo these procedures. She presented with respiratory symptoms that included coughing, wheezing, and dyspnea accompanied by worse symptoms of allergic rhinitis. Worsening of the respiratory symptoms that was refractory to corticosteroids and bronchodilators as well as the sonorous wheeze and stridor on auscultation actually brought the diagnosis of asthma into question and provided a hint of a possible severe obstruction in the major airway, which was a life-threatening emergency. A CT scan confirmed this suspicion and endoscopic resection was immediately performed to relieve the potentially fatal dyspnea. Histological and immunohistochemistry examinations identified the tracheal mass as an IMT.
Definite diagnosis is essential for ensuring optimal treatment and determining the prognosis. Histopathologic differential diagnoses include inflammatory bronchial polyps, inflammatory fibroblastic polyps, fibroepithelial polyps, and so on. Inflammatory bronchial polyps are lined with columnar epithelial cells and are composed of spindle cells arranged in fascicles with marked infiltration of inflammatory cells, primarily eosinophils, and lymphocytes. Medication with antibiotics and corticosteroids in addition to surgical and bronchoscopic excision are among the choices for the treatment of inflammatory bronchial polyps.[7] Inflammatory fibroblastic polyps may resemble a fibromyxoid/vascular pattern of IMT in terms of widely separated stellate-to-plump spindle cells in a myxoid, edematous, or loose delicate fibrous stromal background with a more or less regular rich vascular network and inflammatory cells, especially plasma cells.[8] Inflammatory fibroblastic polyps are predominantly positive for CD34 and negative for ALK1 and desmin in immunohistochemical staining, which may contribute to the discrimination between IMTs and inflammatory fibroblastic polyps.[8] Fibroepithelial polyps lined with normal respiratory epithelium consist of loose edematous fibrovascular stroma with inflammatory cell infiltration, predominantly mononuclear cells. The stromal cells of fibroepithelial polyps exhibit no or limited immunoreactivity for SMA.
The recurrence rate of IMT ranges from less than 2% for lung tumors to 25% for extrapulmonary lesions. Distant metastasis is rare, occurring in less than 5% of cases.[2] ALK expression is considered as a favorable prognostic indicator in IMT. ALK-negative IMT is reported to have higher rates of metastasis and occurs in older patients (mean age 20.1 years).[6] However, local recurrence is irrelevant to ALK expression.[6,9]
During pregnancy, estrogen levels can reach up to 100-fold greater than the normal range. Excess estrogen hormones and obesity have been implicated as risk factors for the development of tumor, which may be due to the promotions of cell proliferation and tissue hyperplasia. We investigated the presence of ER- and progestin receptor-positive cells in the IMT to explore the potential molecular mechanisms. ER-positive spindle cells were detected in the tumor. An increasing number of studies suggest that ER plays a regulatory role in lung cancer and pulmonary vascular diseases.[10,11] The determination of ER expression in the IMT in this case may imply the involvement of ER in the pathogenesis of IMT, and the increased hormone levels during late pregnancy may have been a risk factor for IMT in this woman.
Surgical resection is the mainstay of the treatment for IMT. Crizotinib, as an ALK inhibitor, is proven to be a promising therapy in ALK-rearranged cases with local recurrence and metastasis, and may facilitate the surgical removal for unresectable IMT.[12,13] Interestingly, crizotinib, which is also a ROS1 inhibitor, is applied to a young patient with treatment-refractory ALK-negative IMT and has shown positive therapeutic effects. The treatment strategy during pregnancy is individualized to the symptoms, the duration of pregnancy, and the complications associated with treatment.
In conclusion, we present a rare case of IMT in a pregnant woman misdiagnosed with asthma, urging a more considerate diagnosis for asthma in pregnant women due to the possibility of airway inflammatory tumor during dramatic hormone fluctuation in pregnancy. In this report, a rigorous clinical reasoning and an optimal clinical judgment were involved. The clinical presentation of tracheal IMT may be misleading. Both histology and immunohistochemistry are required to reach an accurate diagnosis. Although only 1 case of tracheal IMT in pregnancy was reported previously,[14] hormone fluctuations during pregnancy and idiopathic ER-α expression confirmed in our case may be the potential risk factors for tracheal IMT.
Acknowledgment
We thank the patient, who requested anonymity, for agreeing to our report and for providing a detailed medical history.
We sincerely thank Dong Chen, PhD and Fei Fan, MD in the Department of Pathology of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their thoughtful discussions.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, ER-α = estrogen receptor-α, IMT = inflammatory myofibroblastic tumor, PDGFRA = platelet-derived growth factor receptor α, SMA = smooth muscle actin.
This project was supported by grants from the National Natural Science Foundation of China (81470002, 81670020, and 81270106).
The authors have no conflicts of interest.
References
- [1].Brunn H. Two interesting benign tumors of contradictory histopathology: remarks on the necessity for maintaining the chest tumor registry. J Thorac Surg 1939;9:119–31. [Google Scholar]
- [2].Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: Where are we now? J Clin Pathol 2008;61:428–37. [DOI] [PubMed] [Google Scholar]
- [3].Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Surabhi VR, Chua S, Patel RP, et al. Inflammatory myofibroblastic tumors: current update. Radiol Clin North Am 2016;54:553–63. [DOI] [PubMed] [Google Scholar]
- [5].Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. World Health Organization Classification of Tumours of Soft Tissue and Bone. World Health Organization Classification of Tumors. Vol 5 IARC Press, 4th edLyon:2013. [Google Scholar]
- [6].Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including alk expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–20. [DOI] [PubMed] [Google Scholar]
- [7].Niimi A, Amitani R, Ikeda T, et al. Inflammatory bronchial polyps associated with asthma: resolution with inhaled corticosteroid. Eur Res J 1995;8:1237–9. [DOI] [PubMed] [Google Scholar]
- [8].Makhlouf HR, Sobin LH. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: How closely are they related to inflammatory fibroid polyps? Hum Pathol 2002;33:307–15. [DOI] [PubMed] [Google Scholar]
- [9].Coffin CM, Patel A, Perkins S, et al. Alk1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001;14:569–76. [DOI] [PubMed] [Google Scholar]
- [10].Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol 2009;36:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lahm T, Albrecht M, Fisher AJ, et al. 17beta-estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Res Crit Care Med 2012;185:965–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gaudichon J, Jeanne-Pasquier C, Deparis M, et al. Complete and repeated response of a metastatic alk-rearranged inflammatory myofibroblastic tumor to crizotinib in a teenage girl. J Pediatr Hematol Oncol 2016;38:308–11. [DOI] [PubMed] [Google Scholar]
- [13].Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in alk-rearranged inflammatory myofibroblastic tumor. N Engl J Med 2010;363:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amir R, Danahey D, Ferrer K, et al. Inflammatory myofibroblastic tumor presenting with tracheal obstruction in a pregnant woman. Am J Otolaryngol 2002;23:362–7. [DOI] [PubMed] [Google Scholar]


