Abstract
Objective
Breast cancer survivors who develop lymphedema report poorer quality of life (QoL) than those without lymphedema. Expressive writing is a potential intervention to address QoL.
Design
Adult women (N=107) with breast cancer and chronic Stage II lymphedema were randomized to writing about thoughts and feelings specific to lymphedema and its treatment (intervention) or about daily activities (control) for four, 20-minute sessions.
Main Outcome Measures
Outcome measures were several indicators of QoL assessed at baseline, one, three, and six months post-intervention (total scores and subscales of Upper Limb Lymphedema 27 and Functional Assessment of Cancer Therapy–Breast). Hypothesized moderators of change in QoL were dispositional optimism, avoidant behaviors, and time since lymphedema diagnosis.
Results
There was no statistically significant intent-to-treat main effects of expressive writing on QoL. Statistically significant moderating effects on change in different indicators of QoL were observed for all three moderators. Expressive writing was more effective for improving QoL in women who were higher on optimism, lower on avoidance, and had less time since a lymphedema diagnosis.
Conclusion
These results provide further evidence that there are subsets of individuals for whom expressive writing is more effective. Future research may investigate targeting expressive writing based on identified moderators.
Keywords: Breast Cancer, Oncology, Lymphedema, Psychology, Writing, Quality of Life
Background
There are approximately 3.1 million breast cancer survivors living in the United States (American Cancer Society, 2014). Lymphedema is a serious problem following breast cancer treatment in a considerable number of women (e.g., 13 to 65%; Gärtner et al., 2010), varying by the type of cancer treatment received. Development of lymphedema is a stressful event and a potentially chronic, disfiguring condition (Megens, Harris, Kim-Sing, & McKenzie, 2001). Breast cancer survivors with lymphedema report a poorer quality of life (QoL) than breast cancer survivors without lymphedema (Coster, Poole, & Fallowfield, 2001; Pusic et al., 2013; Ridner, 2005; Velanovich & Szymanski, 1999). Specific areas of concern include body image, psychological distress, and mobility (Carter, 1997; Coster et al., 2001; Passik & McDonald, 1998).
Unfortunately, even successful treatment for lymphedema that focuses primarily on reduction of limb volume may not fully restore QoL (Ridner, 2005). Evidence supports the association between highly stressful events, such as the development of lymphedema after breast cancer treatment, and long-lasting, negative effects on QoL (Pennebaker & Susman, 1988; Smyth & Greenberg, 2000). Emotional disclosure is integral to many therapeutic modalities designed to help individuals cope with stressful events, and expressive writing offers a cost-effective opportunity to disclose emotions (Smyth & Pennebaker, 2001). Individual studies of women with breast cancer find that those who disclose their thoughts and feelings appear to benefit (e.g., improvements in physical symptoms, QoL; Craft, Davis, & Paulson, 2013; Creswell et al., 2007; Stanton et al., 2002). Yet, there is also substantial evidence of null main effects of written emotional disclosure interventions in women with breast cancer (Jensen-Johansen et al., 2013; Low, Stanton, Bower, & Gyllenhammer, 2010; Walker, Nail, & Croyle, 1999). Two systematic reviews concluded that although some main effects were reported, a majority of studies reported null effects with a meta-analysis of results revealing no statistically significant effects (Merz, Fox, & Malcarne, 2014; Zachariae & O’Toole, 2015). These varied results may be explained by individual differences that affect the likelihood of benefit from emotional disclosure, such as dispositional optimism (Frattaroli, 2006), avoidant behaviors (Stanton et al., 2002), and time since cancer diagnosis (Frattaroli, 2006; Low et al., 2010).
Dispositional optimists generally expect a favorable outcome and are more likely to engage in a self-regulation process (Rasmussen, Wrosch, Scheier, & Carver, 2006). Application of self-regulation theory to emotional disclosure supports that those who engage in rather than avoid the process of exploring and making sense of their feelings may have better QoL (Cameron & Nicholls, 1998). Similarly, written emotional expression has been found to be more effective for women low in avoidance (Stanton et al., 2002). Thus, those who are higher in dispositional optimism and lower in avoidance may benefit more from emotional disclosure.
In addition, a variety of methodological nuances in the design of studies of emotional disclosure interventions may affect outcomes. Meta-analyses across a variety of health and clinical samples have found that disclosure of a current stressful experience is more effective than disclosure of a past experience (Frattaroli, 2006; Smyth, 1998). Yet, it is not yet known if these results specifically apply to people diagnosed with cancer. Lymphedema in breast cancer survivors is an ongoing, chronic trauma; thus, breast cancer survivors who have developed chronic lymphedema may be a group that can achieve maximum benefit from emotional disclosure. In addition, some evidence supports that emotional disclosure implemented in participants’ homes is more effective than disclosure in a controlled laboratory setting (Frattaroli, 2006). Thus, the main purpose of this study was to examine the impact of a home-based expressive writing intervention on QoL in breast cancer survivors with chronic lymphedema.
The pre-specified primary hypothesis was that relative to the control group, individuals in the expressive writing group would report improved QoL post-intervention. One of the secondary aims was selected to further examine possible subsets of individuals for whom expressive writing might be most effective. This aim was to explore the influence of individual difference variables on outcomes associated with the interventions to identify possible subsets of individuals for whom expressive writing was most effective. Optimism and avoidant behaviors were pre-specified variables of interest in the original study aims and time since lymphedema diagnosis was later included based on results of a meta-analysis showing that writing was more efficacious for participants who wrote about more recent stressful events (Frattaroli, 2006). Although lymphedema can be a chronically traumatic condition, some women who have been diagnosed for longer periods of time may have already disclosed and processed the experience of having lymphedema, thus lessening the impact on quality of life (Frattaroli, 2006).
We hypothesized three distinct interaction effects such that those with greater optimism, lower avoidance, or less time since lymphedema diagnosis would experience greater increases in QoL from written emotional disclosure than those with lower optimism, higher avoidance, or greater time since lymphedema diagnosis.
Methods
Participants
The study enrolled community dwelling women recruited through various means from March 2007–December 2009 and study follow-up was completed in January 2011 (Clinical Trials.gov: NCT00400049). Potential participants were screened using a standardized protocol. Women were included if they: (1) were 21–80 years old; (2) could read, write, and speak English; (3) had Stage II lymphedema (i.e., swelling is unrelieved by elevation; untreated arm is hard, does not pit with pressure, skin changes may have taken place) and requires life-long at home self-care such as compression garments (International Society of Lymphedema, 2003); (4) had undergone professional treatment for lymphedema and required life-long self-care; and (5) were willing and able to drive to the study site or be seen in an alternative setting one time. Women were excluded if they: (1) were actively undergoing intravenous chemotherapy or radiation therapy for cancer; (2) had other medical conditions that could cause edema (i.e., congestive heart failure, chronic/acute renal disease, or pulmonale, nephrotic syndrome, nephrosis, liver failure, cirrhosis, pregnancy); (3) had a history of bilateral lymphedema that prohibited extracellular fluid comparison to an unaffected limb; (4) had metastatic breast cancer; (5) were unable to stand upright for measurement of height and weight; (6) had metal implants or pacemakers; or (7) had a history of suicide attempts, recent suicidal ideation, or were taking antipsychotic medication. All study procedures were approved by the Vanderbilt University Institutional Review Board and investigators obtained written informed consent from each participant.
Procedures
This was a two-group, randomized clinical trial (allocation ratio 1:1). Prior to enrollment participants were told the purpose of the study was to help us learn how writing impacts breast cancer survivors with lymphedema. Thus, the participants were blinded to differences in the interventions received to reduce performance bias (Higgins et al., 2011). Participants were randomized to reduce selection bias using a minimization procedure (Conlon & Anderson, 1990). In this procedure, the first participant was assigned to a group with a coin toss and then assigned based upon their Body Mass Index (≥ 30 vs. <30), which was found to be related to quality of life and lymphedema symptom distress in our prior work (Ridner, 2005), and assignment of previous subjects. The principal investigator (S.H.R.) and co-investigators were blinded to assignment and not involved in intervention implementation or data collection to reduce performance bias.
For the initial visit, participants were seen in person in a private location. Study staff measured height and weight, and participants completed baseline assessments of the variables analyzed for this manuscript in addition to other measures of fatigue, psychological distress, activity level, and confidence in body that are not presented. Participants were given the option to complete all other assessments and interventions either on-line or with pencil-and-paper. Both groups wrote for four, twenty-minute sessions spaced over two weeks (i.e., two sessions per week spaced 48 hours apart). A meta-analysis found that writing sessions spaced out over longer periods of time resulted in larger effect sizes for some outcomes (Smyth, 1998), thus we chose, after discussion with our external consultant (Dr. Annette Stanton), to space the writing sessions over a slightly longer period of time than original protocol that implemented writing on four consecutive days (Pennebaker & Beall, 1986). Writing interventions occurred in the privacy of the participant’s home at dates and times selected by participants during their initial visit. Individuals were called by study staff to prompt them when to begin writing and again 20 minutes later as both a safety check and a prompt to stop writing. Each pen and paper writing form, which included the self-administered writing instructions, was mailed back to the study office as soon as feasible. Follow-up data were collected one, three, and six months post intervention on-line via the study website, which reduces study staff influence on responses. Participants were compensated up to $115.00 over their seven months of participation in the study.
The experimental group wrote about their deepest thoughts and feelings regarding lymphedema and its treatment with the same instructions for all four sessions. The active control group wrote objectively about facts related to daily activities with modifications of the topic for four sessions (i.e., eating behaviors, activities during a typical day, use of nicotine/caffeine/alcohol, planned activities) similar to instructions used in other studies (e.g., Greenberg & Stone 1992) and modified by discussions with our study team and external consultant. After each writing session participants in both groups completed a manipulation check. They were asked on a scale of 1 (not at all) to 7 (a great deal): (1) “How personal did you consider your essay to be?” (2) “To what degree did you reveal emotions in your essay?” and (3) “To what degree have you previously refrained from telling others about the subject you covered in your essay?” Participants were also queried as to whether or not they were they were experiencing any distress as a result of writing.
Measures
Quality of Life: Primary Outcome
Upper Limb Lymphedema 27 (ULL-27)
The ULL-27 measures three domains (social, physical, psychological) and total QoL related to upper limb lymphedema (Launois, Megnigbeto, LeLay, Pocquet, & Alloit, 2002). Scores were derived using available items if 75% of the respective domain or total scale items had been completed. This instrument has demonstrated validity in women with lymphedema (Launois et al., 2002). Reliability of the scores in this study at the various times of assessment as measured by the Cronbach’s alpha statistic was as follows: Social=0.80–0.85, Physical=0.90–0.93, Psychological=0.81–0.88, and Overall=0.93–0.95. Higher scores reflect a better QoL.
Functional Assessment of Cancer Therapy–Breast (FACT-B)
The FACT-B is reported as a total score comprised of the five domain scores: social/family well-being, physical well-being, emotional well-being, functional well-being, and additional breast-specific concerns (Brady et al., 1997). Randomly missing item responses on the FACT-B were handled as prescribed by instrument developers (FACIT Administration and Scoring Guidelines, n.d.). The Cronbach’s alphas for the scores at the various times of assessment in this study were: Social/Family=0.81–0.89, Physical=0.78–0.82, Emotional=0.70–0.83, Functional=0.86–0.89, Breast-Specific=0.73–0.76, and Total=0.83–0.88. Higher scores reflect a better QoL.
Individual Differences
Life Orientation Test–Revised (LOT-R)
The 10-item LOT-R assessed dispositional optimism (6 items scored, 4 filler items; Scheier, Carver, & Bridges, 1994). Higher scores represent higher optimism. The LOT-R has been shown to be valid in cancer patients (Cronbach’s alpha=0.78; Carver, Lehman, & Antoni, 2003; Scheier et al., 1994). The Cronbach’s alpha for the scores used in this study was 0.77.
Impact of Event Scale (IES)
The 8-item avoidance subscale of the IES (IES-A) was used to evaluate avoidant responses to a specific traumatic event (i.e., lymphedema; Horowitz, Wilner, & Alvarez, 1979). The Cronbach’s alpha of the IES-A scores in this study was 0.69. This measure is valid (Horowitz et al., 1979), and has been used successfully in cancer populations (Epping-Jordan, Compas, & Howell, 1994).
Demographic and Clinical Characteristics
Demographic characteristics
Age, race, marital status, years of education completed, and income.
Clinical characteristics
Height, weight, stage of cancer, type of cancer and treatment, as well as length of time since diagnosis (of cancer and lymphedema) and since end of treatment (of cancer and lymphedema). Body Mass Index was calculated as kg/m2.
Statistical Analyses
IBM SPSS version 23 was used for all statistical analyses. Frequency distributions were used to summarize nominal and ordinal categorical variables. All continuous demographic, clinical, and study outcome data distributions were skewed, therefore median and inter-quartile range (IQR) were used to summarize those distributions. Study group comparisons of baseline characteristics were conducted using Chi-Square Tests of Independence (categorical) and Mann-Whitney tests (continuous) to evaluate selection bias.
An intention-to-treat analyses of the effect of expressive writing on study outcomes was used for all analyses. That is, every participant was analyzed with their randomly assigned group regardless of compliance, extent of participation, etc. Mixed-level (between: study groups; within: time of assessment) general linear modeling using the log-link function was used to test for group effects on the outcomes. Individual differences analysis of possible moderating effects of baseline optimism and avoidant behavior scores, as well time since lymphedema diagnosis on QoL measures were tested using multiple linear regression. The dependent variable in each model was the change in the QoL value (at 1-, 3-, or 6- months) from baseline. In addition to each of the moderator baseline centered and interaction effects, each moderator analysis included baseline value of the respective QoL measure being analyzed. All data distributions were transformed to meet the normal distribution assumptions of linear regression. Multicollinearity was evaluated for each model and no concerns were observed. Residuals analyses were also conducted and no concerns were observed with transformed data. All two-sided tests of statistical significance maintained a maximum alpha of 0.05. No correction to the critical alpha was made due to the primary focus being on effect sizes (β values).
Results
During the study 144 women with breast cancer met eligiblility criteria, 107 consented, and 104 completed the study (Figure 1). One participant was withdrawn due to cancer recurrence and two were lost to all follow-ups. Overall, 74% of participants were ≤65 years and 13.5% were African American (Table 1). Median time of lymphedema duration was approximately 4 years (range 0 to 26.5 years).
Figure 1.
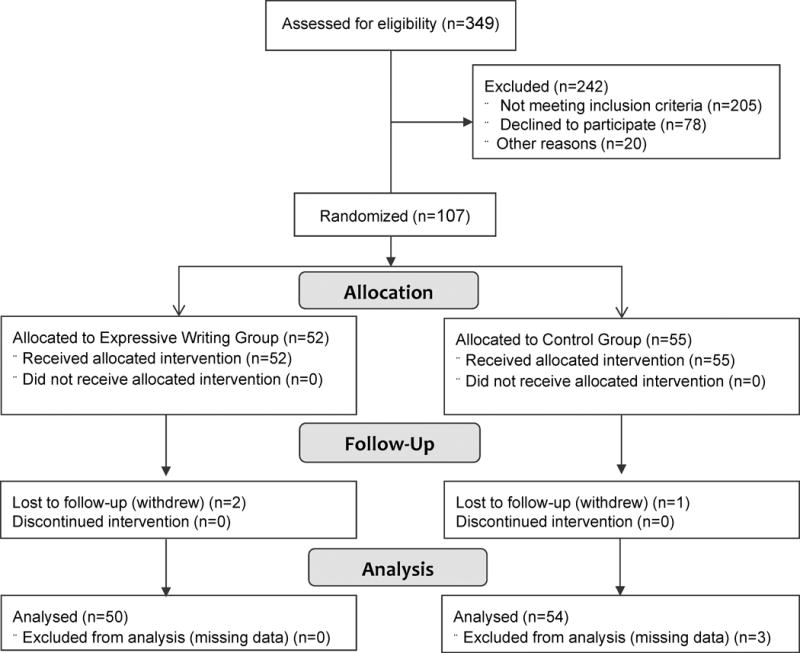
Study Flow Diagram
Table 1.
Characteristics of Participants (N=104)
| Characteristic | Total Sample | Written Disclosure | Control Group |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Age (Median [IQR]), Years | 58.0 (51.6–65.4) | 56.8 (49.2–62.1) | 58.3 (52.9–68.7) |
| Race | |||
| White | 87 (83.7) | 40 (78.4) | 47 (88.7) |
| African American | 14 (13.5) | 10 (19.6) | 4 (7.5) |
| Other | 3 (2.9) | 1 (2.0) | 2 (3.8) |
| Marital Status | |||
| Married/Partnered | 76 (73.1) | 33 (64.7) | 43 (81.1) |
| Other | 28 (26.9) | 18 (35.3) | 10 (18.9) |
| Education | |||
| ≤ 12th Grade | 24 (23.1) | 12 (23.5) | 12 (22.6) |
| > 12th Grade | 80 (76.9) | 39 (76.5) | 41 (77.4) |
| Household income, US $ | |||
| ≤ 30,000 | 9 (8.6) | 6 (11.8) | 3 (5.7) |
| 30,000–60,000 | 29 (27.9) | 13 (25.5) | 16 (30.2) |
| > 60,000 | 55 (52.9) | 26 (51.0) | 29 (54.7) |
| Did not care to answer | 11 (10.6) | 6 (11.8) | 5 (9.4) |
| Body Mass Index (Median [IQR]) | 28.9 (25.8–35.0) | 29.9 (25.1–35.2) | 28.6 (26.0–34.4) |
| Stage at Diagnosis | |||
| I | 24 (23.85) | 11 (22.4) | 13 (25.0) |
| II | 58 (57.4) | 29 (59.2) | 29 (55.8) |
| III | 18 (17.8) | 8 (16.3) | 10 (19.2) |
| IV | 1 (1.0) | 1 (2.0) | 0 (0.0) |
| Surgery Type | |||
| Lumpectomy/segmental | 41 (40.2) | 20 (40.0) | 21 (40.4) |
| Mastectomy | 56 (54.9) | 26 (52.0) | 30 (57.7) |
| Other | 5 (4.9) | 4 (8.0) | 1 (1.9) |
| Chemotherapy | 85 (83.3) | 42 (84.0) | 43 (83) |
| Radiotherapy | 67 (65.7) | 34 (68.0) | 33 (63.5) |
| Lymphedema duration (Median [IQR]), Years | 3.7 (0.9–8.3) | 3.8 (0.9–8.4) | 3.7 (0.8–8.3) |
| Mode of intervention implementation | |||
| Hand written | 30 (28.8) | 14 (27.5) | 16 (30.2) |
| Computer | 74 (71.2) | 37 (72.5) | 37 (69.8) |
Notes. IQR=interquartile range. No statistically significant differences between the study groups were observed (p > 0.05).
Post-writing manipulation checks revealed statistically significant group differences that provided support for intervention fidelity. As shown in Table 2, compared to those in the control group, the expressive writing group rated their essays across the four writing sessions as being more personal, emotional and revealing of issues that had never been previously discussed with anyone before. No adverse events related to the study occurred during study implementation.
Table 2.
Summaries of Intervention Fidelity
| Written Disclosure | Control Group | |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| Personal* | 5.8 (4.7 – 6.8) | 5.0 (2.7 – 6.4) |
| Emotional** | 6.0 (5.7 – 6.8) | 4.5 (3.0 – 5.3) |
| Revealing** | 4.3 (2.7 – 5.3) | 1.8 (1.2 – 3.0) |
Note. Ns for the sessions: Written disclosure=50–51, Control group=51–53.
Group main effect summaries (*p < .05, **p < 0.001) and no statistically significant interaction effects (p > 0.40) indicated intervention fidelity.
Descriptive summaries of the ULL-27 and FACT-B scores for each group at each time of assessment are shown in Table 3. There were no statistically significant main or interaction effects of expressive writing observed on any of the QoL measures (p’s>0.05; Table 4).
Table 3.
Primary Outcome Measures Descriptive Statistics
| Measure | Baseline (T1) | 1 Month (T2) | 3 Months (T3) | 6 Months (T4) | ||||
|---|---|---|---|---|---|---|---|---|
| Writing | Control | Writing | Control | Writing | Control | Writing | Control | |
| Median | Median | Median | Median | Median | Median | Median | Median | |
| IQR | IQR | IQR | IQR | IQR | IQR | IQR | IQR | |
| Upper Limb Lymphedema 27 (ULL-27) | ||||||||
| Physical | 81.3 (n=49) | 85.3 (n=47) | 84.0 (n=47) | 86.7 (n=45) | 82.7 (n=47) | 84.0 (n=45) | 81.3 (n=49) | 84.0 (n=47) |
| 68.0–90.7 | 72.0–92.0 | 69.3–92.0 | 79.3–91.3 | 70.7–90.7 | 68.7–92.0 | 66.7–90.7 | 70.7–89.3 | |
| Psychological | 80.0 (n=48) | 80.0 (n=49) | 81.4 (n=44) | 80.0 (n=49) | 77.1 (n=48) | 80.0 (n=48) | 80.0 (n=48) | 82.9 (n=49) |
| 71.4–91.4 | 71.4–88.6 | 71.4–88.6 | 71.4–91.4 | 66.4–88.6 | 66.3–88.6 | 71.4–91.4 | 67.1–95.7 | |
| Social | 86.0 (n=48) | 84.0 (n=47) | 86.0 (n=46) | 84.0 (n=45) | 84.0 (n=46) | 84.0 (n=47) | 88.0 (n=48) | 84.0 (n=47) |
| 68.0–96.0 | 72.0–96.0 | 71.0–96.0 | 72.0–96.0 | 68.0–93.0 | 76.0–92.0 | 73.0–96.0 | 68.0–96.0 | |
| Overall ULL-27 | 81.5 (n=46) | 83.7 (n=45) | 83.7 (n=41) | 83.7 (n=43) | 79.3 (n=43) | 81.5 (n=43) | 81.1 (n=46) | 84.4 (n=45) |
| 66.5–92.0 | 71.5–90.4 | 70.0–90.4 | 73.3–88.9 | 66.7–89.6 | 70.4–89.6 | 69.3–89.6 | 70.0–88.9 | |
| Functional Assessment of Cancer Therapy - Breast (FACT-B) | ||||||||
| Physical WB | 23.0 (n=50) | 24.0 (n=51) | 23.0 (n=49) | 24.0 (n=51) | 24.0 (n=50) | 24.0 (n=51) | 23.0 (n=50) | 25.0 (n=51) |
| 19.7–25.2 | 21.0–27.0 | 19.0–26.0 | 22.0–26.0 | 19.7–26.0 | 21.0–26.0 | 19.0–26.0 | 20.0–26.0 | |
| Social WB | 23.0 (n=50) | 24.0 (n=51) | 21.0 (n=49) | 23.0 (n=51) | 22.0 (n=50) | 24.0 (n=51) | 23.0 (n=50) | 23.0 (n=51) |
| 17.7–25.3 | 20.0–27.0 | 17.2–25.5 | 19.0–27.0 | 15.7–25.2 | 19.0–27.0 | 18.5–25.2 | 17.0–26.8 | |
| Emotional WB | 19.5 (n=50) | 20.0 (n=51) | 20.0 (n=49) | 20.0 (n=51) | 19.0 (n=50) | 20.0 (n=51) | 20.0 (n=50) | 20.0 (n=51) |
| 16.0–23.0 | 17.0–23.0 | 14.5–22.0 | 17.0–22.0 | 16.7–23.0 | 18.0–22.0 | 17.0–22.0 | 17.0–22.0 | |
| Functional WB | 20.0 (n=50) | 22.0 (n=51) | 21.0 (n=49) | 21.0 (n=51) | 22.0 (n=50) | 21.0 (n=51) | 21.0 (n=50) | 20.0 (n=51) |
| 16.0–25.0 | 17.0–26.0 | 14.5–25.0 | 16.0–25.0 | 17.0–24.0 | 15.0–25.0 | 18.0–24.0 | 15.0–25.0 | |
| Breast Subscale | 25.5 (n=50) | 23.0 (n=51) | 24.0 (n=49) | 24.0 (n=51) | 24.0 (n=50) | 22.0 (n=51) | 25.0 (n=50) | 23.0 (n=51) |
| 20.0–28.0 | 18.0–27.0 | 18.5–28.0 | 19.0–28.0 | 19.0–29.0 | 19.0–27.0 | 20.0–28.3 | 18.0–27.0 | |
| Total FACT-B | 108.5 (n=50) | 114.0 (n=51) | 112.0 (n=49) | 113.0 (n=51) | 114.6 (n=50) | 106.0 (n=51) | 112.7 (n=50) | 112.0 (n=51) |
| 89.5–123.7 | 94.7–125.0 | 86.0–122.2 | 95.0–123.0 | 91.0–125.0 | 95.0–124.0 | 89.0–122.1 | 89.0–122.0 | |
Note. IQR=Interquartile Range; WB=well-being; Data were missing at random.
Table 4.
Effect sizes and statistical probabilities for the main and interaction tests of expressive writing.
| Measure | Group | Time | Interaction | |||
|---|---|---|---|---|---|---|
| d | p-value | d | p-value | d | p-value | |
| Upper Limb Lymphedema 27 (ULL-27) | ||||||
| Physical | 0.11 | 0.332 | 0.18 | 0.423 | 0.06 | 0.948 |
| Psych | <0.01 | 0.071 | 0.14 | 0.603 | 0.14 | 0.748 |
| Social | <0.01 | 0.928 | 0.06 | 0.969 | 0.09 | 0.892 |
| Overall | 0.06 | 0.562 | 0.11 | 0.833 | <0.01 | 0.991 |
| Functional Assessment of Cancer Therapy - Breast (FACT-B) | ||||||
| Physical | 0.19 | 0.054 | 0.06 | 0.973 | 0.06 | 0.949 |
| Social | 0.26 | 0.011 | 0.06 | 0.947 | 0.06 | 0.898 |
| Emotional | 0.09 | 0.413 | <0.01 | 0.986 | 0.06 | 0.970 |
| Functional | <0.01 | 0.738 | 0.06 | 0.920 | 0.09 | 0.842 |
| Breast | 0.22 | 0.027 | <0.01 | 0.995 | 0.11 | 0.742 |
| Total | 0.06 | 0.511 | <0.01 | 0.989 | 0.09 | 0.879 |
Analyses of potential moderator effects of dispositional optimism (LOT-R), avoidant behavior (IES-avoidance), and time since lymphedema diagnosis with the expressive writing intervention on QoL over the course of the study were undertaken. At 3-months, a moderating effect of baseline optimism was observed via an interaction effect of those scores with the study group assignment on the change in overall ULL-27 (β=0.31, p=0.017) such that there was greater positive change in the ULL-27 scores in the expressive writing group for those with more optimistic LOT-R scores at baseline than for those with lower scores (Figure 2a). There was no such relationship within the control group. This moderator effect of optimism on the study group was also apparent for the FACT Breast-specific and Physical subscale scores. Interaction effect coefficients of the LOT-R scores with the intervention group assignment demonstrated fairly consistent coefficients at 1-, 3-, and 6-month assessment points; however, those at 3-months demonstrated the strongest and statistically significant effects (FACT Breast-specific: 1-month, β=0.24, p=0.058; 3-months, β=0.29, p=0.017; 6-months, β=0.27, p=0.033; FACT Physical: 1-month, β=0.21, p=0.071; 3-months, β=0.31, p=0.008; 6-months, β=0.18, p=0.116). No other statistically significant moderating effects of optimism on the QoL measures were observed.
Figure 2a.
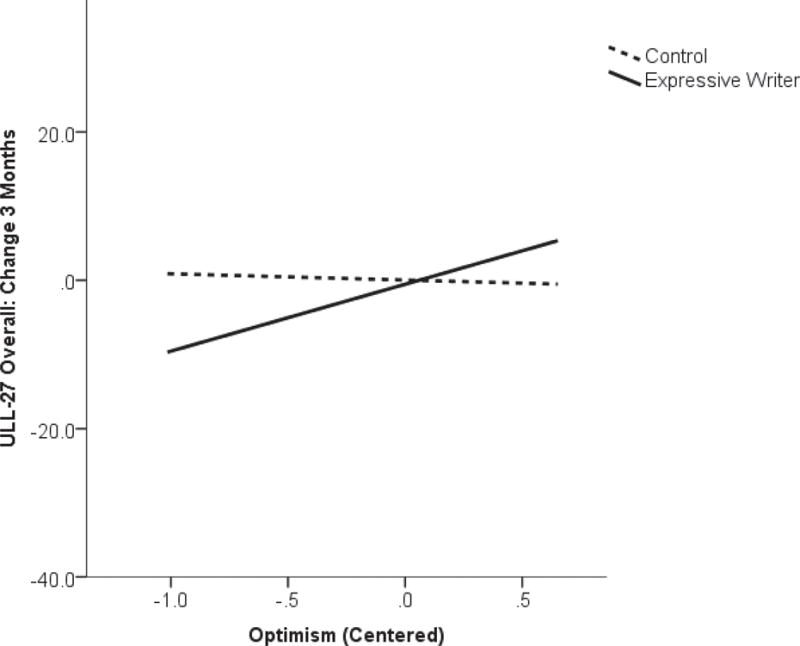
Interaction of baseline levels of optimism with study group on change in quality of life (Upper Limb Lymphedema-27; ULL-27) from baseline to 3-months on study.
Evidence of the moderating effects of baseline avoidant behaviors as measured by the IES-A on the expressive writing intervention’s effect on QoL were also observed. At 1-month, the interaction effect of baseline IES-A and study group on total FACT-B scores was statistically significant (β=−0.40, p=0.007) such that higher avoidant scores in the intervention group at baseline were associated with less change or even worsening FACT-B scores (as most strongly illustrated by the interaction presented in Figure 2b). The opposite pattern of association was observed in the control group. Effects on the FACT-B subscales of social and physical QoL appeared to be driving this overall effect (FACT Social: β=−0.40, p=0.006; FACT Physical: β=−0.40, p=0.048). Furthermore, at 3-months, there was a weaker moderating effect of the IES-A scores on the change in ULL-27 scores (overall ULL-27: β=−0.29, p=0.048, ULL Physical: β=−0.27, p=0.048). No statistically significant moderating effects of IES-A on changes from baseline to 6-month in QoL scores were observed.
Figure 2b.
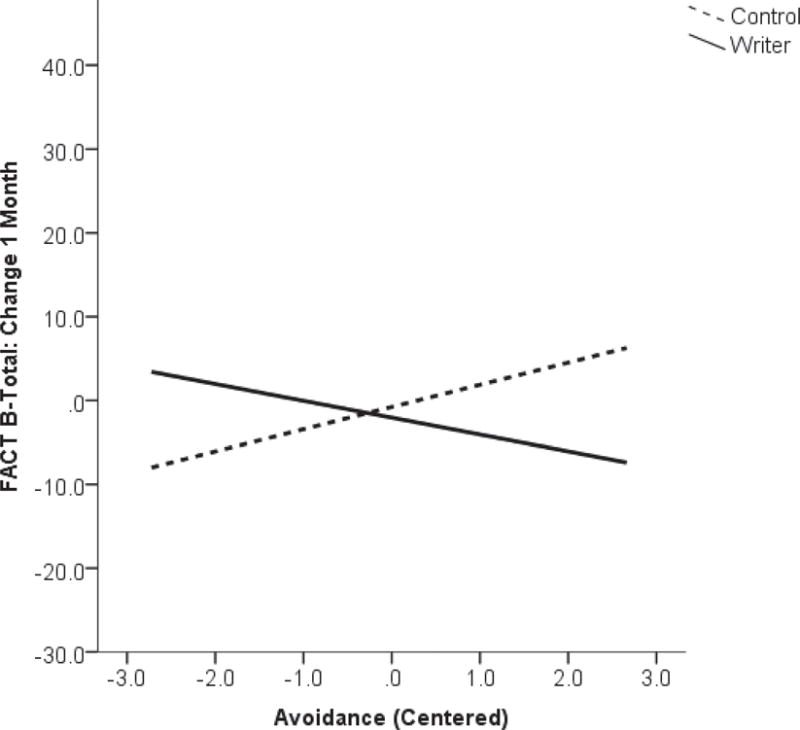
Interaction of baseline levels of avoidant behavior and study group on change in quality of life (Functional Assessment of Cancer Therapy–Breast; FACT-B) from baseline to 1-month on study.
The final moderator analyzed was time since lymphedema diagnosis as an indicator of recency of onset of the stressful event. Statistically significant moderating effects were observed for changes in the overall ULL-27 and total FACT-B scores at 3-months (ULL-27: β=−0.42, p=0.003; FACT-B: β=−0.27, p=0.049) and 6-months (ULL-27: β=−0.33, p=0.011; FACT-B: β=−0.33, p=0.017). The moderating effects were such that participants in the expressive writing group who were more recently diagnosed with lymphedema showed greater improvements in QoL with less change further out from diagnosis. The opposite pattern was observed for changes in ULL-27 scores in the control group (Figure 2c). Subscales of the measures that appeared to be most impacted were the ULL-Social (β=−0.44, p=0.001) and Physical (β=−0.38, p=0.004) domains. The moderating effect of time since diagnosis remained apparent at 6-months for the ULL-Social domain (β=−0.36, p=0.009) and also for the FACT social domain score (β=−0.29, p=0.040) but was reduced somewhat for the ULL-Physical domain (β=−0.27, p=0.052). No statistically significant moderating effects of LOT-R (optimism), IES-A (avoidant behavior), or time since lymphedema diagnosis on the ULL-psychological or the FACT-emotional domains were observed (p>0.05).
Figure 2c.
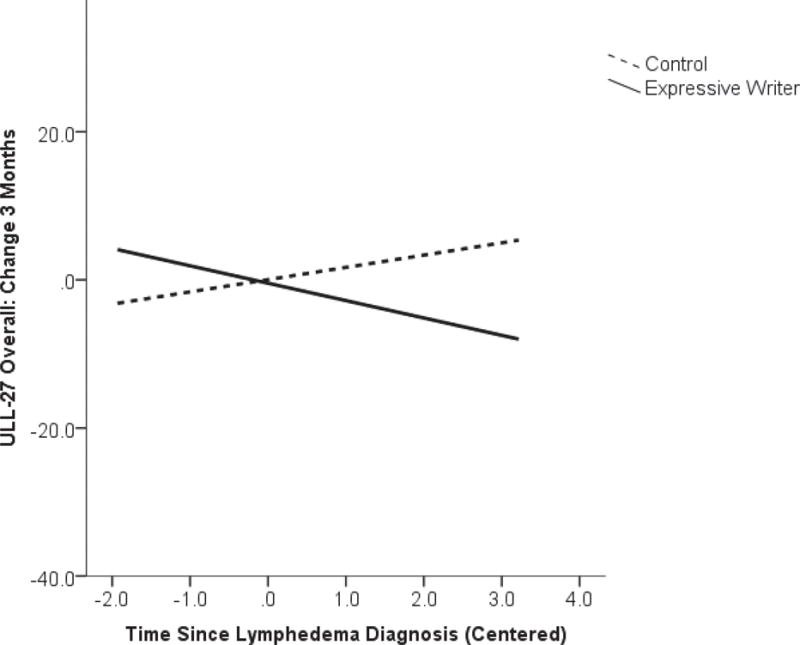
Interaction of time since lymphedema diagnosis and study group on change in quality of life (Upper Limb Lymphedema-27; ULL-27) from baseline to 3-months on study.
Discussion
Our primary hypothesis that the expressive writing condition would have greater improvements in QoL post-intervention relative to the control group was not confirmed. Secondary analyses were consistent with the hypothesized influence of individual differences in dispositional optimism, avoidant behaviors, and time since lymphedema diagnosis on the effect of expressive writing. These results provide further evidence supporting that there are, or may be, subsets of individuals for whom expressive writing is more effective for improving QoL.
Because the null main effect found in this study is consistent with other research (Jensen-Johansen et al., 2013; Low et al., 2010; Walker et al., 1999), including a meta-analysis (Zacharie & O’Toole, 2015), it is possible that expressive writing may be ineffective for improving QoL in women with breast cancer who develop lymphedema. This meta-analysis further revealed that the overall null result is not affected by study quality (Zacharie & O’Toole, 2015). It is also possible, based on prior research, that other factors may explain why we did not detect a differential main effect given the evidence compiled in a meta-analysis of 146 studies supporting efficacy of this intervention in other populations (Frattaroli, 2006). One possibility, based on results of individual studies, is that the writing instructions utilized for the control group participants had a similar effect to those for the experimental group (Craft et al., 2013; Jensen-Johansen et al., 2013). For example, a study of expressive writing in breast cancer patients found that writing specifically about cancer in both a factual and expressive manner had a significant benefit on QoL as compared to writing about a general trauma or not writing at all (Craft et al., 2013). A qualitative analysis of the writing content from the control group for this current study revealed that the writing instructions for the control group also fostered frequent discussion of the effect of cancer on participants’ daily lives (Ridner et al., 2012). Yet, there is no indication in the current study that either set of writing directions was beneficial.
Another possible explanation, that expressive writing may be more efficacious for some individuals than others, was explored as a secondary aim of the current study. Results supported that optimists benefited more from the expressive writing condition than did pessimists, with the most pronounced individual differences evident in physical and breast-specific QoL concerns. This differs from overall results of other studies reviewed in a meta-analysis, which found a reverse relationship such that those who were lower in optimism benefitted more from expressive writing (Frattaroli, 2006). The writing instructions and populations varied in these studies such that two of them were specifically designed to enhance self-regulation or optimism by providing structured guidance for writing (Cameron & Nicholls, 1998) or focusing on a future rather than a current stressor (Mann, 2001). A study comparing emotional disclosure instructions to more structured guidance for writing to enhance self-regulation found that optimists did better in both groups than a control condition, whereas pessimistic individuals only benefitted from the structured guidance intervention (when dividing optimists and pessimists into two separate groups using median splits). Our study provided unstructured emotional disclosure writing instructions about a current stressor, thus relying on participants’ innate ability to self-regulate.
Similarly, our results supported that those who initially avoided engaging in the coping process found expressive writing potentially detrimental as compared to those who were not avoidant. Alternately, those in the control condition who were more avoidant found that writing about facts was more helpful than did those who were not avoidant. Thus, it is not clear whether or not expressive writing may lead to adverse outcomes in some subgroups. This is consistent with another study of women diagnosed with breast cancer, which also found that expressive writing was more effective for women low in avoidance (Stanton et al., 2002). Avoidant behaviors are associated with less narrative structure in expressive writing samples, and an organized narrative is believed to be what produces health effects (Smyth, Anderson, Hockemeyer, & Stone, 2002). Emotional expressiveness, which may be considered a conceptual opposite of avoidance (Niles, Haltom, Mulvenna, Lieberman, & Stanton, 2014), shows a consistent pattern such that those who are more expressive find written expression more useful. Furthermore, avoidance has been found to be a mediator of the effects of expressive writing such that reductions in avoidance explain improvements in symptoms and distress (Milbury et al., 2014; Zakowski, Ramati, Morton, Johnson, & Flanigan, 2004).
Time since lymphedema diagnosis also significantly moderated the effect of expressive writing such that participants in the expressive writing group who were more recently diagnosed with lymphedema showed greater improvements in QoL than those in the control group who were also more recently diagnosed with lymphedema. This result is consistent with that found in other studies in which participants who wrote about more recent traumas or topics had larger effect sizes (Low et al., 2010). Our study tried to recruit a sample currently experiencing the potentially distressful symptom of lymphedema; however, results suggest that a sample of women even more newly diagnosed with lymphedema might have exhibited stronger effects if assigned to the expressive writing condition.
Limitations of the current study included the possibility that it was underpowered. The original study was powered a priori to detect an effect size of d=0.40, a conservative estimate given the current information at the time (Smyth, 1998). With subsequent research and a recent meta-analysis of that research, it appears that the average effect for expressive writing may be considerably smaller (r=0.075, equivalent d=0.15; Frattaroli, 2006; Lipsey & Wilson, 2001). Thus, it might be questioned whether a larger sample may have revealed a statistically significant group by time interaction effect; however, the findings from our data do not show trends to support that assumption. In addition, manipulation check results were based on participants’ own perceptions of their essays, which are subjective and may be biased by factors such as efficacy expectations. However, efficacy expectations were not likely to systematically differ between groups since participants were not informed about differences in the interventions. In this community based study, participants were able to select the time of day and location in their home for each writing session. Thus these conditions were not standardized and could have differed between or within participants. It is unknown if this affected study results. The writing instructions differed between groups such that they were consistent by session for the experimental group and changed by session for the control group to enhance participant engagement. This may have also systematically impacted study results in an unknown direction. This limitation is balanced by the strong adherence and low attrition rates (resulting in reduced attrition bias) that also may have resulted from this high motivation. In addition, this study was conducted exclusively in women, thus the results might not generalize to men.
Future research may investigate targeting the current expressive writing intervention to women more likely to find it useful based on results from the current study (i.e., optimistic, not avoidant, less time since lymphedema diagnosis) and other research (e.g., increased social constraints; Zachariae & O’Toole, 2015) or provide writing instructions that include additional guidance to facilitate the ability to process challenging emotions among those less likely to benefit. For example, participants who were provided guidance to facilitate a self-regulation process or prompted to write about positive thoughts and feelings have found expressive writing more useful than those provided the standard instructions used in the present study (Cameron & Nicholls, 1998; Stanton et al., 2002). Future directions may also include utilizing expressive writing as a component of a multimodal intervention or identifying a different intervention that may be more effective for improving QoL associated with lymphedema.
Acknowledgments
This research was supported by the American Cancer Society under grant number MRSG-07-012-01-CPPB from and the National Center For Complementary and Integrative Health of the National Institutes of Health under award number K01AT008219. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Cancer Society or National Institutes of Health. The authors do not have any conflicts of interest to report.
References
- American Cancer Society. Cancer Treatment and Survivorship Facts and Figures 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast (FACT-B) quality of life instrument. Journal of Clinical Oncology. 1997;3(15):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- Cameron LD, Nicholls G. Expression of stressful experiences through writing: Effects of a self-regulation manipulation for pessimists and optimists. Health Psychology. 1998;17(1):84–92. doi: 10.1037//0278-6133.17.1.84. http://doi.org/http://dx.doi.org/10.1037/0278-6133.17.1.84. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Women’s experiences of lymphedema. Oncology Nursing Forum. 1997;24(5):875–882. [PubMed] [Google Scholar]
- Carver CS, Lehman JM, Antoni MH. Dispositional pessimism predicts illness-related disruption of social and recreational activities among breast cancer patients. Journal of Personality and Social Psychology. 2003;84(4):813–821. doi: 10.1037/0022-3514.84.4.813. [DOI] [PubMed] [Google Scholar]
- Conlon M, Anderson GC. Three methods of random assignment: comparison of balance achieved on potentially confounding variables. Nursing Research. 1990;39(6):376–379. [PubMed] [Google Scholar]
- Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Research and Treatment. 2001;68(3):273–282. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]
- Craft MA, Davis GC, Paulson RM. Expressive writing in early breast cancer survivors. Journal of Advanced Nursing. 2013;69(2):305–315. doi: 10.1111/j.1365-2648.2012.06008.x. http://doi.org/http://dx.doi.org/10.1111/j.1365-2648.2012.06008.x. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Lam S, Stanton AL, Taylor SE, Bower JE, Sherman DK. Does self-affirmation, cognitive processing, or discovery of meaning explain cancer-related health benefits of expressive writing? Personality and Social Psychology Bulletin. 2007;33(2):238–250. doi: 10.1177/0146167206294412. http://doi.org/http://dx.doi.org/10.1177/0146167206294412. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan JE, Compas BE, Howell DC. Predictors of cancer progression in young adult men and women: Avoidance, intrusive thoughts, and psychological symptoms. Health Psychology. 1994;13(6):539–547. doi: 10.1037//0278-6133.13.6.539. http://doi.org/http://dx.doi.org.proxy.library.vanderbilt.edu/10.1037/0278-6133.13.6.539. [DOI] [PubMed] [Google Scholar]
- FACIT Administration and Scoring Guidelines (n.d.). FACIT.org. Retrieved from http://www.facit.org/FACITOrg/Questionnaires
- Frattaroli J. Experimental disclosure and its moderators: A meta-analysis. Psychological Bulletin. 2006;132(6):823–865. doi: 10.1037/0033-2909.132.6.823. http://doi.org/http://dx.doi.org/10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment–a nationwide study of prevalence and associated factors. Breast (Edinburgh, Scotland) 2010;19(6):506–515. doi: 10.1016/j.breast.2010.05.015. http://doi.org/10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Greenberg MA, Wortman CB, Stone AA. Emotional expression and physical heath: Revising traumatic memories or fostering self-regulation? Journal of Personality and Social Psychology. 1996;71(3):588–602. doi: 10.1037//0022-3514.71.3.588. https://doi.org/http://dx.doi.org/10.1037/0022-3514.71.3.588. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ : British Medical Journal. 2011:343. doi: 10.1136/bmj.d5928. https://doi.org/10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed]
- Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- International Society of Lymphedema. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology. 2003;36:84–91. [PubMed] [Google Scholar]
- Jensen‐Johansen MB, Christensen S, Valdimarsdottir H, Zakowski S, Jensen AB, Bovbjerg DH, Zachariae R. Effects of an expressive writing intervention on cancer‐related distress in Danish breast cancer survivors—Results from a nationwide randomized clinical trial. Psycho-Oncology. 2013;22(7):1492–1500. doi: 10.1002/pon.3193. [DOI] [PubMed] [Google Scholar]
- Launois L, Megnigbeto AC, LeLay K, Pocquet K, Alloit F. A specific quality of life scale in upper limb lymphoedema: the ULL-27 Questionnaire. Lymphology. 2002;35:181–187. http://doi.org/doi:10.1016/S1098-3015(11)71503-0. [Google Scholar]
- Lipsey M, Wilson D. Practical Meta-Analysis. Vol. 49. Thousand Oaks, CA: Sage Publications, Inc; 2001. [Google Scholar]
- Low CA, Stanton AL, Bower JE, Gyllenhammer L. A randomized controlled trial of emotionally expressive writing for women with metastatic breast cancer. Health Psychology. 2010;29(4):460–466. doi: 10.1037/a0020153. http://doi.org/10.1037/a0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T. Effects of future writing and optimism on health behaviors in HIV-infected women. Annals of Behavioral Medicine. 2001;23(1):26–33. doi: 10.1207/S15324796ABM2301_5. https://doi.org/http://dx.doi.org/10.1207/S15324796ABM2301_5. [DOI] [PubMed] [Google Scholar]
- Megens AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Archives of Physical Medicine and Rehabilitation. 2001;82(12):1639–1644. doi: 10.1053/apmr.2001.26822. http://doi.org/10.1053/apmr.2001.26822. [DOI] [PubMed] [Google Scholar]
- Merz EL, Fox RS, Malcarne VL. Expressive writing interventions in cancer patients: a systematic review. Health Psychology Review. 2014;8(3):339–361. doi: 10.1080/17437199.2014.882007. https://doi.org/10.1080/17437199.2014.882007. [DOI] [PubMed] [Google Scholar]
- Milbury K, Spelman A, Wood C, Matin SF, Tannir N, Jonasch E, Cohen L. Randomized controlled trial of expressive writing for patients with renal cell carcinoma. Journal of Clinical Oncology. 2014 doi: 10.1200/JCO.2013.50.3532. http://doi.org/10.1200/JCO.2013.50.3532. [DOI] [PMC free article] [PubMed]
- Niles AN, Haltom KEB, Mulvenna CM, Lieberman MD, Stanton AL. Randomized controlled trial of expressive writing for psychological and physical health: the moderating role of emotional expressivity. Anxiety, Stress, and Coping. 2014;27(1):1–17. doi: 10.1080/10615806.2013.802308. http://doi.org/10.1080/10615806.2013.802308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Beall SK. Confronting a traumatic event: toward an understanding of inhibition and disease. Journal of Abnormal Psychology. 1986;95(3):274–281. doi: 10.1037//0021-843x.95.3.274. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Social Science & Medicine. 1988;26(3):327–332. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- Pusic AL, Cemal Y, Albornoz C, Klassen A, Cano S, Sulimanoff I, Mehrara B. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. Journal of Cancer Survivorship. 2013;7(1):83–92. doi: 10.1007/s11764-012-0247-5. http://doi.org/10.1007/s11764-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HN, Wrosch C, Scheier MF, Carver CS. Self-regulation processes and health: the importance of optimism and goal adjustment. Journal of Personality. 2006;74(6):1721–1747. doi: 10.1111/j.1467-6494.2006.00426.x. http://doi.org/JOPY426;10.1111/j.1467-6494.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Supportive Care in Cancer. 2005;13(11):904–911. doi: 10.1007/s00520-005-0810-y. http://doi.org/10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- Ridner SH, Sinclair V, Deng J, Bonner CM, Kidd N, Dietrich MS. Breast cancer survivors with lymphedema: glimpses of their daily lives. Clinical Journal of Oncology Nursing. 2012;16(6):609–614. doi: 10.1188/12.CJON.609-614. http://doi.org/10.1188/12.CJON.609-614. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variables. Journal of Consulting and Clinical Psychology. 1998;66(1):174–184. doi: 10.1037//0022-006x.66.1.174. http://doi.org/http://dx.doi.org.proxy.library.vanderbilt.edu/10.1037/0022-006X.66.1.174. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Anderson CF, Hockemeyer JR, Stone AA. Does emotional non-expressiveness or avoidance interfere with writing about stressful life events? An analysis in patients with chronic illness. Psychology & Health. 2002;17(5):561–569. http://doi.org/http://dx.doi.org.proxy.library.vanderbilt.edu/10.1080/08870440290025777. [Google Scholar]
- Smyth JM, Greenberg MA. Scriptotherapy: The effects of writing about traumatic events. In: Duberstein PR, Masling JM, editors. Psychodynamic Perspectives on Sickness and Health. Washington, DC: American Psychological Association; 2000. pp. 121–160. [Google Scholar]
- Smyth JM, Pennebaker JW. What are the health effects of disclosure? In: Baum A, Revenson TA, Singer JE, editors. Handbook of Health Psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 339–345. [Google Scholar]
- Stanton AL, Danoff-Burg S, Sworowski LA, Collins CA, Branstetter AD, Rodriguez-Hanley A, Austenfeld JL. Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. Journal of Clinical Oncology. 2002;20(20):4160–4168. doi: 10.1200/JCO.2002.08.521. [DOI] [PubMed] [Google Scholar]
- Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. American Journal of Surgery. 1999;177(3):184–187. doi: 10.1016/s0002-9610(99)00008-2. discussion 188. [DOI] [PubMed] [Google Scholar]
- Walker BL, Nail LM, Croyle RT. Does emotional expression make a difference in reactions to breast cancer? Oncology Nursing Forum. 1999;26(6):1025–1032. [PubMed] [Google Scholar]
- Zachariae R, O’Toole MS. The effect of expressive writing intervention on psychological and physical health outcomes in cancer patients: a systematic review and meta-analysis. Psycho-Oncology. 2015;24(11):1349–1359. doi: 10.1002/pon.3802. https://doi.org/10.1002/pon.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowski SG, Ramati A, Morton C, Johnson P, Flanigan R. Written Emotional Disclosure Buffers the Effects of Social Constraints on Distress Among Cancer Patients. Health Psychology. 2004;23(6):555–563. doi: 10.1037/0278-6133.23.6.555. http://doi.org/http://dx.doi.org/10.1037/0278-6133.23.6.555. [DOI] [PubMed] [Google Scholar]


