Abstract
With few exceptions, nutritional and dietary interventions generally impact upon both old-age quality of life and longevity. The life prolonging effects, commonly observed with dietary restriction reportedly are linked to alterations in protein intake and specifically limiting the dietary intake of certain essential amino acids. There is however a paucity of data methodically evaluating the various essential amino acids on health- and lifespan and the mechanisms involved. Rodent diets containing either lower methionine content, or tryptophan, than that found in commercially available chow, appear to elicit beneficial effects. It is unclear whether all of these favorable effects associated with restricted intake of methionine and tryptophan are due to their specific unique properties or if restriction of other essential amino acids, or proteins in general, may produce similar results. Considerably more work remains to be done to elucidate the mechanisms by which limiting these vital molecules may delay the onset of age-associated diseases and improve quality of life at older ages.
Keywords: methionine restriction, tryptophan, dwarf mice, naked mole-rat, delayed aging, healthspan
1.1 Introduction
For more than eighty years, a key focus in aging research has centered on the impact of dietary restriction on modulating lifespan. Across evolutionary distant phyla containing yeast, flies, worms, mice, monkeys and potentially humans, nutritional and dietary interventions generally impact upon longevity and quality of life at older ages. With few exceptions, life extending benefits of caloric restriction have been described numerous times in both vertebrates and invertebrates and with benefits evident at various levels of dietary restriction and/or intermittent feeding regimes and even when these interventions start in middle age (Liao et al., 2010; see Masoro, 2005; Le Couteur et al., 2016; Lee and Longo, 2016 for reviews). Although these improvements in longevity are attributed to simply a reduction in calorie intake, several studies gaining traction have challenged this dogma and shown that, life prolonging effects are not due to carbohydrate and lipid dietary restriction but rather appear to be specifically due to restricting protein intake (Grandison et al., 2009; Levine et al., 2014; Mair et al., 2005; Min and Tatar, 2006; reviewed in Sanchez-Roman and Barja, 2013). Indeed, in 90% of the studies involving protein restriction in laboratory rats and mice, a ~20% lifespan extension was observed (Pamplona and Barja, 2006). Very few studies have dissected this relationship between protein restriction and lifespan so as to assess the role of the individual essential amino acids (EAA; i.e., those that cannot be synthesized de novo in humans and require dietary sources). Indeed, in mammals there are relatively few reports in which a specific amino acid has been selectively reduced in the diet followed by examination of physiological parameters related to aging and life extension. Despite this large gap in our understanding of the role of the various EAAs, the delay of age-associated diseases and life prolonging effects of protein restriction are primarily attributed to restricting two of the EAAs, namely tryptophan (Segall, 1977; Sidransky, 1986) and methionine (Zimmerman et al., 2003; Orentreich et al., 1993). Understanding the mechanisms that are altered by restriction of these amino acids and how they impact upon aging may provide critical information towards finding mechanisms to modulate both lifespan and healthspan.
1.2 Tryptophan restriction
Tryptophan is commonly found in protein rich foods such as turkey, chicken, fish eggs, and red meat. However, it is also abundant in dairy products, chocolate, oats, dried dates, bananas, seeds and nuts (e.g., almonds, sunflower seeds, pumpkin seeds). Tryptophan is of critical importance in growth, development and reproduction and is also the precursor for serotonin, a signaling molecule involved in numerous blood, bone, bowel, and brain functions (Fernstrom and Wurtman, 1997). Tryptophan may thus influence memory, mood and learning through this serotonin pathway as well as affect stress responses and aging (Markus, 2008; Ruddick et al., 2006). In excess, tryptophan can be both toxic and carcinogenic, traits attributed to the effects of excess serotonin and its modulation of cell growth, fibrosis and inflammation and its direct effects on pituitary function, in particular the adrenocorticotrophin and thyroid axes (Hiraku et al., 1995; King et al., 1997; Welford et al., 2016).
Work performed 40 years ago by Timiras and coworkers (Zimmerman et al., 2003; Segall and Timiras, 1976; Segall et al., 1983; Ooka et al., 1988) described studies in rats where tryptophan-deficiency was created by limiting this amino acid to 30–40% in the diet from weaning to 24–30 months of age. Aging features appeared to be delayed in some organs like liver, heart and ovary but not in others (kidney, lung, aorta) while survival appeared to be increased. However, longevity was assessed following a return to the control diet partway through the experiments, thus it is unknown how long the animals may have survived if left on a tryptophan deficient diet. Early deaths occurred in the tryptophan deficient group but rats surviving past the first year outlived control groups. Also, low levels of tryptophan caused reductions in overall diet intake thus confounding the results.
Other studies on tryptophan-poor diets revealed that rats increased their period of fertility and fecundity and showed higher levels of testosterone. Metabolic rate is also increased with dietary tryptophan depletion as is the pelage condition and hair growth (Segall et al., 1983; Ooka et al., 1988; Ashley and Curzon, 1981). These indicators of extended healthspan are likely linked to the reported increased lifespan observed in lab rodents (Segall et al., 1983).
Marked decreases in brain serotonin as the underlying mechanism for the delayed aging was mostly ruled out as pharmacologic antagonists did not affect growth to the same extent as tryptophan restriction while side effects impacted the length of these studies. Of note is that gut microbes contribute to the tryptophan levels in mice, as microbiota depletion experiments have shown that the tryptophan metabolic pathway is altered in adult brains (Desbonnet et al., 2015). In addition, tryptophanase activity is increased in germ-free mice (microbiome-depleted) thus increasing serum levels of tryptophan (Wikoff et al., 2009). Many studies have been conducted using acute tryptophan deficiency but outcomes were not aging related. Overall, it will be important to validate the longevity data in rodents on tryptophan deficient diets to solidify the potential contribution of this amino acid to our understanding of its role in aging-related processes.
1.3 Methionine restriction
An abundance of work has been reported examining aging-related outcomes and longevity in rodents consuming methionine deficient diets. Foods with the highest amounts of methionine per total protein content include beef, cereals, dairy, eggs, and brazil nuts. In contrast, foods with the lowest levels by comparison would be those that predominantly make up a low protein, vegan diet. Symbiotic microorganisms in the gastrointestinal tract can synthesize methionine, but it not known whether this serves as a significant source of methionine for humans (Lee and Hase, 2014). The microbiome serves as an important source of methionine and other proteins in Caenorhabditis elegans and other organisms (Cabreiro et al., 2013). This microbial food source is most notably important for herbivores maintained on a low quality (low protein/high fiber) diet (Bennett and Faulkes, 2000; Torrallardona et al., 1996).
Methionine is notably the first amino acid present in nuclear-encoded proteins, as the methionine codon signals the start of protein translation. Methionine is essential for the production of cysteine which in turn is the precursor of glutathione (GSH) a key component of detoxification pathways, and S-adenosylmethionine (SAM), a critical methyl-donor for methylation of various molecules including DNA and proteins (Figure 1). Activation of the transsulfuration pathway, downstream of methionine recycling, leads to the production of hydrogen sulfide (H2S) gas, that is itself implicated in the beneficial effects of methionine restriction (Hine et al., 2015).
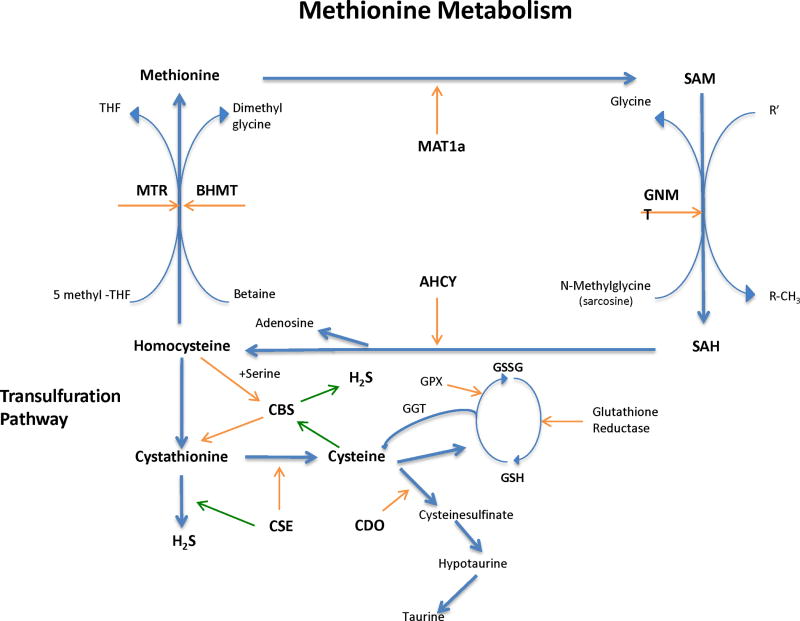
Figure 1.
The methionine metabolic pathway. We begin with methionine and include remethylation of homocysteine to methionine and transsulfuration of homocysteine to cysteine. In addition, the fate of cysteine incorporation to cysteine sulfinate through to taurine and hydrogen sulfide (green arrows) is shown as well as the contribution of cysteine from glutathione degradation pathways. MAT1a - methionine adenosyltransferase 1a; SAM – S-adenosylmethionine; GNMT – glycine N-methyltransferase; SAH – S-adenosyltransferase; AHCY – adenosylhomocysteine hydrolase; CBS – cystathionine beta-synthase; CSE – cystathionine gamma-lyase; CDO – cysteine dioxygenase; GGT – gamma-glutamyl transpeptidase; GPX – glutathione peroxidase; GSH – glutathione; GSSG – glutathione disulfide; H2S – hydrogen sulfide; MTR – methionine synthase; BHMT – betaine homocysteine S-methyltransferase; THF - tetrahydrofolate.
1.4 Methionine catabolism: The transsulfuration pathway
The liver is the main organ involved in methionine metabolism. During the methionine cycle, methionine is converted to SAM, S-adenosylhomocysteine (SAH), and homocysteine, and thereafter it is either recycled via remethylation to methionine or further modified via the irreversible transsulfuration pathway to form cysteine. Cysteine, rather than methionine, may be the critical player inducing direct affects upon body size, adiposity and associated endocrine signaling. In support of this premise is the observation that dietary supplementation with cysteine attenuates the metabolic effects and energy balance perturbations observed during methionine restriction (Elshorbagy et al., 2011; Gomez et al., 2015). These effects could be directly linked to changes in the transsulfuration pathway. Both cystathionine beta-synthase (CBS), and cystathionine gamma-lyase (CSE) enzymes are upregulated during methionine restriction giving rise to elevated levels of H2S and other downstream effectors such as glutathione and related compounds (Kabil and Banerjee, 2010; McIsaac et al., 2012; Petti et al., 2012). H2S is considered a gasotransmitter, modulating many molecular pathways involved in mitochondrial function, cytoprotection, inflammation and apoptosis (Wallace and Wang, 2015). It also regulates second messenger systems and activates ATP-dependent potassium ion channels, thereby affecting many physiological and pathophysiological processes. H2S levels are elevated in cardiovascular disease, rheumatoid arthritis and tumor growth (Peter et al., 2013; Muniraj et al., 2014; Hellmich et al., 2015). Despite this increase in pathological states, numerous reports suggest that high levels of H2S may extend longevity. For example, C. elegans based studies in which exogenous levels of H2S are elevated lead to an increase in the lifespan (Miller and Roth, 2007). Similarly, augmented levels of H2S have been associated with increased lifespan in Drosophila and also with caloric restriction (Hine et al., 2015; Kabil et al., 2011). In contrast the naked mole-rat has very low circulating levels of H2S and low CBS activity under non-stressed conditions but also shows increased sensitivity of CBS to SAM activation (Dziegelewska et al., 2016).
Enhanced activity of the transsulfuration pathway and resulting H2S production may have hormetic effects activating various cytoprotective and/or nutrient signaling pathways (e.g., target of rapamycin [TOR], eukaryotic initiation factor 2 [eIF2α]). For example, H2S induces protein S-sulfhydration whereby cysteine residues within proteins are modified such that the -SH from the sufhydryl donor forms a persulfide (-SSH) that may impact upon its function. S-sulfhydration of Kelch-like ECH-associated protein 1 (KEAP1), the negative regulator of the master cytoprotective transcription factor, nuclear factor erythroid 2-related factor 2 (NRF2), results in the activation of this important cytoprotective factor. This leads to increased detoxification, molecular chaperone levels and augmented ubiquitin proteasome system (UPS) proteolysis and in doing so helps protect against senescence (Yang et al., 2013). Another possible mechanism involves mitochondrial cytochrome enzymes, as H2S binds with iron and inhibits both respiration and ROS formation conferring protection against oxidative damage (Pietra et al., 2011). Similar protective effects of elevated H2S are also observed in invertebrates, fungi, and torpid animals (Robertson et al., 2015; Miller and Roth, 2007; Blackstone et al., 2005). Moreover, the preternaturally long-lived naked mole-rats have high levels of expression of the critical enzymes involved in cysteine and H2S formation, namely CSE and CBS, suggestive of elevated levels of H2S, although surprisingly observed serum levels are very low (Lewis et al., 2013). Resultant enhanced sulfhydration and antioxidant properties (Salmon et al., 2005) may also confer resistance to endogenous ROS and other oxidative stressors (Harper et al., 2011) and also contribute to extended longevity and stress resistance commonly observed in longer lived mutants of model organisms, including yeasts and worms (Hine et al., 2015; Miller and Roth, 2007; Salmon et al., 2005; Harper et al., 2011; Calvert et al., 2009). In humans, high H2S levels reportedly improve clinical outcome in response to ischemic reperfusion injury. However, high and moderately elevated levels of H2S have also been shown to inhibit cytochrome c oxidase, react with other heme- and sulfur-containing proteins and contribute to disease thus, the amount of this gasotransmitter is likely tightly controlled and needs further study (Ueki et al., 2011; Roman et al., 2013; Dorman et al., 2002; Goubern et al., 2007). Moreover, enhanced resistance to oxidative stress is only one piece of the puzzle in terms of lifespan extension (Perez et al., 2009; Dai et al., 2014; Cunningham et al., 2015; Speakman et al., 2015). Methionine restriction may itself directly activate NRF2. Nrf2 is a key regulator of several hundred cytoprotective molecules, including molecular chaperones, antioxidants, detoxicants and proteasome subunits (Lewis et al., 2010). Efficient removal of damaged proteins via the UPS may contribute substantially to a well maintained proteome and prolonged healthspan that may similarly contribute to lifespan extension (Calvert et al., 2009; Hourihan et al., 2013).
Both CYS3 and CYS4 are highly conserved in naked mole-rats (Altschul et al., 1990) and it has been shown that the gene product, CBS, overexpression is sufficient to extend lifespan in worms as can exogenous H2S (Hine et al., 2015). In animals, especially animals consuming a large proportion of plant material, it is important to note that the bacteria and protozoa symbionts of the gut also produce H2S, and while tempting to speculate that this is an integral component of the health benefits of a vegetarian diet, it has not as yet been determined if H2S produced by the microbiome causally contributes to longevity and if it has similar effect to that produced endogenously. While tantalizing, the beneficial effects of H2S associated with methionine restriction remain poorly understood. Nevertheless, the genes associated with the transsulfuration pathway may be potential therapeutic targets in the quest for ways to retard the aging process and extend the period of good health.
1.5 Rodent studies of methionine restriction
The earliest aging studies restricting methionine in rodents were conducted by Orentreich and colleagues (Zimmerman et al., 1993; Orentreich et al., 1993; Richie et al., 1994) and showed that lifespan was significantly extended in animals subjected to an 80% restriction. Since that time, many reports have focused on physiological parameters contributing to delayed aging as well as potential disease resistance following short and long-term intake of diets deficient in methionine. Lifespan extension is observed both in mice started on these diets shortly after weaning and in mice started on the diets at 12 months of age, suggesting that the underlying mechanisms are not necessarily growth or developmentally regulated (Sun et al., 2009; Brown-Borg et al., 2014a; Miller et al., 2005). Although absolute differences exist in the changes reported in mice, they trend in the same direction and may be partly due to the particular background strains utilized.
Rats and mice fed diets with low methionine weigh less and have altered body composition when compared to rodents fed methionine-replete food. Introduction of the methionine-restricted diet either at one month or 12 months of age resulted in lower body weights compared to animals on normal chow (Orentreich et al., 1993; Sun et al., 2009; Miller et al., 2005). Moreover, several studies suggest these rodents are more physically active than those fed methionine-sufficient diets but also show improved healthspan (Plaisance et al., 2010; Lees et al., 2014). Also, cataract development appears to be retarded slightly but significantly in mice on methionine-restricted diets (Miller et al., 2005). There is an age-dependent increase in T-cell subsets in mice and with methionine restriction, this age-related change seems to be slowed in comparison to mice ingesting higher amounts of methionine (0.43%; Miller et al., 2005). Although there is an abundance of pathology and necropsy data in pro-longevity mutant mice, few studies have systematically examined animals on methionine-restricted diets. One study observed that mice fed methionine-restricted diets exhibited similar types of illnesses as control mice but at slightly later ages (45 days) while others showed that methionine restriction delays prostate cancer development in a prostate cancer model (Miller et al., 2005; Sinha et al., 2014). In rats, chemically induced colon cancer is inhibited with methionine-restricted diets (Komninou et al., 2006). In short-living, growth hormone transgenic mice, methionine restriction significantly decreased the incidence of liver and kidney tumors in addition to lengthening lifespan by more than 50% (Brown-Borg et al., 2014b). Significant anti-inflammatory gene expression profiles in both liver and white adipose tissue were observed in mice consuming methionine-restricted diets (Wanders et al., 2014). Clearly, overall rodent health appears to be improved with this dietary intervention.
1.5.1 Alterations in hormone profiles with methionine restriction
Not only did methionine restriction in mice lead to a 10–20% increase in maximum lifespan (Orentreich et al., 1993; Miller et al., 2005) and improve healthspan, but sustained insulin sensitivity and metabolic rates were also observed during aging. Strikingly, although methionine restricted mice ate more than their experimental controls, within three weeks of methionine restriction, they showed improved responses to glucose tolerance testing rapidly removing the glucose bolus from the blood and presumably funneling this into cells (Lees et al., 2014; Malloy et al., 2006).
Rodents consuming low methionine diets exhibit reductions in plasma IGF1, insulin, leptin, thyroxine, and increases in adiponectin and fibroblast growth factor 21. Insulin like growth factor 1 levels are decreased by about one-third in mice and two-thirds in rats when compared to animals fed normal chow (Plaisance et al., 2010; Miller et al., 2005; Malloy et al., 2006). Insulin concentrations in the plasma are markedly decreased in both rats and mice fed methionine-restricted diets (by 85% and 75%, respectively) while thyroxine or T4 levels are reduced by 25% in mice and no difference was found in methionine-restricted rats (Miller et al., 2005; Malloy et al., 2006). Insulin sensitivity is greater in animals subjected to methionine restriction as glucose levels are lower and adiponectin and FGF21 are increased (Miller et al., 2005; Lees et al., 2014; Malloy et al., 2006). Directional changes in expression of both this growth factor and IGF1 may contribute to the observed changes in body composition and size of methionine-restricted mice as well as concomitant resistance to various cancers (Ables et al., 2012).
The levels of endocrine hormones in methionine-restricted animals are very similar to the levels observed in several long-living strains of mice supporting their underlying role in delaying or slowing processes that contribute to aging. Dwarf mice share many phenotypes in common with methionine-restricted mice. Both mouse models show similar increases in longevity and resistance to cancer as well as enhanced stress resistance (Brown-Borg et al., 1996). It is possible that this shared phenotype reflects similar perturbations in methionine metabolism, in keeping with the hypothesis that upregulation of the methionine pathway and its downstream components (e.g., the transsulfuration pathway and formation of cysteine) are key players in extending both lifespan and the period of good health (Uthus and Brown-Borg, 2006). One significant finding is that growth hormone signaling is necessary for methionine restriction to contribute to lifespan extension in rodents as long living GH deficient (Ames dwarf) and GH resistant (growth hormone receptor knockout) mice do not benefit with further life extension when fed methionine-restricted diets (Brown-Borg et al., 2014b).
1.5.2 Changes in lipid metabolism with methionine restriction
Reductions in dietary methionine intake also impact lipid metabolism and nutrient signaling. Components of lipid metabolism are altered via reductions in hormones due to the decrease in fat mass while serum cholesterol and triglyceride levels are observed to be lower in comparison to normal chow-fed animals (Elshorbagy et al., 2011; Malloy et al., 2006). The direction of change in fatty acid composition of CNS and liver membranes in methionine-restricted mice are indicative of an increased resistance to oxidative damage (Jove et al., 2013). In this same study, glycerophospholipid and sphingolipids were altered thereby influencing lipid raft assembly and cellular signaling in methionine-restricted mice. These changes in sphingolipids and its associated ceramide signaling pathways may also contribute to improved tolerance to oxidative stress. Similarly, methionine restriction modulates membrane phospholipid composition and this too may influence susceptibility to lipid peroxidation (Jove et al., 2013). The bi-lipid layer of cell membranes from methionine-restricted mice has a lower double bond index and less oxidation-prone docasohexanoic acid [DHA] resulting in membrane phospholipids that are more resistant to oxidative damage (Jove et al., 2013; Hulbert et al., 2006). Similarly, long-lived species such as naked mole-rats (Mitchell et al., 2007; Hulbert et al., 2006) and parrots (Pamplona et al., 1996; Pamplona et al., 2005) as well as Ames dwarf mice show lower proportions of DHA in their cell membranes and a lower peroxidation index is observed in their tissues (Pamplona et al., 2005; Hulbert et al., 2007; Montgomery et al., 2012; Valencak and Ruf, 2013).
1.5.3 Altered energy balance with methionine restriction
Food consumption is increased in animals on methionine-restricted diets whether expressed in absolute terms or per gram of body weight (Orentreich et al., 1993; Miller et al., 2005; Malloy et al., 2006; Hasek et al., 2010). Fat mass is significantly reduced however lean mass does not appear to be affected by these diets (Malloy et al., 2006; Hasek et al., 2010; Elshorbagy, 2014). In terms of bone mass, rodents consuming a methionine-restricted diet had lower bone mineral content and a lower cancellous bone volume but also exhibited lower bone resorption markers, and bone mineralization rates (Huang et al., 2014). Thus, in balance, methionine restriction impacts bone mineralization but not bone turnover per se and decreases fat mass resulting in lighter weight animals (Huang et al., 2016).
Mice consuming low methionine diets exhibit higher levels of average daily energy expenditure compared to control fed animals. Enhanced metabolic flexibility (i.e., effectiveness of substrate switching between fasting and fed states; fat to carbohydrate utilization) may be linked to improvement in glucose metabolism for fatty acid oxidation appears to be downregulated in methionine restriction (Plaisance et al., 2010; Hasek et al., 2010; Anthony and Gietzen, 2013; Orgeron et al., 2014; Wanders et al., 2015). Increased metabolic rate of methionine-restricted mice is however not observed in mice lacking uncoupling protein (UCP1; Wanders et al., 2015), suggesting that methionine-restricted mice exhibit higher levels of thermogenesis and possibly may have higher body temperatures. Increased thermogenesis rather than ATP formation may be causally linked to the observed decline in oxidative damage in methionine-restricted rats (Maddineni et al., 2013; Sanchez-Roman et al., 2012). Redox signaling through ubiquinone 9 and NRF2-dependent phase II antioxidants, including NAD(P)H dehydrogenase quinone 1 (NQO1), glutathione-S-transferase (GST), and heme oxygenase 1 (HO1) is also upregulated in methionine-restricted mice, traits shared with other long-lived vertebrates (Lewis et al., 2010; Jove et al., 2013; Mitchell et al., 2007; Brown-Borg et al., 2015).
Fat deposition was shown to be limited by methionine restriction even when fed to obesity-prone animals due to the increase in total energy expenditure (Valencak and Ruf, 2013). Many of these changes reflect the altered costs of maintenance and growth when dietary methionine is low. Growth factor signaling and nutrient signaling are therefore clearly integrated and detect nutrient levels influencing the organism’s responses to its environment. Low amino acid levels are detected by mammalian target of rapamycin (mTOR), an intracellular sensor and regulator of protein synthesis, cell growth and proliferation. Downstream effectors of mTOR, TORC1 and TORC2 coordinate anabolic and catabolic processes in response to energy status, nutrients and growth (Laplante and Sabatini, 2012). In contrast to other longevity mutants however, phosphorylated mTOR and AMPK (energy status sensor) are similar between methionine restriction and mice fed methionine-replete diets (Sun et al., 2009; Brown-Borg, 2015; Dominick et al., 2015; Gesing et al., 2011; Sharp and Bartke, 2005; Wang and Miller, 2012; Kim and Guan, 2011). The lack of a difference in AMPK may explain the decrease in fatty acid oxidation described in these animals as opposed to the upregulation found in long living dwarf mice (Perrone et al., 2012; Stauber et al., 2005; Bartke and Westbrook, 2012). Adiponectin and FGF21 are higher in methionine- restricted mice and typically activate AMPK in peripheral tissues stimulating fatty acid oxidation and utilization (Chau et al., 2010). Other mechanisms likely contributing to the methionine-restricted-mediated increase in energy expenditure include beta adrenergic receptor activation and UCP1 (Plaisance et al., 2010). It appears that either some of the sensors of nutrients and energy status do not detect the lack of methionine nor the change in energy status or that mRNA and protein levels of these sensors are not indicative of their action suggesting that the effects of methionine restriction are complex. Recently, Leib and Knight (2015) showed that the proposed amino acid sensor, GCN2, plays no role in dietary essential amino acid sensing, contrary to earlier reports.
1.5.4 Changes in mitochondrial function with methionine restriction
Mitochondria are intimate players in aging and lifespan and exhibit altered function in several mouse models of extended longevity (aging mutants). Mitochondrial encoded proteins of the respiratory chain complexes are enriched for methionine in mice, with reports that methionine itself serves as an antioxidant when ROS production is high (Bender et al., 2008). Altered mitochondrial function and concomitant reduced production of ROS and toxic oxidized macromolecules has been proposed to be causally linked to the increased lifespan of methionine-restricted mice (Carol et al., 2008; Lopez-Torres and Barja, 2008).
Uncoupled respiration is increased in tissues from rodents consuming methionine-restricted diets while enzyme components of oxidative phosphorylation are reduced or unchanged (Plaisance et al., 2010; Hasek et al., 2010; Caro et al., 2008; Sanz et al., 2006). Mitochondrial reactive oxygen species (ROS) generation and oxidative damage to proteins and DNA are also lower in methionine-restricted animals (Sanchez-Roman and Barja, 2013; Maddineni et al., 2013; Bender et al., 2008; Caro et al., 2008; Lopez-Torres and Barja, 2008; Sanz et al., 2006; Caro et al., 2009; Yang et al., 2015). Glutathione is decreased but no differences in antioxidative enzymes have been observed (Richie et al., 1994; Maddineni et al., 2013). Aspects of mitochondrial function reported for methionine-restricted animals are similar to some of the other interventions that impact aging and lifespan such as mild uncoupling of respiration and oxidative phosphorylation, reduced ROS production as well as reduced oxidative damage yet other mechanisms are present as antioxidant protection is less apparent.
1.5.5 Enhanced cytoprotection in methionine restricted rodents
Cytoprotection is an important physiological component that plays a pivotal role in attenuating the functional declines that occur during aging and is causally linked to species longevity (Lewis et al., 2010; Brown-Borg, 2006). Similarly enhanced stress resistance has been linked to prolonged lifespans in numerous studies and is common in longevity mutants and interventions that promote long-life (Kapahi et al., 1999; Murakami et al., 2003; Harper et al., 2006; 2007; 2011; Lewis et al., 2012). Studies using skin fibroblasts from methionine-restricted mice were not found to be resistant to cytotoxic agents in contrast to similar studies in long-living mice (Salmon et al., 2005; Harper et al., 2006; Murakami et al., 2003). However, hepatocytes from methionine-restricted mice showed contrary results and resisted the toxic effects of acetaminophen when compared to normal chow fed and dwarf mouse cells (Harper et al., 2006). Both in vivo and in vitro studies reveal that the activity of the master regulator of cytoprotective factors, NRF2, is elevated in methionine-restricted mice, a feature shared with long-lived species (Zhang et al., 2010; Lin et al., 2012; Lewis et al., 2014). Increased Nrf2 activity and concomitant enhanced expression of genes with an antioxidant response element would lead to enhanced antioxidant and detoxification capacities, although the exact mechanisms behind this relationship are unknown. Increased Nrf2 activity thus likely explains the higher levels of glutathione S-transferase, heme oxygenase 1, and NAD(P)H dehydrogenase quinone 1 in methionine-restricted mice (Jove et al., 2013). Clearly, methionine-restricted rodents share these stress resistance mechanisms with other long-living experimental mouse models, implicating common mechanisms in their lifespan extension, lower incidence of cancer and later onset of age-associated diseases (Komninou et al., 2006; Sinha et al., 2014). What is not known is if these common mechanisms of extending mouse lifespan are equally applicable to long-lived humans and other long-lived species, or if these are mechanisms employed by short-lived animal models that naturally have poor defenses against endogenous and environmental stressors and thus, aging.
1.6 Other long-lived model organisms
To date methionine restriction has not been undertaken in any long–lived mammal and indeed almost nothing is known about methionine biology outside that of laboratory rodents. Very little is known about methionine and the effects of methionine restriction on metabolism in non-traditional model organisms. Circulating levels of methionine in the long-lived naked mole-rat are one-third of that observed in C57Bl/6 mice and liver tissue methionine levels are also markedly lower than those measured by mass spectrometry in mouse livers (McIsaac et al., 2016; Ma et al., 2015). The physiological relevance of these low levels is not known. This may reflect divergent regulation of methionine metabolism although it may also simply indicate the low levels of methionine present in their fruit and vegetable diet. In addition, tissue and plasma levels of methionine are only part of the picture. The flux of methionine within tissues in various organisms lends important information about utilization and regulation of this pathway. The flux of methionine is markedly higher in long-living Ames mice compared to wild type mice indicating that both transmethylation and transsulfuration are enhanced (Uthus and Brown-Borg, 2006). Further work in long-living mammals will address the importance of these findings.
1.7 Methionine restriction in humans
Published reports on the effects of methionine restriction on human health are few and far between. More common are case reports identifying the effects of mutations in components of the methionine pathway. Fortunately, many of these are rare. Hypermethioninemia is caused when mutations in methionine adenosyltransferase, glycine N-methyltransferase, adenosylhomocysteine hydrolase or cystathionine beta synthase genes occur (Baric et al., 2004; Furujo et al., 2012; Chien et al., 2015; Motzek et al., 2016). Low methionine diets are prescribed for patients with hypermethioninemia that are considered high risk for a cardiovascular event or neurologic deficit keeping in mind the potential to impact S-adenosylmethionine and potential neurological outcomes.
In vitro studies using human cell lines strongly suggest a similar effect of methionine restriction on replicative lifespan and delayed senescence, to that observed in yeasts where both chronological and replicative lifespan are increased in response to methionine restriction (Johnson and Johnson, 2014; Koziel et al., 2013). A thirty-fold reduction in methionine content of the cell culture media resulted in a 1.75 increase in the Hayflick limit, number of cumulative population doublings before a cell becomes senescent (Koziel et al., 2014). This increase in replicative lifespan of human cells was accompanied by a lower growth rate as well as reduced p16 levels and decreased oxygen consumption (Koziel et al., 2014). The one in vivo study involving humans reported that methionine restriction can increase fat oxidation in obese adults with metabolic syndrome (Plaisance et al., 2011). Clinically, more relevant work is needed to understand the potential therapeutic use of methionine restriction to delay age-related physiological decline.
1.8 Branched chain amino-acid diets
There have been studies showing that different macronutrient ratios within the diet impact longevity. Solon-Biet and coworkers (2014, 2015) showed that longevity could be increased and metabolic outcomes improved in ad libitum fed animals by changing the relative proportions of macronutrients to induce mTOR inhibition. As the scientific community looks to further understand the role of dietary restriction and protein restriction, in particular, related studies describing the role of branched chain amino acid (BCAA) diets on metabolism and aging have been conducted (Valerio et al., 2011; Fontana et al., 2016). Rodent diets that specifically reduce the BCAA improved glucose tolerance, pyruvate tolerance, and body composition but did not induce an increase in serum FGF21 (Fontana et al., 2016). These investigators also conducted a clinical trial feeding low protein to 19 males for six weeks and found that this diet decreased body weight, fat mass, lowered fasting blood glucose levels and increased FGF21 in the serum. Additional studies indicate that FGF21 may be the key regulator of the metabolic effects induced by low protein diets (Laeger et al., 2016). A prospective cohort study in humans also suggests that in addition to the amount of the protein in the diet, the source of that protein is also important in terms of metabolic outcomes and mortality (Song et al., 2016). This report concluded that there was a positive association with high animal protein intake and mortality while high plant-based protein intake was inversely associated with mortality when at least one life style risk factor was present.
1.9 Conclusion
Substantial evidence has accumulated in laboratory rodents that there are numerous benefits to a diet lower in methionine and possibly also tryptophan, than found in commercially available chow. It is not known whether the beneficial effects associated with restricted intake of these two amino acids are due to their unique properties or if restriction of other essential amino acids may produce similar phenotypes. Recent, albeit limited, evidence suggests that reducing other essential amino acids or protein in general, may induce similar effects at least under stressful conditions such as renal ischemia (Fontana et al., 2016; Song et al., 2016; Ables et al., 2014). Substantially more work remains to be done to elucidate if all individual essential amino acids behave similarly and to understand the mechanisms by which limiting these vital molecules improve health span and delay the onset of age-associated maladies.
Highlights.
In rodent diets, methionine and tryptophan restriction benefit health and lifespan.
Low dietary methionine affects multiple systems including glucose, lipid and energy metabolism.
Possible mechanisms include alterations in methionine metabolism, mTOR and Nrf2 signaling.
Acknowledgments
This work was supported by the NIH (AG034206-HMBB; AG038509-HMBB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ables GP, Brown-Borg HM, Buffenstein R, Church CD, Elshorbagy AK, Gladyshev VN, Huang TH, Miller RA, Mitchell JR, Richie JP, Rogina B, Stipanuk MH, Orentreich DS, Orentreich N. The first international mini-symposium on methionine restriction and lifespan. Front Gen. 2014;5:122. doi: 10.3389/fgene.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted c57bl/6j mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PloS One. 2012;7(12):e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, Gietzen DW. Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care. 2013;16:96–101. doi: 10.1097/MCO.0b013e32835b618b. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ashley DV, Curzon G. Effects of long-term low dietary tryptophan intake on determinants of 5-hydroxytryptamine metabolism in the brains of young rats. J Neurochem. 1981;37(6):1385–1393. doi: 10.1111/j.1471-4159.1981.tb06306.x. [DOI] [PubMed] [Google Scholar]
- Baric I, Fumic K, Glenn B, Cuk M, Schulze A, Finkelstein JD, James SJ, Mejaski-Bosnjak V, Pazanin L, Pogribny IP, Rados M, Sarnavka V, Scukanec-Spoljar M, Allen RH, Stabler S, Uzelac L, Vugrek O, Wagner C, Zeisel S, Mudd SH. S-adenosylhomocysteine hydrolase deficiency in a human, a genetic disorder of methionine metabolism. PNAS U S A. 2004;101(12):4234–9. doi: 10.1073/pnas.0400658101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Westbrook R. Metabolic characteristics of long-lived mice. Front Gen. 2012;3:288. doi: 10.3389/fgene.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Hajieva P, Moosmann B. Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria. PNAS. 2008;105(43):16496–16501. doi: 10.1073/pnas.0802779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett NC, Faulkes CG. African mole-rats: Ecology and eusociality. Cambridge University Press; Cambridge; New York: 2000. [Google Scholar]
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice, is stress resistance a common factor? Age (Dordr) 2006;28(2):145–62. doi: 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM. Reduced growth hormone signaling and methionine restriction, interventions that improve metabolic health and extend life span. Ann NYAS. 2015;1363(1):40–9. doi: 10.1111/nyas.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy S, Wonderlich JA, Armstrong V, Rojanathammanee L. Altered dietary methionine differentially impacts glutathione and methionine metabolism in long-living growth hormone-deficient Ames dwarf and wild-type mice. Longev Healthspan. 2014a;3:10. doi: 10.1186/2046-2395-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy S, Wonderlich JA, Rojanathammanee L, Kopchick JJ, Armstrong V, Raasakka D. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell. 2014b;36:1019–1027. doi: 10.1111/acel.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in c. Elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through nrf2 signaling. Circ Res. 2009;105(4):365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Gomez J, Sanchez I, Naudi A, Ayala V, López-Torres M, Pamplona R, Barja G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuv Res. 2009;12(6):421–34. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- Caro P, Gomez J, Lopez-Torres M, Sanchez I, Naudi A, Jove M, Pamplona R, Barja G. Forty percent and eighty percent methionine restriction decrease mitochondrial ros generation and oxidative stress in rat liver. Biogeron. 2008;9(3):183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. PNAS. 2010;107(28):12553–8. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Abdenur JE, Baronio F, Bannick AA, Corrales F, Couce M, Donner MG, Ficicioglu C, Freehauf C, Frithiof D, Gotway G, Hirabayashi K, Hofstede F, Hoganson G, Hwu WL, James P, Kim S, Korman SH, Lachmann R, Levy H, Lindner M, Lykopoulou L, Mayatepek E, Muntau A, Okano Y, Raymond K, Rubio-Gozalbo E, Scholl-Bürgi S, Schulze A, Singh R, Stabler S, Stuy M, Thomas J, Wagner C, Wilson WG, Wortmann S, Yamamoto S, Pao M, Blom HJ. Mudd’s disease (MAT I/III deficiency), a survey of data for MAT1A homozygotes and compound heterozygotes. Orphanet J Rare Dis. 2015;20:10–99. doi: 10.1186/s13023-015-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GM, Roman MG, Flores LC, Hubbard GB, Salmon AB, Zhang Y, Gelfond J, Ikeno Y. The paradoxical role of thioredoxin on oxidative stress and aging. Arch Biochem Biophys. 2015;576:32–8. doi: 10.1016/j.abb.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Long Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Dominick G, Berryman DE, List EO, Kopchick JJ, Li X, Miller RA, Garcia GG. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology. 2015;156:565–575. doi: 10.1210/en.2014-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung and nasal epithelium. Toxic Sci. 2002;65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- Dziegelewska M, Holtze S, Vole C, Wachter U, Menzel U, Morhart M, Groth M, Szafranski K, Sahm A, Sponholz C, Dammann P, Huse K, Hildebrandt T, Platzer M. Low sulfide levels and a high degree of cystathionine β-synthase (CBS) activation by S-adenosylmethionine (SAM) in the long-lived naked mole-rat. Redox Biol. 2016;8:192–198. doi: 10.1016/j.redox.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshorbagy AK. Body composition in gene knockouts of sulfur amino acid-metabolizing enzymes. Mamm Genome. 2014;25(9–10):455–63. doi: 10.1007/s00335-014-9527-x. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Smith AD, Drevon CA, Refsum H, Perrone CE. Cysteine supplementation reverses methionine restriction effects on rat adiposity: Significance of stearoyl-coenzyme a desaturase. J Lipid Res. 2011;52(1):104–112. doi: 10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD, Wurtman RJ. Brain serotonin content: Physiological regulation by plasma neutral amino acids. Obes Res. 1997;5:377–380. doi: 10.1002/j.1550-8528.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Fontana L, Cummings N, Arriola Apelo SI, Alexander CM, Kimple ME, Lamming DW. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Reports. 2016;15:520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furujo M, Kinoshita M, Nagao M, Kubo T. Methionine adenosyltransferase I/III deficiency, neurological manifestations and relevance of S-adenosylmethionine. Mol Gen Metab. 2012;107(3):253–6. doi: 10.1016/j.ymgme.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Gesing A, Masternak MM, Wang F, Joseph AM, Leeuwenburgh C, Westbrook R, Lewinski A, Karbownik-Lewinska M, Bartke A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Geron A Biol Sci Med Sci. 2011;66A:1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Gomez J, Lopez Torres M, Naudi A, Mota-Martorell N, Pamplona R, Barja G. Cysteine dietary supplementation reverses the decrease in mitochondrial ros production at complex i induced by methionine restriction. J Bioenerg Biomemb. 2015;47(3):199–208. doi: 10.1007/s10863-015-9608-x. [DOI] [PubMed] [Google Scholar]
- Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in drosophila. Nature. 2009;462(7276):1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J Exp Biol. 2011;214(Pt 11):1902–1910. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging, Influence of genes and nutrition. Mech Age Dev. 2006;127(8):687–694. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6(1):1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasek BE, Stewart LK, Henegan TM, Boudreau A, Lenard NR, Black C, Shin J, Hypens P, Malloy VL, Plaisance EP, Krajcik RA, Orentreich N, Gettys TW. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Phys Reg Integ Comp Phys. 2010;299:R728–739. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine beta-synthase/hydrogen sulfie inhibition in cancer. Antiox Redox Sig. 2015;22(5):424–428. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, Mair WB, Mitchell JR. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraku Y, Inoue S, Oikawa S, Yamamoto K, Tada S, Nishino K, Kawanishi S. Metal-mediated oxidative damage to cellular and isolated DNA by certain tryptophan metabolites. Carcinogenesis. 1995;16(2):349–356. doi: 10.1093/carcin/16.2.349. [DOI] [PubMed] [Google Scholar]
- Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antiox Redox Sig. 2013;19(5):465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285(29):21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH, Ables GP. Dietary restrictions, bone density, and bone quality. Ann NYAS. 2016;1363(1):26–39. doi: 10.1111/nyas.13004. [DOI] [PubMed] [Google Scholar]
- Huang TH, Lewis JL, Lin HS, Kuo LT, Mao SW, Tai YS, Chang MS, Ables GP, Perrone CE, Yang RS. A methionine-restricted diet and endurance exercise decrease bone mass and extrinsic strength but increase intrinsic strength in growing male rats. J Nutr. 2014;144(5):621–630. doi: 10.3945/jn.113.187922. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Faulks SC, Buffenstein R. Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J Geron A Biol Sci Med Sci. 2006;61(10):1009–1018. doi: 10.1093/gerona/61.10.1009. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Faulks SC, Harper JM, Miller RA, Buffenstein R. Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech Age Dev. 2006;127(8):653–657. doi: 10.1016/j.mad.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death, Metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87(4):1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Johnson FB. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PloS One. 2014;9(5):e97729. doi: 10.1371/journal.pone.0097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove M, Ayala V, Ramirez-Nunez O, Naudi A, Cabre R, Spickett CM, Portero-Otin M, Pamplona R. Specific lipidome signatures in central nervous system from methionine-restricted mice. J Proteome Res. 2013;12(6):2679–2689. doi: 10.1021/pr400064a. [DOI] [PubMed] [Google Scholar]
- Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxi Redox Sig. 2011;15(2):363–72. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Rad Biol Med. 1999;26(5–6):495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Guan KL. Amino acid signaling in TOR activation. Ann Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- King CM, Land SJ, Jones RF, Debiec-Rychter M, Lee MS, Wang CY. Role of acetyltransferases in the metabolism and carcinogenicity of aromatic amines. Mutat Res. 1997;376(1–2):123–128. doi: 10.1016/s0027-5107(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Komninou D, Leutzinger Y, Reddy BS, Richie JP., Jr Methionine restriction inhibits colon carcinogenesis. Nutr Cancer. 2006;54(2):202–208. doi: 10.1207/s15327914nc5402_6. [DOI] [PubMed] [Google Scholar]
- Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, Jansen-Durr P. Mitochondrial respiratory chain complex i is inactivated by nadph oxidase nox4. Biochem J. 2013;452(2):231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- Koziel R, Ruckenstuhl C, Albertini E, Neuhaus M, Netzberger C, Bust M, Madeo F, Wiesner RJ, Jansen-Durr P. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell. 2014;13(6):1038–1048. doi: 10.1111/acel.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Munzber H, Morrison CD. Metabolic responses to dietary protein restriction require an increase in FGF21 that is dleayed by the absence of GCN2. Cell Reports. 2016;16:707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mole Life Sci: CMLS. 2016;73(6):1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Longo V. Dietary restriction with and without caloric restriction for healthy aging. 2016 doi: 10.12688/f1000research.7136.1. F1000Res. pii.F1000 Faculty Rev-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10(6):416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13(5):817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DE, Knight ZA. Re-examination of Dietary Amino Acid Sensing Reveals a GCN2-Independent Mechanism. Cell Rep. 2015;13(6):1081–9. doi: 10.1016/j.celrep.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in igf-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Andziak B, Yang T, Buffenstein R. The naked mole-rat response to oxidative stress: Just deal with it. Antiox Redox Sig. 2013;19(12):1388–1399. doi: 10.1089/ars.2012.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integ Comp Biol. 2010;50(5):829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hornsby PJ, Buffenstein R. Stress resistance in the naked mole-rat, The bare essentials - a mini-review. Gerontology. 2012;58(5):453–462. doi: 10.1159/000335966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of nrf2 signaling and longevity in naturally long-lived rodents. PNAS. 2015;112(12):3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9(1):92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AH, Chen HW, Liu CT, Tsai CW, Lii CK. Activation of nrf2 is required for up-regulation of the pi class of glutathione s-transferase in rat primary hepatocytes with l-methionine starvation. J Agri Food Chem. 2012;60(26):6537–6545. doi: 10.1021/jf301567m. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Barja G. Lowered methionine ingestion as responsible for the decrease in rodent mitochondrial oxidative stress in protein and dietary restriction possible implications for humans. Biochim Biophys acta. 2008;1780(11):1337–1347. doi: 10.1016/j.bbagen.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Ma S, Yim SH, Lee SG, Kim EB, Lee SR, Chang KT, Buffenstein R, Lewis KN, Park TJ, Miller RA, Clish CB, Gladyshev VN. Organization of the mammalian metabolome according to organ function, lineage specialization, and longevity. Cell Metab. 2015;22(2):332–343. doi: 10.1016/j.cmet.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddineni S, Nichenametla S, Sinha R, Wilson RP, Richie JP., Jr Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp Bio Med. 2013;238(4):392–399. doi: 10.1177/1535370213477988. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3(7):e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Markus CR. Dietary amino acids and brain serotonin function: Implications for stress-related affective changes. Neuromolecular Med. 2008;10:247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Age Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McIsaac RS, Lewis KN, Gibney PA, Buffenstein R. From yeast to human, Exploring the comparative biology of methionine restriction in extending eukaryotic life span. Ann NYAS. 2016;1363(1):155–170. doi: 10.1111/nyas.13032. [DOI] [PubMed] [Google Scholar]
- McIsaac RS, Petti AA, Bussemaker HJ, Botstein D. Perturbation-based analysis and modeling of combinatorial regulation in the yeast sulfur assimilation pathway. Mol Biol Cell. 2012;23(15):2993–3007. doi: 10.1091/mbc.E12-03-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in caenorhabditis elegans. PNAS USA. 2007;104(51):20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, t4, igf-i and insulin levels, and increases hepatocyte mif levels and stress resistance. Aging Cell. 2005;4(3):119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Age Dev. 2006;127(7):643–6. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Buffenstein R, Hulbert AJ. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (heterocephalus glaber), A comparative study using shotgun lipidomics. Exp Geron. 2007;42(11):1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Hulbert AJ, Buttemer WA. Metabolic rate and membrane fatty acid composition in birds, A comparison between long-living parrots and short- living fowl. J Comp Phys B, Biochem Sys Env Phys. 2012;182(1):127–137. doi: 10.1007/s00360-011-0603-1. [DOI] [PubMed] [Google Scholar]
- Motzek A, Knezevic J, Switzeny OJ, Cooper A, Baric I, Beluzic R, Strauss KA, Puffenberger EG, Mudd SH, Vugrek O, Zechner U. Abnormal hypermethylation at imprinting control regions in patients with S-adenosylhomocysteine hydrolase (AHCY) deficiency. PloS One. 2016 doi: 10.1371/journal.pone.0151261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniraj N, Stamp LK, Badiei A, Hegde A, Cameron V, Bhatia M. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int J Rheum Dis. 2014 doi: 10.1111/1756-185x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17(11):1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech Age Dev. 1988;43(1):79–98. doi: 10.1016/0047-6374(88)90099-1. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123(2):269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Orgeron ML, Stone KP, Wanders D, Cortez CC, Van NT, Gettys TW. The impact of dietary methionine restriction on biomarkers of metabolic health. Prog Mol Biol Transl Sci. 2014;121:351–376. doi: 10.1016/B978-0-12-800101-1.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: The protein and methionine connection. Biochim Biophys acta. 2006;1757(5–6):496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Sanz A, Ayala V, Vasileva E, Barja G. Protein and lipid oxidative damage and complex i content are lower in the brain of budgerigar and canaries than in mice. Relation to aging rate. Age. 2005;27(4):267–280. doi: 10.1007/s11357-005-4562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Prat J, Cadenas S, Rojas C, Perez-Campo R, Lopez Torres M, Barja G. Low fatty acid unsaturation protects against lipid peroxidation in liver mitochondria from long-lived species, The pigeon and human case. Mech Age Dev. 1996;86(1):53–66. doi: 10.1016/0047-6374(95)01673-2. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochem Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DA, Plummer JD, Chittur SV, Mohney R, Vignola K, Orentreich DS, Orentreich N. Genomic and metabolic responses to methionine-restricted and methionine-restricted, cysteine-supplemented diets in Fischer 344 rate inguinal adipose tissue, liver and quadriceps muscle. J Nutrigen Nutrigenom. 2012;5:132–157. doi: 10.1159/000339347. [DOI] [PubMed] [Google Scholar]
- Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc. 2013;2(5):e000387. doi: 10.1161/JAHA.113.000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti AA, McIsaac RS, Ho-Shing O, Bussemaker HJ, Botstein D. Combinatorial control of diverse metabolic and physiological functions by transcriptional regulators of the yeast sulfur assimilation pathway. Mol Biol Cell. 2012;23(15):3008–3024. doi: 10.1091/mbc.E12-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri R, Roman-Morales E, Lopez-Garriga J. Hydrogen sulfide and hemeproteins: Knowledge and mysteries. Antiox Redox Sig. 2011;15(2):393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisance EP, Greenway FL, Boudreau A, Hill KL, Johnson WD, Krajcik RA, Perrone CE, Orentreich N, Cefalu WT, Gettys TW. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endo Metab. 2011;96(5):E836–E840. doi: 10.1210/jc.2010-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisance EP, Henagan TM, Echlin H, Boudreau A, Hill KL, Lenard NR, Hasek BE, Orentreich N, Gettys TW. Role of beta-adrenergic receptors in the hypoerphagic and hypermetabolic responses to dietary methionine restriction. Am J Phys Reg Integ Comp Phys. 2010;299:R740–R750. doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP, Leutzinger Y, Jr, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Robertson LT, Trevino-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, Simpson SJ, Mitchell JR. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J Nutr. 2015;145(8):1717–1727. doi: 10.3945/jn.114.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman HB, Hirschberger LL, Krijt J, Valli A, Kožich V, Stipanuk MH. The cysteine dioxygenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS(−) production and evidence of pancreatic and lung toxicity. Antiox Redox Sig. 2013;19(12):1321–36. doi: 10.1089/ars.2012.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8(20):1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick JJ, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Phys Endo Metab. 2005;289:E23–29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roman I, Barja G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp Geron. 2013;48(10):1030–1042. doi: 10.1016/j.exger.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roman I, Gomez A, Perez I, Sanchez C, Suarez H, Naudi A, Jove M, Lopez-Torres M, Pamplona R, Barja G. Effects of aging and methionine restriction applied at old age on ros generation and oxidative damage in rat liver mitochondria. Biogerontology. 2012;13(4):399–411. doi: 10.1007/s10522-012-9384-5. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Segall P. Long-term tryptophan restriction and aging in the rat. Aktuelle Gerontol. 1977;7(10):535–538. [PubMed] [Google Scholar]
- Segall SE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Age Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Segall PE, Timiras PS, Walton JR. Low tryptophan diets delay reproductive aging. Mech Age Dev. 1983;23(3–4):245–252. doi: 10.1016/0047-6374(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Geron A Biol Sci Med Sci. 2005;60:293–200. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- Sidransky H. Role of tryptophan in carcinogenesis Adv. Exp Med Biol. 1986;206:187–207. doi: 10.1007/978-1-4613-1835-4_16. [DOI] [PubMed] [Google Scholar]
- Sinha R, Cooper TK, Rogers CJ, Sinha I, Turbitt WJ, Calcagnotto A, Perrone CE, Richie JP., Jr Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in tramp mice. Prostate. 2014;74(16):1663–1673. doi: 10.1002/pros.22884. [DOI] [PubMed] [Google Scholar]
- Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Blount JD, Bronikowski AM, Buffenstein R, Isaksson C, Kirkwood TB, Monaghan P, Ozanne SE, Beaulieu M, Briga M, Carr SK, Christensen LL, Cochemé HM, Cram DL, Dantzer B, Harper JM, Jurk D, King A, Noguera JC, Salin K, Sild E, Simons MJ, Smith S, Stier A, Tobler M, Vitikainen E, Peaker M, Selman C. Oxidative stress and life histories, unresolved issues and current needs. Ecol Evol. 2015;5(24):5745–57. doi: 10.1002/ece3.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber AJ, Brown-Borg H, Liu J, Waalkes MP, Laughter A, Staben RA, Coley JC, Swanson C, Voss KA, Kopchick JJ, Corton JC. Constitutive expression of peroxisome proliferator-activated receptor alpha-regulated genes in dwarf mice. Mol Pharm. 2005;67:681–694. doi: 10.1124/mol.104.007278. [DOI] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Geron A Biol Sci Med Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrallardona D, Harris CI, Fuller MF. Microbial amino acid synthesis and utilization in rats: The role of coprophagy. British J Nutr. 1996;76(5):701–709. doi: 10.1079/bjn19960077. [DOI] [PubMed] [Google Scholar]
- Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Phys Endo Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living ames dwarf mouse. Mech Age Dev. 2006;127(5):444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencak TG, Ruf T. Phospholipid composition and longevity, lessons from Ames dwarf mice. Age (Dordr) 2013;35(6):2303–13. doi: 10.1007/s11357-013-9533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, D’Antona G, Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and helathspan: an evolutionary perspective. Aging. 2011;3(5):464–478. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nature Rev Drug Dis. 2015;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- Wanders D, Burk DH, Cortez CC, Van NT, Stone KP, Baker M, Mendoza T, Mynatt RL, Gettys TW. Ucp1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J. 2015;29(6):2603–2615. doi: 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Ghosh S, Stone KP, Van NT, Gettys TW. Transcriptional impact of dietary methionine restriction on systemic inflammation, relevance to biomarkers of metabolic disease during aging. Biofactors. 2014;40(1):13–26. doi: 10.1002/biof.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Miller RA. Augmented autophagy pathways and mTOR modulation in fibroblasts from long-lived mutant mice. Autophagy. 2012;8:1273–1274. doi: 10.4161/auto.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford RWD, Vercauteren M, Trebaul A, Cattaneo C, Eckert D, Garzotti M, Sieber P, Segrestaa J, Studer R, Groenen PMA, Nayler O. Serotonin biosynthesis as a predictive marker of serotonin pharmacodynamincs and disease-induced dysregulation. Sci Rep. 2016;6:30059. doi: 10.1038/srep30059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HB, Xu YY, Zhao XN, Zou SW, Zhang Y, Zhang M, Li JT, Ren F, Wang LY, Lei QY. Acetylation of MAT IIα represses tumour cell growth and is decreased in human hepatocellular cancer. Nature Comm. 2015;6:6973. doi: 10.1038/ncomms7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antiox Redox Sig. 2013;18(15):1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Tox App Pharm. 2010;245(3):326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Geron. 2003;38(1–2):47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]