Abstract
The objective of this review was to systematically appraise the existing published literature about community-based cervical cancer screening programs that have used visual inspection methods using acetic acid (VIA) in India. All peer reviewed journal articles till December 2015 were searched per PRISMA guidelines. Articles reporting results from cervical cancer screening programs in community-based settings, conducted in India, and using VIA were included in this review. The search resulted in 20 articles to be included in the review with a total of 313,553 women at 12 unique urban and rural sites across India. Seventeen (85%) studies were cross-sectional and three studies were randomized controlled trials; most studies compared accuracy of VIA with other screening tests such as visual inspection using Lugol’s Iodine (VILI), HPV DNA, and cytology. Of studies that reported test accuracy for CIN Grade 2+, the VIA sensitivity values ranged from 16.6 – 82.6% and specificity ranged from 82.1 – 96.8%. Women between age groups of 30–59 years were recruited using motivational one-on-one counseling and local support staff. All studies conducted diagnostic follow-up using colposcopy and guided biopsies, when necessary. Three major themes were identified that facilitated implementation of screening programs in a community-based setting: standardized training that maintained competency of test providers; collaborations with community-based organizations that used health education for recruitment of participants; and employing the screen-and-treat method to reduce loss to follow-up. Summarized evidence presented in this review could substantially influence future implementation and sustainment of cervical cancer screening programs at a national level.
Keywords: Cervical Cancer, Screening, Acetic Acid, Visual Inspection, Health Planning, Implementation, India
Introduction
According to the World Health Organization estimates, approximately 122,844 new cases and 67,544 deaths were due to cervical cancer in India, accounting for nearly 1/3rd of the global cervical cancer deaths in 2014.[1] Epidemiological and laboratory research has clearly established that a persistent infection with Human Papillomavirus (HPV) causes most cases of cervical cancer and the past decade has focused on primary prevention using HPV vaccinations, which have shown promising results.[2, 3] Although there has been substantial progress in primary prevention strategies, an optimal effect on incidence and mortality due to cervical cancer can only be achieved by the addition of secondary prevention strategies, which include screening for precancerous and cancerous cervical lesions in women above 30 years of age.[4] For developing countries like India, it is critical that they achieve relatively high screening coverage rates as well as ensure that screen-positive women receive appropriate diagnostic and treatment services.
Establishing a quality assured cytology screening program, with national coverage can prove to be very challenging and probably beyond the capacity and resources available for India.[5] Moreover, underlying pelvic infections resulting in cervical smear abnormalities along with inherent difficulties in efficiently performing the different steps in cytology screening, which requires significant training and experience, can result in low sensitivity for the performance of Pap smears.[6] Repeated, yearly testing can improve the sensitivity of the Pap smears as seen in the US but can require significant resources.[7] Accumulating evidence on HPV testing as a screening strategy, highlights the test to be the most objective and reproducible of all cervical screening tests.[8] The test however, is expensive (approximately $20 US Dollars per test) and requires a sophisticated laboratory infrastructure which can be difficult to setup in primary care settings in India. On the other hand, visual inspection methods using acetic acid (VIA) and Lugol’s iodine (VILI) have shown to be well accepted by women in India and the incidence of discomfort and pain during VIA is less than that reported for when Pap smears are conducted.[9, 10]
For large scale screening of populations, visual inspection methods have been extensively studied and proven to be effective, especially in the low- and middle-income countries. Visual methods involve the application of acetic acid (VIA) or Lugol’s iodine (VILI) on the cervix to enhance the ability to detect the presence of pre-cancerous lesions thereby enabling the detection of cervical cancer at earlier stages.[11] It is now well established that with training, a physician or even a healthcare worker can identify acetowhite (with VIA) or mustard yellow (with VILI) lesions on the cervix, which are indicative of cancerous or precancerous tissue. Several studies in India have demonstrated that VIA and VILI have comparable sensitivity and specificity to cytology while offering the advantages of being simple to perform and cost-effective for large scale implementation.[12] A randomized controlled trial in India has shown a 30% reduction in cervical cancer incidence [11] and a modeling study showed that even a single VIA test at 35 years of age can significantly decrease the risk of mortality from and incidence of advanced cervical cancer when compared to no screening.[13]
The Government of India’s Ministry of Health and Family Welfare, recently launched the Operational Framework for the Management of Common Cancers which includes the use of VIA in primary care settings across India.[14] However, awareness about cervical cancer among the public is very low and there are only a few centers with cancer screening facilities throughout the country, which makes early detection and treatment very difficult. Furthermore, to move forward on this framework, it is important to consider the existing evidence in a critical manner. Public health evidence is usually the result of observation, theory and experiments, and the usefulness of this evidence may vary by the stakeholder type. Three distinct categories of scientific evidence have been proposed: (a) type 1 focuses on the causes of disease and the magnitude of risk factors, (b) type 2 on the relative impact on specific interventions, but Brownson and colleagues specifically emphasize (c) type 3 evidence, which shows how and under which “contextual” conditions, were the interventions implemented and how they were received.[15]
In promoting evidence-based public health, contextual information is information that is needed to adapt and implement an evidence-based intervention in a setting or population. Contextual information can be critical for moving clinical interventions to population-level and policy level interventions. To date, there have been no systematic reviews of published literature on community based cervical cancer programs in India that could provide this contextual information. For this review, we sought to answer two specific questions concerning the context in which cervical cancer screening is delivered: How were community-based cervical cancer screening programs implemented in India and what were the barriers and facilitators to implementing community-based cervical cancer screening programs using VIA methodology in India?
Methods
Protocol and registration
The protocol for this review was registered with the PROSPERO International Prospective Register of Systematic Reviews (No. CRD42016032601). This review was conducted and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[16]
Information sources and search strategy
The initial database search was conducted by one author (PA) and the search strategy is provided in Appendix A. The electronic databases included Medline, Embase, PsychInfo and Cochrane Database of Systematic Reviews searched using the OVID platform up to December 31st, 2015. Gray literature was not included, as they did not meet the standards associated with peer-reviewed publications. Conference abstracts were excluded since complete information about the implementation of the project was not available. We also excluded case studies, commentaries, proposed studies, protocol papers and editorials. Reference lists for all retrieved studies and table of contents for high-yield journals were also searched. English language restrictions were applied to the search. The search strategy was first created for Medline, which was then adapted for other database searches.
Eligibility criteria and study selection
Two authors (PA and NM) independently screened all the citations. Titles and abstracts were first screened for inclusion; when eligibility could not be ascertained, full-text articles were screened. Disagreements were resolved by consensus. To be included in the review, peer-reviewed journal articles had to report results from cervical cancer screening programs, which were community-based, conducted in India, and used visual inspections methods using acetic acid (VIA). Hospital-based studies, studies that did not include VIA as one of the screening methods and those that were conducted outside India, were excluded.
After removal of duplicates, abstracts were screened according to the eligibility criteria and a total of 20 research papers were found eligible to be included in this review.[5, 6, 9, 10, 17–32] We included observational, program effectiveness, acceptability and feasibility studies so as to provide a rich discussion about implementation of VIA in community-based settings in India. An additional 10 papers provided details about the 20 unique studies and were deemed to be critical for this review.[8, 11, 12, 22, 33–37]
Data collection process
When an article reported studies done in different locations, only information pertinent to India was extracted. Implementation data was primarily extracted from the discussion section in the articles that spoke about the authors experience in implementing the study. Data were extracted using a standardized form, which included the following variables: authors, year of publication, screening coverage, recruitment strategies, study methods and infrastructure, screening test accuracy and outcomes, diagnosis and treatment follow-up and implementation factors.
Risk of bias in individual studies
Methodological quality was assessed independently by two reviewers using the Effective Public Health Practice Project Group’s tool.[38] Disagreements were resolved by consensus. The tool allows studies to be rated on the following components: participation selection, study design, control of confounders, blinding, data collection methods, loss to follow-up, intervention integrity and analyses. Cumulative scores were reported as: strong (no component rated weak), moderate (one component rated weak), and weak (two or more components rated weak).
Results
Study characteristics
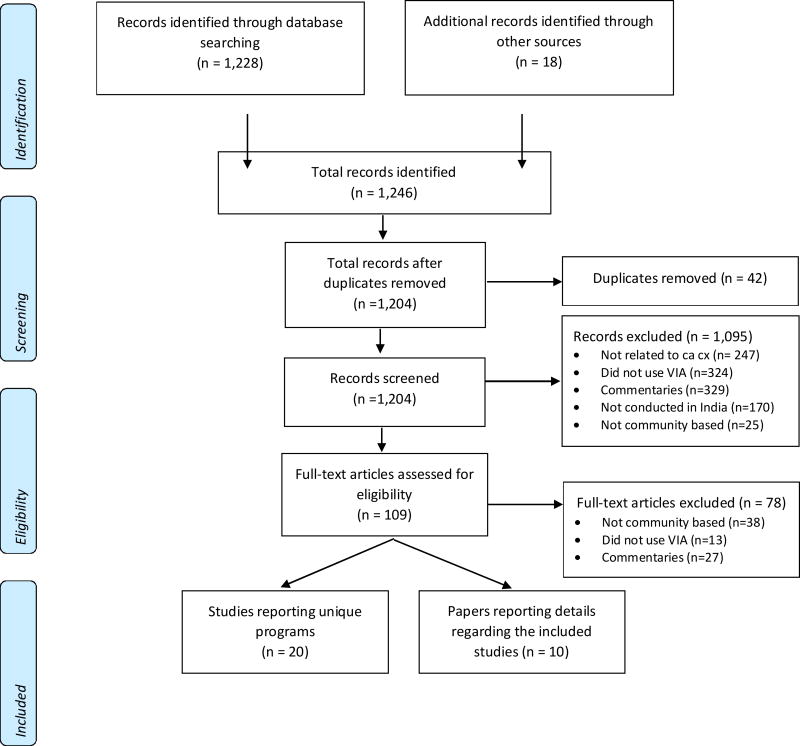
Figure 1 describes the literature search strategy, and characteristics of the studies included in this review are summarized in Table 1. The initial search of the databases resulted in 1,228 citations. From a manual search, an additional 18 abstracts were identified resulting in 1,246 citations in total. Of these, 42 were duplicates. The final selection of 20 studies included a total of 313,553 women at 12 unique sites across India.[5, 6, 9–11, 17–22, 24–30, 32, 39]. Seventeen were cross-sectional studies [5, 6, 9, 10, 17–21, 24–30, 39] and three were randomized controlled trials.[11, 22, 32]
Fig 1. PRISMA 2009 Flow Diagram.
Implementing Community-based Cervical Cancer Screening Programs using Visual Inspection Methods in India: A Systematic Review
Table 1.
Characteristics of studies included in this review
| First Author |
Ye ar |
Durat ion of study |
Commun ity |
Location | Age range |
Screen ing tests |
Screening conducted by |
Num ber of scree ned wome n |
Refere nce standar d |
Sensitivity | Specificity | Screen positivit y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cross Sectional Studies[5, 6, 9, 10, 17–21, 24–31]
| ||||||||||||
| Sankaranarayanan et al.[17] | 1998 | 2 years | Unclear | Thiruvanathapuram (KL) | Not Reported | VIA, Cytology | Cytotechnicians | 3000 | Not Reported | Not Reported | ~92.2% | 9.93% |
| Basu et al.[6] | 2003 | 2 years 6 months | Urban | Kolkata (WB) | 30 – 64 years | VIA, VIAM, Cytology | Female Health Workers | 5843 | Histology & Colposcopy | CIN II+: 55.7% | CIN II+: 82.1% | 18.70% |
| Sankaranarayanan et al.[18] | 2003 | 3 years | Unclear | Thiruvanathapuram (KL) | 25 – 65 years | VIA, VILI, Cytology | Female Health Workers | 4444 | Histology & Colposcopy | CIN II+: 82.6% | CIN II+: 86.5% | HT: 15.8% |
| Sankaranarayanan et al.[19] | 2004a | 5 years | Urban & Rural | Kolkata (WB), Jaipur (RJ), Mumbai (MH), Thiruvanathapuram (KL) | 25 – 65 years | VIA, VILI | Female Health Workers, Nurses, Cytotechnicians | 31,154 | Histology & Colposcopy | HSIL: 73% | HSIL: 84.8% | 16.20% |
| Sankaranarayanan et al.[20] | 2004b | 4 years | Urban | Kolkata (WB) & Mumbai (MH) | 25 – 65 years | VIA, VIAM | Female Health Workers | 18,675 | Histology & Colposcopy | HSIL: 60.3% | HSIL: 86.8% | 14.10% |
| Basu et al.[9] | 2006 | 1 year | Rural | Kolkata (WB) | 30 – 65 years | VIA | Female Health Workers | 2184 | Not Reported | Not Reported | Not Reported | 11.30% |
| Kamal et al.[21] | 2007 | 7 years | Urban | Nagpur (MH) | *Repoductive age group | VIA, Cytology | Multipurpose Health workers | 1347 | Colposcopy | Colpo + : 29.6% | Colpo +: 92.1% | 12.00% |
| Rural | 2392 | Colposcopy | Colpo + : 26.2% | Colpo +: 88% | 16% | |||||||
| Bhatla et al.[5] | 2009 | 1 year 6 months | Rural | Faridabad (HR) | 25 – 59 years | VIA, VILI, Cytology | Female Health Workers | 3000 | Not Reported | Not Reported | Not Reported | 14.20% |
| Gravitt et al.[24] | 2010 | 2 years 6 months | Rural | Medchal (AP) | > 25 years | VIA, Cytology, HPV DNA | Gynecologists | 2331 | Histology | CIN II+: 16.65% CIN III+: 31.56% | CIN II+: 87.36% CIN III+: 87.45% | 12.90% |
| Kumar et al.[10] | 2011 | 5 months | Urban | Mumbai (MH) | 30 – 65 years | VIA, VILI, CBE, OVE | Primary Health Workers | 182 | Not Reported | Not Reported | Not Reported | Not Reported |
| Deodhar et al.[25] | 2012 | 10 months | Rural | Solapur (MH) | 30 – 49 years | VIA, VIL, Cytology | Nurses | 5648 | Histology | CIN II+: 64.5% | CIN II: 84.2% | 16.90% |
| Basu et al.[26] | 2013 | i) 4 years ii) 2 years ii) 2 years | Rural | Kolkata (WB) | 30 – 65 years | VIA, HC2 | Female Health Workers | 35,308 | Not Reported | Not Reported | Not Reported | 1.37% |
| Jeronimo et al.[27] | 2014 | Unclear | Urban & Rural | Dadri (UP) & Hyderabad (AP) | 30 – 59 years 30 – 49 years | VIA, Cytology , CareHPV (Self collected), CareHPV (Physician collected) | Unclear | 4925 | Histology | CIN II+: 21.9% CIN III+: 7.69 | CIN II+: 94.6 CIN III+: 94.5% | 5.00% |
| Ghosh et al.[31] | 2014 | 3 years | Unclear | Kolkata (WB) | 30 – 60 years | VIA, HC2 | Female Health Worker | 30,773 | Not Reported | Not Reported | Not Reported | 7.50% |
| Satyanarayana et al.[28] | 2014 | 3 years | Rural | Dadri (UP) | 30 – 60 years | VIA, VILI, Cytology | Not described. | 4198 | Histology | CIN-II+: 54.5% CIN III+: 50.% | CIN II+: 96.8% III+: 96.7% | 9.70% |
| Basu et al.[29] | 2015 | 4 years 4 months | Rural | Kolkata (WB) | 30 – 60 years | VIA, HC2 | Female Health Workers | 39,740 | Histology & Colposcopy | CIN II+: 61.0%; CIN III: 59.9%; CIN III+: 67.9% | CIN II+: 93.4%; CIN III: 93.1%; CIN III+: 93.2% | 7.10% |
| Poli et al.[30] | 2015 | 7 years | Rural | Hyderabad (AP) | 26 – 60 years | VIA | Auxiliary Nurse Midwives | 18,869 | Not Reported | Not Reported | Not Reported | 10.75% |
|
| ||||||||||||
|
Randomized Control Trials[11, 22, 32]
| ||||||||||||
| Shastri et al.[32] | 2013 | 16 years | Urban | Mumbai (MH) | 35 – 64 years | VIA, CBE | Primary Health Workers | 67070 | Not Reported | Not Reported | Not Reported | 1.75 – 1.91% |
| Sankaranarayanan et al.[11] | 2007 | 3 years 6 months | Rural | Dindigul (TN) | 30 – 59 years | VIA | Nurses | 31,343 | Not Reported | Not Reported | Not Reported | 9.90% |
| Nene et al.[22] | 2007 | 4 years | Rural | Osmanabad (MH) | 30 – 59 years | VIA, Cytology, HPV DNA | Nurses | 26,755 | Not Reported | Not Reported | Not Reported | 14% |
VIA = Visual Inspection with Acetic Acid, VILI = Visual Inspection with Lugol’s Iodine, VIAM = Visual Inspection with Acetic acid under Magnification, CBE = Clinical Breast Examination, OVE = Oral Visual Examination, UP = Uttar Pradesh, MH = Maharashtra, WB = West Bengal, HR = Haryana, AP = Andhra Pradesh, RJ = Rajasthan, KL = Kerala, TN = Tamil Nadu.
Synthesis of results from individual studies
Screening coverage
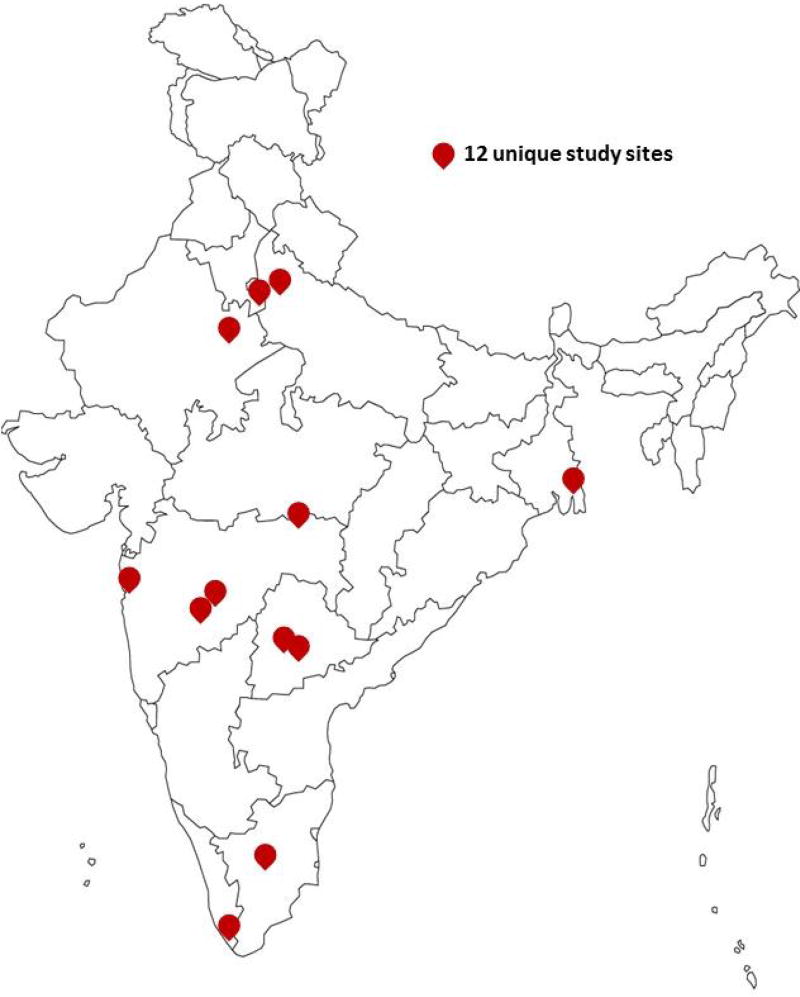
Of the 20 studies, 10 were conducted in rural areas [5, 9, 11, 22, 24–26, 28–30], four in urban areas [6, 10, 20, 32], three in urban and rural areas [19, 21, 27], while three studies did not specify the type of community where the study was conducted.[17, 18, 39] As seen in Fig 2, the twenty studies included 12 unique study sites all across India. Eleven studies did not provide information on the number of eligible women in the communities being studied.[6, 9, 10, 17, 19–21, 27, 29–31] Gravitt et al.[24] used census lists to enumerate the target population in the community, while four other studies [5, 8, 11, 26] conducted household surveys to enumerate the eligible population. Shastri et al.[32] used both census lists and conducted household surveys of the 20 clusters included in their trial. Of the nine studies [5, 11, 12, 22, 24–26, 28, 32] that provided the number of eligible women in the communities being studied, the participation rates ranged from 41.6% to 78.6%.
Fig 2.
Map of India showing the twelve unique study sites
Seventeen studies included women in the age groups of 30–59 years.[5, 6, 9, 10, 17–24, 26, 28–31] Of the remaining three, one study [32] included 35–64 year old women while two other studies [25, 27] set their inclusion age group to be from 30–49 years. Overall, among the 20 studies included in this review, six studies [5, 18–20, 24, 30] included women below 30 years of age (starting at 25 years) and nine studies [6, 9, 10, 18–20, 24, 26, 32] included women above the age of 60 years. All studies recruited women who were asymptomatic and/or apparently healthy, ever married, non-pregnant, intact uterus, and with no previous history of cervical cancer. However, two studies noted the presence of symptomatic women in their study sample. One study reported 83.1% of the total study sample to be symptomatic [28], whereas another reported some women to be symptomatic (with symptoms such as persistent vaginal discharge, post-coital bleeding and irregular bleeding) without specifying the actual number.[31]
Recruitment strategies
Of the 20 studies, four studies did not describe their recruitment strategies nor provide any information on who conducted the recruitment.[17, 21, 26, 31] The remaining 16 studies, used motivational and/or health education campaigns that were either group or one-on-one counselling for recruiting women into the study. Nine studies used audiovisual (e.g. radio, films, etc.) or written (e.g. pamphlets, brochures, etc.) information media for recruitment.[6, 18–20, 22, 27–29, 32] Eight studies [10, 11, 20, 22, 24, 27, 30, 32] collaborated with local organizations (e.g. NGO’s, women’s self-help groups, etc.) and/or involved local leaders (e.g. social or religious leaders, village panchayats, civic leaders, etc.) during recruitment, and only one study conducted by Nene, et al. included family members and husbands when recruiting women from the communities.[22] For recruitment, nine studies used health workers (e.g. Auxiliary Nurse Midwives (ANMs) or ASHA workers), [5, 6, 11, 19, 20, 22, 25, 28, 30], seven studies used social workers [6, 9, 10, 18, 28, 29, 32], and seven studies used field workers and/or volunteers [6, 11, 22, 24, 27–29].
Study methods and infrastructure
As shown in Table 1, 17 studies were cross-sectional [5, 6, 9, 10, 17–21, 24–31] and three studies were randomized controlled trials. [22, 23, 32] Of the 17 cross-sectional studies, 11 compared VIA with other screening methods such as VILI, VIA with low-level magnification, Pap smears and HPV DNA tests.[5, 6, 17–21, 25, 27–29] One study measured the prevalence of Human Papillomavirus (HPV) infection and Cervical Intraepithelial Neoplasia (CIN) in a previously unscreened population, [26] and another study evaluated the performance of colposcopy in further evaluating VIA or HPV DNA positive women in the community.[31] Four observational studies were described as program effectiveness, acceptability and or feasibility of implementing a VIA screening program in a community-based setting.[9, 10, 24, 30]
Three studies did not provide any information on the screening site infrastructure.[18, 25, 31] Of the 17 studies that did provide information, Kumar et al. used mobile vans to provide screening services in the community [10], while Gravitt et al. provided transportation services for women from their villages to the medical center.[24] In the 15 remaining studies, seven studies [6, 20, 22, 26–29] used government public health setups (e.g. primary healthcare centers, urban health centers, district hospitals) and others used convenient locations to set-up temporary, open access screening clinics in target communities.[5, 9, 17, 19, 21, 23, 30, 32]
Screening test providers and performance
Two studies did not mention who conducted the screening test. [9, 28] Of the 18 studies that did provide the information, Gravitt et al. used physicians, 14 studies [5, 10, 17, 18, 21–23, 25–27, 29–32] used health workers (including nurses, ANM and cytotechnicians) and five studies [5, 23, 29, 30, 39] used both healthcare workers and physicians. These studies usually mentioned medical supervision or cross-examination by medical officers as a method of quality assurance in reporting of VIA results. The three remaining studies used non-health care workers such as high school or university graduates in arts and science.[6, 19, 20]
Among the 20 studies included in the review, six studies did not provide any information on the training provided to individuals conducting the screening.[9, 10, 26, 27, 29, 31] In 14 studies that provided training, 10 studies [5, 6, 17, 19, 20, 23–25, 30, 32] reported using the manual developed by the International Agency for Research on Cancer (IARC) [40] and most of these studies provided refresher training prior to starting the study or on an annual basis. Only four studies reported evaluating the training for screening providers.[5, 6, 21, 22] Since Kumar et al. had the study objective of measuring the overall acceptance and satisfaction levels among women undergoing health education and screening in their program, the authors did not report screening test outcomes or screen positivity. The range for screen positivity was between 1.37% and 18.7% (Table 1). Only ten studies reported the sensitivity and specificity for VIA (Table 2).[6, 18–21, 24, 25, 27–29] Of the studies that reported test accuracy at CIN Grade 2+, the VIA sensitivity ranged from 16.6% to 82.6%, and specificity 82.1% to 96.8%. At CIN Grade 3+, the sensitivity ranged from 7.7% to 67.9%, and specificity from 87.4% to 96.7%.
Table 2.
Criteria for test accuracy studies
| Authors | Year | Reference Standard used |
Colposcopy done on |
Colposcopists blinded to VIA results |
Disease threshold |
|---|---|---|---|---|---|
| Basu et al.[6] | 2003 | Histology/ colposcopy* | All women | No | CIN II+ |
| Sankaranarayanan et al.[18] | 2003 | Histology/ colposcopy* | All women | Yes | CIN II+ |
| Sankaranarayanan et al. [19] | 2004 | Histology/ colposcopy* | All women | Yes | HSIL |
| Sankaranarayanan et al.[20] | 2004 | Histology/ colposcopy* | All women | Yes | HSIL |
| Kamal et al. [21] | 2007 | Colposcopy | All women | Yes | Colposcopy + |
| Gravitt et al.[24] | 2010 | Histology | VIA + | No | CIN II+ |
| Deodhar et al.[25] | 2012 | Histology | VIA + | No | CIN II+ |
| Satyanarayana et al.[28] | 2014 | Histology | VIA + | No | CIN II+ |
| Jeronimo et al.[27] | 2014 | Histology | VIA + | No | CIN II+ |
| Basu et al.[29] | 2015 | Histology/ colposcopy* | VIA + | No | CIN II+ |
Colposcopy results were used as the final diagnosis where histology was not available.
Of the ten studies that provided information on test accuracy, four studies used histology [24, 25, 27, 28], five studies used histology and/or colposcopy[6, 18–20, 29], and one study used colposcopy [21] as their reference standard. For the five studies that used histology and/or colposcopy – study authors reported using colposcopy findings as the reference standard for women without a biopsy. Five of the 10 studies conducted a colposcopy on all the screened women, whereas four studies conducted colposcopy only on women who were VIA positive. Gravitt et al.[24] conducted colposcopy on a random sample of 20% of the women who screened negative to obtain data for correction of verification bias. Four of the six studies that used colposcopy findings as a reference standard, blinded the colposcopists to the VIA results. Seven studies used CIN Grade 2+ as the threshold to assess disease status. Kamal et al.[21] used colposcopy positivity as their disease threshold and defined it as dense, acetowhite epithelium with coarse punctations, thick leukoplakia, atypical vessels and colposcopically suspect invasive cancer or frank growth. Two studies used a histological diagnosis of High-grade Squamous Intraepithelial Lesion (HSIL) as their disease threshold.
Diagnosis and Treatment
All studies included in this review conducted the diagnostic follow-up using colposcopy and guided biopsies, when necessary (Table 3). Fourteen studies offered same-visit colposcopy and biopsies to women who needed them.[5, 6, 9, 10, 18–23, 25, 29, 30, 39] Basu et al.[26] did not report when colposcopy was performed, whereas Kamal et al.[21] conducted the study in two different sites (urban and rural) but did not have colposcopy available on the same day in the rural site. In these studies, the loss to follow-up ranged from 0 to 1.2%. In the five studies that did not conduct the diagnostic follow-up on the same visit, loss to follow-up for diagnosis ranged from 10% to 70.9%. Amongst the six studies that provided data on biopsies, the loss to follow-up ranged from 2.6% to 38% with participant refusal for biopsy, being the most commonly cited reason.[6, 9, 24–26, 39] Six studies did not provide any information on the number of women lost to follow-up for diagnostic evaluations.[5, 10, 19, 26, 27, 30]
Table 3.
Diagnostic and Treatment Follow-up
| First Author |
Year | Diagno stic follow- up |
Same day diagnosis/R eferred |
Loss to follow -up for colpos copy |
Loss to follo w-up for biops y |
Reasons for loss to follow up |
Inform ation regardi ng treatme nt provide d? |
Same visit cryothe rapy |
Treatment strategy
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIN1 | CIN2/3 | Invasiv e cancer |
|||||||||
| Sankaranarayanan et al.[17] | 1998 | Colposcopy and Histology | Referred | 10% | Not reported | Not reported | Yes | Not reported | 77 cases of mild dysplasia were considered as false positives and not treated. | ||
| Basu et al.[6] | 2003 | Colposcopy and Histology | Same day | 0% | 11.60 % | Biopsies were not obtained or inadequate or inconclusive in these cases | Yes | Unclear | Of 336 women detected with low grade lesions, 127 had cryotherapy and two had LEEP. A total of 207 (61.6%) did not receive treatment | Of the 122 women detected with high grade lesions, 48 had cryotherapy, 20 had LEEP, and three had conisation. A total of 51 (41.8%) women did not receive treatment. | |
| Sankaranarayanan et al.[18] | 2003 | Colposcopy and Histology | Same day | 0% | Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Sankaranarayanan et al.[19] | 2004 | Colposcopy and Histology | Same day | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Sankaranarayanan et al.[20] | 2004 | Colposcopy and Histology | Same day | 0% | Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Basu et al.[9] | 2006 | Colposcopy and Histology | Same day | 0% | 4.40 % | Women refused | Yes | No | Treated appropriately after biopsy results | ||
| Kamal et al.[21] | 2007 | Colposcopy | Same day | 0% | Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Colposcopy | Referred | 84% | Not reported | Referral | Not reported | Not reported | Not reported | ||||
| Nene et al.[22] | 2007 | Colposcopy and Histology | Same day | 1.40% | Not reported | Not reported | Yes | No | 80% of the women with CIN 1 received treatment | Women with moderate to severe CIN (CIN2+) were treated with cryotherapy as eligible and/or referred for further management. Overall, 85% with CIN 2 and 88% with CIN 3 received treatment and approximately 15% were lost to follow-up. | Not reported |
| Sankaranarayanan et al.[23] | 2007 | Colposcopy and Histology | Same day | 1.20% | Not reported | Not reported | Yes | Yes | A central referral center was established at the CFCHC, Ambillikai, with facilities for treatment of detected lesions by loop electrosurgical excision procedure (LEEP) and cold knife conisation. When lesions were too extensive for cryotherapy, women were given an appointment to visit the referral center for LEEP or cold knife conisation by doctors. | Women with suspected invasive cancer were referred to the clinical oncology department of the CFCHC or to other cancer treatment facilities near the district for investigations, staging and treatment. | |
| Bhatla et al.[5] | 2009 | Colposcopy and Histology | Same day | Not reported | Not reported | Not reported | Yes | No | 9/37 (24.3%) women diagnosed with CIN 1 were lost to follow up | All 20 women with CIN 2+ underwent treatment. 0% lost to follow-up | |
| Gravitt et al.[24] | 2010 | Colposcopy and Histology | Referred | 44% | 38% | Women refused | Yes | No | No info provided for CIN 1 | Of the 19 women who had CIN2+, we provided LEEP for 2, hysterectomy for 9, and referral to the cancer hospital for radiation therapy for 4 women who had invasive cancer. Four women (21%) refused treatment despite several direct visits by the study gynecologist for direct counseling. | Not reported |
| Kumar et al.[10] | 2011 | Colposcopy and Histology | Same day | Not reported | Not reported | Not reported | Yes | No | Referred to tertiary care center | ||
| Deodhar et al.[25] | 2012 | Colposcopy and Histology | Same day | 0% | 3.60 % | Refusal | Yes | Unclear | No info provided for CIN 1 | Of 112, 98 received LEEP and 1 received LEEP + hysterectomy and 13 (11.6%) were lost to follow up. | Invasive cancers were referred to a tertiary hospital for further investigations and management. |
| Basu et al.[26] | 2013 | Colposcopy and Histology | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | ||
| Shastri et al.[32] | 2013 | Colposcopy and Histology | Referred | 20.60 % | Not reported | Predictors of non-compliance: increasing age, manual labourers, illiteracy, language barriers, one time screening participation, women referred for both breast and cervical cancer | Yes | No | Compliance to treatment for pre-invasive cancer is 80.67% | Compliance to treatment for invasive cancer was 85.93% | |
| Ghosh et al.[31] | 2014 | Colposcopy and Histology | Same day | 0% | 3.10% | Not reported | Not reported | Not reported | Not reported | ||
| Jeronimo et al.[27] | 2014 | Colposcopy and Histology | Referred | Not reported | Not reported | Not reported | Yes | No | Not reported | Women with moderate to severe CIN (CIN2+) were treated with cryotherapy as eligible and/or referred for further management. | Not reported |
| Satyanarayana et al.[28] | 2014 | Colposcopy and Histology | Referred | 70.90 % | Not reported | Maybe referral | Yes | No | Referred to tertiary care center | ||
| Basu et al.[29] | 2015 | Colposcopy and Histology | Same day | 0% | 2.60% | Refusal | Yes | No | CIN1 = 1880 were advised for yearly f/ups | The total patients diagnosed with CIN 2 + were 230 of which 37 (16.1%) were loss to follow up at CNCI. | Of the 48 women diagnosed with invasive cancer, 6 (12.5%) were loss to follow up |
| Poli et al.[30] | 2015 | Colposcopy and Histology | Same day | Not reported | Not reported | Not reported | Yes | No (only first two years) | Cryotherapy where eligible | Women requiring treatment for HSIL other than cryotherapy or with invasive cancer were referred to a higher center for appropriate treatment. | Not reported |
Two studies reported providing cryotherapy during the same visit.[23, 30] Sankaranarayanan et al.[23] reported using nurses to provide cryotherapy during the same visit, whereas Poli et al.[30] provided cryotherapy in the same visit for the first two years but discontinued it for the remainder of the study citing logistical issues with gas supply needed for cryotherapy. Six studies did not provide information about the treatment given to women who tested positive on VIA.[18–21, 26, 31] Six studies reported referring women to further management to tertiary care centers.[9, 10, 23, 27, 28, 30] In the remaining eight studies, compliance to treatment ranged from 58.2% to 100% for women diagnosed with CIN Grade 2, 3, or invasive cancer.[5, 6, 17, 22, 24, 25, 29, 32] Six studies provided details about treatment given to women diagnosed with CIN Grade 1/pre-invasive cancer, with reported treatment compliance rates of 39.4% to 80.6%.[5, 6, 22, 29, 30, 32]
Implementation barriers and facilitators
Six major themes were identified related to implementation barriers to community-based screening programs: 1) limitations of the VIA test performance in terms of sensitivity and specificity; 2) logistical and infrastructure challenges; 3) non-participation of women; 4) competency levels of healthcare workers providing the screening tests; 5) integration of cervical cancer screening with breast/oral cancer screening and delivery of other healthcare services; and 6) difficulties encountered in diagnostic and treatment follow-up.
Satyanarayana et al.[28] discussed the possibility of missing cases and reported a 50% sensitivity for VIA in a realistic rural community setting. Jeronimo et al.[27] reported lower specificity for VIA and as a consequence discussed the possibility ≥50% of the women being referred to either colposcopy or unnecessary treatment as compared to other screening tests like Pap smears or HPV DNA testing. A 2003 study reported the lack of criteria to define VIA positivity as a barrier to implementing cervical cancer screening programs.[18] Logistical issues in the studies included ensuring uninterrupted cryo gas supply in the field clinics for treatment,[30] to selecting screening study sites based on accessibility by road, availability of health centers and permission required from local health authorities.[26] Gravitt et al.[24] in their study in rural Andhra Pradesh reported that 58% of the eligible women refused to participate in the study. In the focus groups conducted by the authors, reluctance to participate was reported as being related to perception that there was no need to go to the clinic when they have no symptoms.[24]
While reporting on competency levels of individuals conducting the screening tests, Sankaranarayanan et al.[19] highlighted the heterogeneous service delivery conditions that play an important role in implementing cervical cancer screening programs in the real world settings. Specifically, they discussed the issues of variable educational backgrounds of test providers and variable lengths of experience of colposcopists and pathologists, which could impact the interpretation of subjective tests such as VIA. None of the studies included in this review described the colposcopy related experience levels of the colposcopists. In Basu et al.[9] study, women participating in the screening program expected treatment for other health problems they were experiencing and were disappointed to note that the program only provided cervical cancer screening. Thus, the study authors discussed that cervical cancer screening could not be run as a stand-alone program, and needed to be integrated with existing primary health services. The studies conducted by Kamal et al.[21] and Satyanarayana et al.[28] reported higher rates of loss to follow-up when diagnostic follow-up with colposcopy and/or biopsies were not provided on the same visit. Bhatla et al. in their study of 3,000 women, reported 2/3rds of the VIA positive women received treatment within four months of diagnosis, thus highlighting a long delay between screening and treatment.[5] They discussed the possibility of minimizing delays with a single visit ‘screen and treat’ approach reducing the number of women not receiving treatment.
Three major themes were identified in this review that facilitated implementation of cervical cancer screening programs in a community-based setting: 1) standardization of training that maintained competency of test providers; 2) collaborations with community-based organizations and health education delivery for recruitment of participants; and 3) employing the ‘screen and treat’ method to reduce loss to follow-up. Several studies [5, 6, 17, 19, 20, 23–25, 30, 32] reported using the manual developed by the International Agency for Research on Cancer [40] to provide VIA screening training to healthcare providers. Using this manual and providing regular refresher trainings for the staff delivering the screening allowed the programs to maintain high levels of competency among test providers throughout the duration of the study. Studies in this review mentioned the importance of establishing collaborations with community-based organizations and local leaders in recruiting study participants.[10, 24, 27] Only one study [23] was able to provide evidence for the effectiveness, safety and acceptability of the ‘screen and treat’ method in a cervical cancer screening program in a low resource setting. Their study was able to achieve a relatively high compliance rate (74.8%) to treatment when compared to other studies not providing treatment at the same visit.
Risk of bias across studies
Table 4 presents the details of bias assessment. Three studies had a strong rating (no components rated weak), and one was assigned a moderate rating (one component rated weak), and 16 were assigned a weak rating (two or more components rated weak). The three studies that were rated strong were all randomized controlled trials. The most common risk of bias in all the studies reported was selection bias and non-randomization.
Table 4.
Risk bias assessment in included studies.
| No. | Author, year | Selection Bias | Study Design | Confounders | Blinding | Data collection methods |
Withdrawals and drop-outs |
Final rating |
|---|---|---|---|---|---|---|---|---|
| 1. | Sankaranarayanan et al., 1998[17] | +++ | +++ | + | + | +++ | + | 3 |
| 2. | Basu et al., 2003[6] | +++ | +++ | + | + | + | +++ | 3 |
| 3. | Sankaranarayanan et al., 2003[18] | +++ | +++ | + | + | + | + | 3 |
| 4. | Sankaranarayanan et al., 2004[19] | +++ | +++ | + | + | + | +++ | 3 |
| 5. | Sankaranarayanan et al., 2004[20] | +++ | +++ | + | + | + | + | 3 |
| 6. | Basu et al., 2006[9] | +++ | +++ | + | + | + | +++ | 3 |
| 7. | Kamal et al., 2007[21] | +++ | +++ | + | + | + | +++ | 3 |
| 8. | Nene et al., 2007[22]* | ++ | + | + | + | + | + | 1 |
| 9. | Sankaranarayanan et al., 2007[11]* | ++ | + | + | + | + | + | 1 |
| 10. | Bhatla et al., 2009[5] | +++ | +++ | + | + | + | +++ | 3 |
| 11. | Gravitt et al., 2010[24] | +++ | +++ | + | + | + | +++ | 3 |
| 12. | Kumar et al., 2011[10] | +++ | +++ | + | + | + | +++ | 3 |
| 13. | Deodhar et al., 2012[25] | ++ | +++ | + | + | + | + | 2 |
| 14. | Basu et al., 2013[26] | ++ | +++ | + | + | + | +++ | 3 |
| 15. | Shastri et al., 2013[32]* | + | + | + | + | + | ++ | 1 |
| 16. | Ghosh et al., 2014 [31] | +++ | +++ | + | + | + | + | 3 |
| 17. | Jeronimo et al., 2014[27] | +++ | +++ | + | + | + | + | 3 |
| 18. | Satyanarayanan et al., 2014[28] | ++ | +++ | + | + | + | +++ | 3 |
| 19. | Basu et al., 2015[29] | +++ | +++ | + | + | + | + | 3 |
| 20. | Poli et al., 2015[30] | +++ | +++ | + | + | + | +++ | 3 |
strong;
moderate;
weak;
randomized controlled trial; 1= strong; 2=moderate; 3=weak
Discussion
Findings from this systematic review of 20 studies with a total of 313,553 women that were screened at 12 unique urban and rural sites across India that used VIA provides contextual information on how screening programs can be implemented in community-based settings across India. Studies that reported test accuracy for CIN Grade 2+, the VIA sensitivity values ranged from 16.6 – 82.6% and specificity ranged from 82.1 – 96.8%. Most studies provided same-visit colposcopy and biopsy with minimal loss to follow-up but only two studies described providing same-visit cryotherapy.
Almost 40% of the studies in our review used media, group and one-on-one counseling, and local social support to recruit women into the screening programs, which represents substantial investment prior to the implementation of screening programs in the community. The studies also reported substantial use of infrastructure in setting up screening programs in the communities including transportation and establishing screening sites, which required several collaborations. As evidence in the Tamil Nadu Cervical Cancer Screening Pilot Project, efforts to mobilize women for participation were restricted due to a lack of health education.[41] On the other hand Shastri et al. attributed high levels of participation, diagnosis and treatment compliance to effective health education programs.[32] When translating evidence from research studies into real world program settings, it is critical that program planners consider the human and logistical capital required for successful implementation of cervical cancer screening programs.
Sauvaget et al. in their review reported that the screening provider’s background (e.g. physicians, nurses, health workers) did not influence the test accuracy of VIA.[42] That cannot be addressed as the test providers in the studies included in this review had varying backgrounds and expertise, and none of the studies reported on the providers’ experience levels, which may affect VIA test outcomes. Furthermore, authors frequently reported a learning curve, in the sense that VIA positivity rates were higher in the earlier stages compared to the later stages when conducting studies over a period of few years. VIA being a subjective test required the staff to develop some degree of experience prior to getting comfortable in delivering accurate test results.
An important consideration in this regard is the focus of screening programs to provide adequate training to the test providers. When studies reported providing training, the IARC manual was consistently used. However, not all studies provided refresher trainings or evaluated their training. Based on their experience in conducting screening programs, the Alliance for Cervical Cancer Prevention (ACCP) recommended providing screener training using a competency-based curriculum, combining both didactic and hands-on approaches, and conducting the trainings in a clinical setting similar to the service delivery conditions of the program site.[43] Studies included in this review did not provide information about the training of the test providers based on these criteria.
When screening tests such as VIA are being evaluated for large scale implementation, they need to be reliable and have good test characteristics in addition to being convenient, safe and acceptable by target community members.[44] Test reliability assesses the degree to which repeated measurements of the test yields the same result, and the accuracy of a test (specificity and sensitivity) is measured using cross-sectional studies with adequate sample size. Previous reviews, not focused on Indian or community-based settings, have reported sensitivity and specificity values of 79–82% and 91–92% by Sauvaget et al.[42], 79–83% and 84–85% by Arbyn et al.[45], 77% and 82% by Mustafa et al.[46], and 71.8% and 79.4% by Sritipsukho et al.[47] Compared to previous reviews, the sensitivity values reported in the included studies were lower and for specificity were in the same range when compared to previous reviews. Variation in test providers training, light source when conducting the VIA test in the field settings, and the preparation and storage of diluted acetic acid have previously been reported as possible explanations for wide variations in sensitivity and specificity for VIA conducted in community-based settings.[48]
According to Mahe & Gaffkin, several basic features are necessary to ensure internal validity in cross-sectional studies reporting test characteristics, including: a) final disease status data should be obtained for all subjects; b) all test results must be determined independently of previous results; c) the reference standard used to determine the disease status should be accurate; and d) the full “spectrum” of the disease should be included in the study.[49] In the cross-sectional studies that reported test accuracy, six used colposcopy as the reference standard and provided it to all screened women. However, the ability of colposcopy to categorize pre-cancer and cancers is poor and can cause inflation of the sensitivity values of the tests.[39, 50] More importantly, abnormalities on colposcopy are likely to be correlated with VIA positivity since both tests are subjective and rely on the visual inspection.[42] Two of the six studies using colposcopy as a reference standard did not blind the colposcopists to the VIA findings, which may have introduced ascertainment bias in these studies. Five of the ten studies reporting test accuracy did not apply the reference standard test to all screened women indicating the possibility of verification bias in the included studies. Furthermore, the quality and accuracy of the disease definition could be substantially affected by the experience levels of the colposcopists or pathologists interpreting histology, which was not reported consistently in the studies included in this review.
In low resource settings, visual inspection approaches offer a distinct advantage of immediate availability of screening test results, which provides health care professionals an opportunity to offer treatment during the same visit; widely known as the same day “screen-and-treat” approach. This approach has shown to reduce the likelihood of failure to follow-up and prevent advanced disease as demonstrated in several studies from India and other low-and middle-income countries included in a review.[41] Only two studies included in this review reported using the ‘screen-and-treat’ approach, with Poli et al. having to discontinue the approach after the initial two years due to logistical issues of ensuring uninterrupted cryo gas supply. Furthermore, treating women without confirming the diagnosis can result in considerable overtreatment as demonstrated in a meta-analysis, which concluded that if VIA alone was used to screen women, compared to Pap smears, 58 more per 1000 women would receive treatment unnecessarily for CIN grade 2–3 lesions.[46] Furthermore, a meta-analysis reported that 14.8% (65/439) of the women treated with cryotherapy had infertility and 12 reported spontaneous abortions in 210 pregnancies.[51] Scaling-up programs will require planners to keep in mind that if diagnostic services are provided at the screening site, fewer women would need to be referred, thereby reducing loss to follow-up. In waiting for diagnostic confirmation, this model also prevents overtreatment by cryotherapy, which has been reported in the ‘screen and treat’ model.[52] This may require creative implementation strategies that involve a wide variety of stakeholders such as social scientists, family members, and both public and private healthcare partnerships.
Most of the evidence in this review was derived from cross-sectional studies conducted in controlled community settings with limited information on the adaptation and translation of an effective intervention in socially intact groups or communities. Two studies in this review [28, 31] included information from symptomatic women in their study, which may bias the evidence regarding VIA effectiveness. However, the information synthesized in this review will be critical as national programs are implemented and evaluated for sustainability. It is also possible that research studies using VIA methods with non-significant findings may not have been accepted for publication and might have led to publication bias. We also did not include any qualitative studies or conference abstracts since complete information about the screening programs was not available. Nonetheless, to the best of our knowledge, this is the only review that focused on reviewing VIA based cervical cancer screening programs in community-based settings across India.
Overall, VIA based screening programs implemented in these studies were found to be appropriate, acceptable and feasible in community-based settings in India. Implementation barriers and facilitators presented in this review could substantially influence the future implementation of cervical cancer programs at a national level in India. The lower test accuracy values highlight the challenges involved in providing VIA in a community-based setting. A concern for over-diagnosis resulting in psychological distress for the false positives and may lead to over-treatment is evident if the ‘screen and treat’ approach is implemented in future screening programs.
Supplementary Material
Highlights.
Cervical cancer screening programs using visual inspection methods in India are reviewed
Focus of the review is to understand implementation of community based screening programs
For visual inspection, specificity and sensitivity values range from 82.1–96.8% and 16.6–82.6% respectively
Standardized training for community health workers was critical to screening test accuracy
Logistical and infrastructural challenges were identified as most common barriers to implementation
Acknowledgments
Funding: Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health [under training grant # TW009338]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A. Medline through Ovid Search String
“Cervical Neoplasm, Uterine” OR “Cervical Neoplasms, Uterine” OR “Neoplasm, Uterine Cervical” OR “Neoplasms, Uterine Cervical” OR “Uterine Cervical Neoplasm” OR “Neoplasms, Cervical” OR “Cervical Neoplasms” OR “Cervical Neoplasm” OR “Neoplasm, Cervical” OR “Neoplasms, Cervix” OR “Cervix Neoplasms” OR “Cervix Neoplasm” OR “Neoplasm, Cervix” OR “Cancer of the Uterine Cervix” OR “Cancer of the Cervix” OR “Cervical Cancer” OR “Uterine Cervical Cancer” OR “Cancer, Uterine Cervical” OR “Cancers, Uterine Cervical” OR “Cervical Cancer, Uterine” OR “Cervical Cancers, Uterine” OR “Uterine Cervical Cancers” OR “Cancer of Cervix” OR “Cervix Cancer” OR “Cancer, Cervix” OR “Cancers, Cervix”
AND
“Early Detection of Cancer” OR “Cancer Early Detection” OR “Cancer Screening” OR “Screening, Cancer” OR “Cancer Screening Tests” OR “Cancer Screening Test” OR “Screening Test, Cancer” OR “Screening Tests, Cancer” OR “Test, Cancer Screening” OR “Tests, Cancer Screening” OR “Early Diagnosis of Cancer” OR “Cancer Early Diagnosis”
AND
“India”
Total search results = 1228
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Systematic Review registration number: CRD42016032601
All authors contributed substantially to the conception and design of the manuscript. PA and NM were responsible for the acquisition of data and the analysis. All authors contributed to the interpretation of data. All authors contributed to drafting the article and revising it critically for important intellectual content. All authors have provided the final approval of the version to be published.
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.WHO. [Accessed 3/12/2014];India Country Profile for Cancer. 2014 http://www.who.int/cancer/country-profiles/ind_en.pdf?ua=1.
- 2.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Hariri S, Markowitz LE, Dunne EF, Unger ER. Population Impact of HPV Vaccines: Summary of Early Evidence. Journal of Adolescent Health. 2013;53(6):679–682. doi: 10.1016/j.jadohealth.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24(Supplement 3):S171–S177. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Bhatla N, Gulati A, Mathur SR, Rani S, Anand K, Muwonge R, Sankaranarayanan R. Evaluation of cervical screening in rural North India. International Journal of Gynecology & Obstetrics. 2009;105(2):145–149. doi: 10.1016/j.ijgo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Basu P, Sankaranarayanan R, Mandal R, Roy C, Das P, Choudhury D, Bhattacharya D, Chatterjee R, Dutta K, Barik S. Visual inspection with acetic acid and cytology in the early detection of cervical neoplasia in Kolkata, India. International Journal of Gynecological Cancer. 2003;13(5):626–632. doi: 10.1046/j.1525-1438.2003.13394.x. [DOI] [PubMed] [Google Scholar]
- 7.Vesco KK, Whitlock EP, Eder M, Lin J, Burda MBU, Senger CA, Holmes RS, Fu R, Zuber S. Screening for cervical cancer: a systematic evidence review for the US Preventive Services Task Force. Lancet Oncol. 2011;12(7):663–72. [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A. HPV screening for cervical cancer in rural India. New England Journal of Medicine. 2009;360(14):1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 9.Basu P, Ghoshal M, Chattopadhyay K, Mittal S, Das P, Choudhury D, Chattopadhyay U. Cervical screening by visual inspection with acetic acid (VIA) is well accepted by women-results from a community-based study in rural India. Asian Pac J Cancer Prev. 2006;7(4):604–608. [PubMed] [Google Scholar]
- 10.Kumar Y, Mishra G, Gupta S, Shastri S. Cancer screening for women living in urban slums--acceptance and satisfaction. Asian Pac J Cancer Prev. 2011;12(7):1681–5. [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370(9585):398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Nene BM, Dinshaw KA, Mahe C, Jayant K, Shastri SS, Malvi SG, Chinoy R, Kelkar R, Budukh AM. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. International Journal of Cancer. 2005;116(4):617–623. doi: 10.1002/ijc.21050. [DOI] [PubMed] [Google Scholar]
- 13.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, Wright TC. Cost-effectiveness of cervical-cancer screening in five developing countries. New England Journal of Medicine. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 14.Mo.H.a.F Welfare. [Accessed 03/01/2017];Operational Framework: Management of Common Cancers. 2016 http://cancerindia.org.in/cp/images/PDF/Operational_Framework_Management_of_Common_Cancers.pdf.
- 15.Brownson RC, Fielding JE, Maylahn CM. Evidence-based public health: a fundamental concept for public health practice. Annual review of public health. 2009;30:175–201. doi: 10.1146/annurev.publhealth.031308.100134. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementThe PRISMA Statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, Parkin DM, Nair MK. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer. 1998;83(10):2150–2156. [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, Chithrathara K, Parkin DM, Nair MK. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol’s iodine (VILI) in cervical cancer screening in Kerala, India. International Journal of Cancer. 2003;106(3):404–408. doi: 10.1002/ijc.11245. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, Sharma R, Dolo A, Shastri SS, Nacoulma M. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. International Journal of Cancer. 2004;110(6):907–913. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 20.Sankaranarayanan R, Shastri SS, Basu P, Mahé C, Mandal R, Amin G, Roy C, Muwonge R, Goswami S, Das P. The role of low-level magnification in visual inspection with acetic acid for the early detection of cervical neoplasia. Cancer detection and prevention. 2004;28(5):345–351. doi: 10.1016/j.cdp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Kamal MM, Sapkal RU, Sarodey CS, Munshi MM, Alsi YD, Chande MA, Hingway SR, Dandige S, Kane US, Kshirsagar R. Comparative study of four candidate strategies to detect cervical cancer in different health care settings. Journal of Obstetrics and Gynaecology Research. 2007;33(4):480–489. doi: 10.1111/j.1447-0756.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Nene B, Jayant K, Arrossi S, Shastri S, Budukh A, Hingmire S, Muwonge R, Malvi S, Dinshaw K, Sankaranarayanan R. Determinants of women s participation in cervical cancer screening trial, Maharashtra, India. Bulletin of the World Health Organization. 2007;85(4):264–272. doi: 10.2471/BLT.06.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sankaranarayanan R, Rajkumar R, Esmy PO, Fayette JM, Shanthakumary S, Frappart L, Thara S, Cherian J. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. British journal of cancer. 2007;96(5):738–43. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gravitt PE, Paul P, Katki HA, Vendantham H, Ramakrishna G, Sudula M, Kalpana B, Ronnett BM, Vijayaraghavan K, Shah KV. Effectiveness of VIA, Pap, and HPV DNA testing in a cervical cancer screening program in a peri-urban community in Andhra Pradesh, India. PLoS One. 2010;5(10):e13711. doi: 10.1371/journal.pone.0013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deodhar K, Sankaranarayanan R, Jayant K, Jeronimo J, Thorat R, Hingmire S, Muwonge R, Chiwate A, Deshpande R, Ajit D. Accuracy of concurrent visual and cytology screening in detecting cervical cancer precursors in rural India. International Journal of Cancer. 2012;131(6):E954–E962. doi: 10.1002/ijc.27633. [DOI] [PubMed] [Google Scholar]
- 26.Basu P, Mittal S, Bhaumik S, Mandal SS, Samaddar A, Ray C, Siddiqi M, Biswas J, Sankaranarayanan R. Prevalence of high-risk human papillomavirus and cervical intraepithelial neoplasias in a previously unscreened population—A pooled analysis from three studies. International Journal of Cancer. 2013;132(7):1693–1699. doi: 10.1002/ijc.27793. [DOI] [PubMed] [Google Scholar]
- 27.Jeronimo J, Bansil P, Lim J, Peck R, Paul P, Amador JJ, Mirembe F, Byamugisha J, Poli UR, Satyanarayana L. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. International Journal of Gynecological Cancer. 2014;24(3):576–585. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satyanarayana L, Asthana S, Bhambani S, Sodhani P, Gupta S. A comparative study of cervical cancer screening methods in a rural community setting of North India. Indian J Cancer. 2014;51(2):124–8. doi: 10.4103/0019-509X.138172. [DOI] [PubMed] [Google Scholar]
- 29.Basu P, Mittal S, Banerjee D, Singh P, Panda C, Dutta S, Mandal R, Das P, Biswas J, Muwonge R. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. International Journal of Cancer. 2015;137(4):859–867. doi: 10.1002/ijc.29458. [DOI] [PubMed] [Google Scholar]
- 30.Poli UR, Bidinger P, Gowrishankar S. Visual inspection with acetic acid (via) screening program: 7 years experience in early detection of cervical cancer and pre-cancers in rural South India. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2015;40(3):203. doi: 10.4103/0970-0218.158873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh I, Mittal S, Banerjee D, Singh P, Dasgupta S, Chatterjee S, Biswas J, Panda C, Basu P. Study of accuracy of colposcopy in VIA and HPV detection-based cervical cancer screening program. Aust N Z J Obstet Gynaecol. 2014;54(6):570–5. doi: 10.1111/ajo.12282. [DOI] [PubMed] [Google Scholar]
- 32.Shastri SS, Mittra I, Mishra G, Gupta S, Dikshit R, Badwe RA. Effect of visual inspection with acetic acid (VIA) screening by primary health workers on cervical cancer mortality: A cluster randomized controlled trial in Mumbai, India. ASCO Annual Meeting Proceedings. 2013:2. [Google Scholar]
- 33.Thulaseedharan JV, Malila N, Hakama M, Esmy PO, Cheriyan M, Swaminathan R, Muwonge R, Sankaranarayanan R. Socio demographic and reproductive risk factors for cervical cancer-a large prospective cohort study from rural India. Asian Pacific Journal of Cancer Prevention. 2012;13(6):2991–2995. doi: 10.7314/apjcp.2012.13.6.2991. [DOI] [PubMed] [Google Scholar]
- 34.Vedantham H, Silver MI, Kalpana B, Rekha C, Karuna B, Vidyadhari K, Mrudula S, Ronnett BM, Vijayaraghavan K, Ramakrishna G. Determinants of VIA (Visual Inspection of the Cervix After Acetic Acid Application) positivity in cervical cancer screening of women in a peri-urban area in Andhra Pradesh, India. Cancer Epidemiology Biomarkers & Prevention. 2010;19(5):1373–1380. doi: 10.1158/1055-9965.EPI-09-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinshaw K, Mishra G, Shastri S, Badwe R, Kerkar R, Ramani S, Thakur M, Uplap P, Kakade A, Gupta S. Determinants of compliance in a cluster randomised controlled trial on screening of breast and cervix cancer in Mumbai, India. Oncology. 2008;73(3–4):145–153. doi: 10.1159/000126497. [DOI] [PubMed] [Google Scholar]
- 36.Dinshaw K, Mishra G, Shastri S, Badwe R, Kerkar R, Ramani S, Thakur M, Uplap P, Kakade A, Gupta S, Ganesh B. Determinants of Compliance in a Cluster Randomised Controlled Trial on Screening of Breast and Cervix Cancer in Mumbai, India. Oncology. 2007;73(3–4):154–161. doi: 10.1159/000126498. [DOI] [PubMed] [Google Scholar]
- 37.Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, Miller AB, Daniel EE, Gupta S, Uplap P. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. International journal of cancer. 2010;126(4):976–984. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 38.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. Journal of evaluation in clinical practice. 2012;18(1):12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh P, Gandhi G, Kochhar P, Zutshi V, Batra S. Visual inspection of cervix with Lugol’s iodine for early detection of premalignant & malignant lesions of cervix. The Indian journal of medical research. 2012;136(2):265. [PMC free article] [PubMed] [Google Scholar]
- 40.Wesley RSRS, editor. A practical manual on visual screening for cervical neoplasia. 2003. [Google Scholar]
- 41.Krishnan S, Madsen E, Porterfield D, Varghese B. Advancing Cervical Cancer Prevention in India: Implementation Science Priorities. The Oncologist. 2013;18(12):1285–1297. doi: 10.1634/theoncologist.2013-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011;113(1):14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 43.A.f.C.C.P. (ACCP) Cervical cancer library - Screening and treatment - Featured resources, 2016. 2016 http://www.rho.org/screening.htm.
- 44.Mahé C, Gaffikin L. Screening test accuracy studies: how valid are our conclusions? Application to visual inspection methods for cervical screening. Cancer Causes & Control. 16(6):657–666. doi: 10.1007/s10552-005-0296-4. [DOI] [PubMed] [Google Scholar]
- 45.Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, Nouhou H, Sakande B, Wesley R, Somanathan T. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. International journal of cancer. 2008;123(1):153–160. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 46.Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, Gandhi S, Chen Y, Cheung A, Hopkins J. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. International Journal of Gynecology & Obstetrics. 2015 doi: 10.1016/j.ijgo.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Sritipsukho P, Thaweekul Y. Accuracy of visual inspection with acetic acid (VIA) for cervical cancer screening: a systematic review. Journal of the Medical Association of Thailand= Chotmaihet thangphaet. 2010;93:S254–S261. [PubMed] [Google Scholar]
- 48.Parashari A, Singh V. Reasons for variation in sensitivity and specificity of visual inspection with acetic acid (VIA) for the detection of pre- cancer and cancer lesions of uterine cervix. Asian Pac J Cancer Prev. 2013;14(12):7761–2. doi: 10.7314/apjcp.2013.14.12.7761. [DOI] [PubMed] [Google Scholar]
- 49.Mahé C, Gaffikin L. Screening test accuracy studies: how valid are our conclusions? Application to visual inspection methods for cervical screening. Cancer Causes & Control. 2005;16(6):657–666. doi: 10.1007/s10552-005-0296-4. [DOI] [PubMed] [Google Scholar]
- 50.Pretorius RG, Kim RJ, Belinson JL, Elson P, Qiao Y-L. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. Journal of lower genital tract disease. 2006;10(1):5–9. doi: 10.1097/01.lgt.0000192694.85549.3d. [DOI] [PubMed] [Google Scholar]
- 51.Santesso N, Mustafa RA, Wiercioch W, Kehar R, Gandhi S, Chen Y, Cheung A, Hopkins J, Khatib R, Ma B, Mustafa AA, Lloyd N, Wu D, Broutet N, Schünemann HJ. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. International Journal of Gynecology & Obstetrics. 2016;132(3):266–271. doi: 10.1016/j.ijgo.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Sankaranarayanan R. ‘See-and-treat’works for cervical cancer prevention: what about controlling the high burden in India? The Indian journal of medical research. 2012;135(5):576. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.