Abstract
Background
The optimal level of sodium intake remains controversial.
Objective
To determine whether examination of left ventricular (LV) longitudinal and circumferential strain (LS, CS) and e′ velocity can provide insight into thresholds for the detrimental effects of estimated sodium intake (ESI) on subclinical cardiovascular disease.
Methods
We performed speckle-tracking analysis on Hypertension Genetic Epidemiology study echocardiograms with available urinary sodium data (N = 2,996). We evaluated the association between ESI and LS, CS, and e′ velocity using multivariable-adjusted linear mixed effects models (to account for relatedness among subjects) with linear splines (spline 1: ESI ≤3.7g/day, spline 2: ESI >3.7g/day based upon visual inspection of fractional polynomial plots of the association between ESI and indices of strain and e′ velocity). We performed mediation analysis to understand the indirect effects of systolic blood pressure (SBP) and serum aldosterone on the relationship between ESI and strain and e′ velocity.
Results
Mean age was 49 ± 14 years, 57% were female, 50% were African American, and 54% had hypertension. The median (25th–75th percentile) ESI was 3.73 (3.24–4.25) g/day. ESI >3.7g/day was associated with larger left atrial and LV dimensions (p <0.05). After adjusting for speckle-tracking analyst, image quality, study site, age, sex, smoking status, alcohol use, daily blocks walked, diuretic use, estimated glomerular filtration rate, LV mass, ejection fraction, and wall motion score index, ESI >3.7g/day was associated with all strain parameters and e′ velocity in (p <0.05 for all comparisons), but ESI ≤3.7g/day was not (p >0.05 for all comparisons). There were significant interactions by potassium excretion for CS. Mediation analysis suggested that SBP explained 14% and 20% of the indirect effects between ESI and LS and e′ velocity, respectively, while serum aldosterone explained 19% of the indirect effects between ESI and LS.
Conclusions
ESI >3.7 g/day is associated with adverse cardiac remodeling and worse systolic strain and diastolic e′ velocity.
Keywords: Urinary sodium, sodium intake, strain, echocardiography
Introduction
Only a small percentage of the world’s population meets current sodium intake goals of 1.5–2.3 g/day (1–4). Findings from several recent studies, however, have challenged these recommendations (5–7). A large prospective cohort study showed a J-shaped relationship, whereby an estimated sodium intake (ESI) of 3–6 g/day resulted in the lowest risk for death and cardiovascular events (6). Conversely, longitudinal results from the Trial of Hypertension Prevention (TOHP) trials suggested that ESI is linearly related with mortality. Furthermore, the mechanistic underpinnings of such associations are poorly understood. For example, it has been shown that the relationship between ESI and mortality is not simply mediated by higher blood pressure, a known consequence of increased sodium intake (2,8). Since there are no published clinical trials dedicated to determining whether low sodium intake reduces cardiovascular events, we must rely on well-executed, observational analyses and sound biological plausibility to determine optimal sodium intake (8). Whether urinary sodium, a surrogate marker of ESI, is associated with subclinical measures of cardiac dysfunction, such as myocardial strain, remains unknown. Strain is a sensitive indicator of cardiomyocyte health that correlates with fibrosis, cardiomyocyte hypertrophy, and abnormal calcium transients within cardiomyocytes (9), and therefore may provide insight into the relationship between ESI and cardiovascular disease.
We thus sought to study the relationship between ESI and indices of cardiac mechanics and hypothesized that higher ESI is associated with worse systolic strain and impaired myocardial relaxation in individuals free of clinical heart failure. We leveraged the Hypertension Genetic Epidemiology Network (HyperGEN) Study, a large population- and family-based study—which included overnight urine collections from which urine sodium concentrations were measured, and in which we have previously performed speckle-tracking echocardiographic analysis (10–13).
Methods
Study Population
HyperGEN, part of the National Institutes of Health Family Blood Pressure Program (FBPP), is a cross-sectional study consisting of 5 U.S. sites, with 4 participating in an ancillary echocardiographic study (Salt Lake City, Utah; Forsyth County, North Carolina; Minneapolis, Minnesota; and Birmingham, Alabama). The goal of HyperGEN was to identify and characterize the genetic basis of familial hypertension (14). Study eligibility required a diagnosis of hypertension prior to the age of 60 and ≥1 sibling willing to participate in the study. Hypertension was defined by an average systolic blood pressure ≥140 mmHg or an average diastolic blood pressure ≥90 mmHg (on ≥2 separate clinic visits) or by self-reporting treatment for hypertension. A random sample of normotensive individuals who represented the source cohort from which the HyperGEN affected sibships were identified was also recruited. Individuals with a history of type 1 diabetes mellitus or severe chronic kidney disease were excluded due to the high risk of secondary forms of hypertension. All HyperGEN study participants gave written informed consent, and the HyperGEN study was approved by each study site’s local institutional review board.
Clinical Characteristics
Demographic, clinical, and laboratory data were collected during the initial HyperGEN visit. Height, weight, and blood pressure were measured by trained personnel using a standardized protocol. Three consecutive, seated blood pressure measurements in each arm were obtained per person, and the second and third values were averaged and used for analysis (14). Histories of myocardial infarction, transient ischemic attack, and stroke were obtained by self-report. Diabetes mellitus was defined by fasting glucose ≥126 mg/dl, use of hypoglycemic medication, or a self-reported history. Dyslipidemia was defined by use of lipid lowering medication, low-density lipoprotein cholesterol ≥160 mg/dl, triglycerides >150 mg/dl, or high density lipoprotein cholesterol <40 mg/dl (for men) or <50 mg/dl (for women). Obesity was defined by a BMI ≥30 kg/m2. Chronic kidney disease was defined by an estimated glomerular filtration rate ≤60 ml/min/1.73m2.
Estimated Sodium Intake
Urinary electrolytes were measured from overnight urine collections (15). We extrapolated 24-hour urinary sodium and potassium excretion using the method of Tanaka, since this method has reported the least bias when overnight urine specimens are analyzed (16). The 24-hour urinary sodium was used as a surrogate of sodium intake.
Conventional Echocardiography
Echocardiography (including 2D, M-mode, and Doppler imaging) was acquired on all study participants using standardized acquisition protocols and stored in analog format (high grade, medical quality videocassette tapes) at the time of study visit (17,18). Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (ASE) (19,20). LV ejection fraction (LVEF) was calculated using the biplane method of discs. LV mass was calculated using the linear method recommended by the ASE and indexed to body surface area. LV hypertrophy was defined by a LV mass index >95 g/m2 in women or > 115 g/m2 in men. Diastolic function was quantitated using early diastolic (E) and late/atrial diastolic (A) transmitral velocities, E/A ratio, isovolumic relaxation time, and E deceleration time.
Digitization of Echocardiograms and Interpretation of Image Quality
Archived echocardiograms in analog format were converted to digital format using the TIMS 2000 DICOM System (Foresight Imaging, Chelmsford, Massachusetts). Cine loops of 2–4 cardiac cycles from the parasternal short axis (papillary muscle level) and apical four chamber views were digitized at a high rate and stored offline in DICOM format. Each study was assessed for image quality by an experienced operator, blinded to all other clinical and echocardiographic data, using a 4-point scale based on the degree of endocardial border visualized (1 = 0–25%; 2 = 25%–50%; 3 = 50%–75%; 4 = 75%–100%), similar to scales used previously (21,22).
Two-Dimensional Speckle-Tracking Analysis
Digitized cine loops were analyzed using 2D wall motion tracking software (2D Cardiac Performance Analysis [CPA], TomTec v4.5, Unterschleisshein, Germany). After isolating the highest quality cardiac cycle by visual estimation, the endocardial and epicardial borders were traced at end-systole in each view. Computerized speckle-tracking analysis was performed and endocardial and epicardial border tracings were manually adjusted to optimize tracking. Indices of LV mechanics included peak longitudinal and circumferential strain, as well as average speckle-tracking derived e’ (STe′) velocities. For ease of display, strain values were converted to absolute values. Lower absolute strain values, lower STe′ velocities, and higher E/STe′ ratios were used to indicate worse cardiac function. Strain and STe′ values in our study are lower than what has been reported in the literature. For strain measurements, this may be due to differences in vendor used for post-processing software (23). The relatively low frame rates of the digitized echocardiograms (~30 fps) could also lead to lower measured values of strain and e′ velocity. In addition, for STe′ velocity, the underestimation may be due measurement of average segmental e′ tissue velocity, as opposed to tissue Doppler imaging which measures peak e′ tissue velocity. Despite these limitations, we have demonstrated that correlations between strain values measured prospectively and after digitization are high, especially for longitudinal strain and STe′ velocities (10). There is high correlation between the two methods, though consistent underestimation of e′ velocity by speckle-tracking (Online Figure 1). Furthermore, we have also been able to reproduce many known associations between several cardiovascular risk factors and strain and e′ velocity (derived from analog-to-digital conversion, followed by speckle-tracking) in HyperGEN, which supports the accuracy of our methods.
Images used for speckle-tracking analysis were generally of high quality. In the parasternal short-axis and apical 4 chamber views, 84% and 96% of images had an image quality score of ≥2, respectively, indicating good image quality for the majority of myocardial segments. Data on interobserver and intraobserver reliability shows excellent intraclass correlation coefficients for speckle-tracking parameters (Online Table 1) (10,11).
Statistical Analysis
We first created fractional polynomial plots to explore the relationship between ESI and strain, STe′, and E/STe′ ratio. A change in the slope near 3.7 g/day of ESI for reduced absolute strain values was observed in each of the plots. We repeated these analyses using restricted cubic splines with 3 knots placed at the 10th, 50th, and 90th percentiles of ESI (Online Figure 2). Clinical characteristics, laboratory data, and both conventional echocardiographic parameters and speckle-tracking parameters were then calculated for the total cohort and stratified by ESI (≤3.7 g/day vs. >3.7 g/day). This cutpoint was chosen based upon visual inspection of the plots as well as dichotomization of the group (median ESI was 3.73 g/day, which was rounded to 3.7 g/day for ease of clinical interpretation). Continuous data are presented as mean ± standard deviation. Categorical variables are presented as a count and percentage. We compared clinical data between groups using t-tests for normally distributed continuous variables (or non-parametric equivalent when appropriate) and Chi-squared tests for categorical variables (or Fisher’s exact test when appropriate).
For our primary analyses, we used regression models with linear splines (spline 1 ≤3.7 g/day, spline 2 >3.7 g/day) to determine whether ESI was associated with worse indices of strain and STe′. Significant differences between spline slopes were confirmed for each parameter using the mkspline command and marginal option in Stata (p <0.05 for all comparisons). All regression analyses used linear mixed effects models to account for relatedness among HyperGEN participants. Multivariable model 1 adjusted for speckle-tracking analyst, image quality, study site (which accounts for differences in sonographers and echocardiography equipment) as fixed effects and family membership as a random effect. Additional covariates were selected using a combination of clinical relevance and association with ESI either in previous studies or the present one. The additional covariates included in our multivariable model 1 included age, sex, smoking status, alcohol use, numbers of blocks walked per day, diuretic use, estimated glomerular filtration rate, LV mass, LVEF, and wall motion score index. We repeated our analyses using fractional polynomial regression (Online Table 2). We also created multiplicative interaction terms to determine whether there were sex, race, hypertension, or potassium excretion (<2g/day vs. >2g/day) interactions with urinary sodium in their association with strain and STe′. An interaction term P <0.10 in the multivariable regression model was considered significant and explored further.
Since systolic blood pressure and serum aldosterone are along the putative causal pathway between ESI and adverse strain and STe′, we performed mediation analysis to understand these indirect effects. The following models all adjusted for model 1 covariates plus: systolic blood pressure (model 2) and serum aldosterone (model 3) (2,8,24). We calculated the proportion explained by the intermediate factors as follows: 100% x [Beta-coefficientmodel – Beta-coefficientmodel+intermediate factor]/[Beta-coefficientmodel] (25). We used the likelihood ratio test to determine whether the addition of the intermediary factor resulted in a statistically significant change in the model estimates.
Beta-coefficients are displayed per 1-gram increase in ESI. A 2-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata v.12.1 (StataCorp, College Station, Texas).
Results
Characteristics of Study Participants
Descriptive characteristics of the study sample from HyperGEN are displayed in Table 1, dichotomized by ESI. The study cohort consisted of 2996 participants. The mean age was 49±14 years, 57% were female, and 50% were African American. Comorbidities were common, and medication use reflected standard therapies used for these comorbidities. The mean blood pressure was 126 ± 21/72 ± 11 mmHg, and obesity was common (mean BMI 31 ± 7 kg/m2, 47% obese [BMI ≥30 kg/m2]). Laboratory results revealed largely preserved kidney function (estimated glomerular filtration rate 86 ± 20 ml/min/1.73 m2) and a median ESI (25th–75th percentile) of 3.73 (3.24–4.25) g/day and median (25th–75th percentile) potassium intake of 1.55 (1.32–1.78) g/day. In general, participants with higher ESI were older, were more often white, more frequently had comorbidities, had higher blood pressure, and higher BMI (p <0.05 for all comparisons).
Table 1.
Clinical, Physical, and Laboratory Characteristics of the 2996 HyperGEN Participants by Estimate Sodium Intake
| Estimate sodium intake | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | All Levels (N=2996) | 3.7≤g/day (N=1457) | >3.7g/day (N=1539) | P-value |
| Age, y | 49±14 | 47±14 | 51±13 | <0.001 |
| Female, n (%) | 1716 (57) | 855 (59) | 861 (56) | 0.13 |
| Race/Ethnicity, n (%) | <0.001 | |||
| White | 1476 (49) | 630 (43) | 846 (55) | |
| African-American | 1512 (50) | 825 (57) | 687 (45) | |
| Other | 7(1) | 2 (<1) | 5 (<1) | |
| Blocks walked per day | 6 (2–12) | 6 (2–12) | 6 (2–12) | 0.84 |
| Comorbidities, n (%) | ||||
| Hypertension | 1604 (54) | 690 (47) | 914 (59) | <0.001 |
| Dyslipidemia | 1780 (59) | 818 (56) | 962 (63) | <0.001 |
| Obesity | 1394 (47) | 532 (37) | 862 (56) | <0.001 |
| Current drinking | 583 (24) | 308 (27) | 275 (22) | 0.003 |
| Current smoking | 527 (18) | 296 (20) | 231 (15) | <0.001 |
| Diabetes mellitus | 475 (16) | 172 (12) | 303 (20) | <0.001 |
| Chronic kidney disease | 226 (8) | 113 (8) | 113 (7) | 0.66 |
| Myocardial infarction | 155 (5) | 61 (4) | 94 (6) | 0.02 |
| Transient ischemic attack or stroke | 122 (4) | 56 (4) | 66 (4) | 0.52 |
| Medications, n (%) | ||||
| Anti-hypertensive medication | 1363 (46) | 585 (40) | 778 (51) | <0.001 |
| ACE-inhibitor | 541 (18) | 230 (16) | 311 (20) | 0.002 |
| Angiotensin receptor blocker | 64 (2) | 25 (2) | 39 (3) | 0.13 |
| Beta-blocker | 321 (11) | 137 (9) | 184 (12) | 0.023 |
| Calcium channel blocker | 621 (21) | 265 (18) | 356 (23) | 0.001 |
| Loop diuretic | 169 (6) | 87 (6) | 82 (5) | 0.45 |
| Thiazide diuretic | 358 (12) | 162 (11) | 196 (13) | 0.17 |
| Anti-hyperglycemic medication | 288 (10) | 95 (7) | 193 (13) | <0.001 |
| Statin | 218 (7) | 88 (6) | 130 (8) | 0.011 |
| Physical examination | ||||
| Systolic blood pressure, mm Hg | 126±20 | 123±20 | 128±20 | <0.001 |
| Diastolic blood pressure, mm Hg | 72±11 | 71±11 | 73±11 | 0.003 |
| Body-mass index, kg/m2 | 31±7 | 29±6 | 32±7 | <0.001 |
| Waist circumference, cm | 101±17 | 97±16 | 106±17 | <0.001 |
| Laboratory data | ||||
| Estimated GFR, ml/min/1.73m2 | 87±20 | 86±20 | 87±20 | 0.67 |
| Fasting glucose, mg/dl | 105±42 | 100±36 | 110±47 | <0.001 |
| Low density lipoprotein, mg/dl | 119±34 | 118±35 | 120±34 | 0.13 |
| Urinary sodium, g/day | 3.78±0.81 | 3.13±0.44 | 4.38±0.56 | <0.001 |
| Urinary potassium, g/day | 1.58±0.35 | 1.45±0.30 | 1.70±0.36 | <0.001 |
| Aldosterone, pg/ml | 8.4±7.1 | 8.2±7.3 | 8.6±7.0 | 0.16 |
ACE, angiotensin-converting enzyme inhibitor; GFR, glomerular filtration rate
Table 2 lists both the conventional and speckle-tracking echocardiographic parameters of the study participants. Average LV structure fell within normal limits (LV end-systolic volume 51 ± 22 ml; LV end-diastolic volume 129 ± 30 ml; LV mass index 83 ± 22 kg/m2), though one-fifth had evidence of LV hypertrophy. EF was preserved (62 ± 8%) in the majority of participants. Participants with higher ESI had larger LV and left atrial dimensions and slightly lower EF (p <0.05 for all comparisons).
Table 2.
Two-Dimensional, Doppler, and Speckle-Tracking Echocardiographic Parameters of the 2996 HyperGEN Participants by Estimate Sodium Intake
| Estimate sodium intake | ||||
|---|---|---|---|---|
|
| ||||
| Conventional echocardiographic parameter | All Levels (N=2996) | 3.7≤g/day (N=1457) | >3.7g/day (N=1539) | P-value |
| LV end-diastolic volume, ml | 129±30 | 125±30 | 133±31 | <0.001 |
| LV end-systolic volume, ml | 51±22 | 49±22 | 53±22 | <0.001 |
| LV mass index, g/m2 | 83±22 | 82±21 | 85±23 | <0.001 |
| LV hypertrophy, n (%) | 586 (20) | 261 (18) | 325 (21) | 0.027 |
| Left atrial diameter, cm | 3.5±0.5 | 3.4±0.5 | 3.5±0.5 | <0.001 |
| Pulse pressure/stroke volume, mL/mmHg | 0.71±0.23 | 0.70±0.23 | 0.72±0.22 | 0.06 |
| LV ejection fraction, % | 62±8 | 62±8 | 61±8 | 0.03 |
| E velocity, cm/s | 74±19 | 75±19 | 73±19 | 0.013 |
| A velocity, cm/s | 65±18 | 64±18 | 66±18 | 0.003 |
| E/A ratio | 1.24±0.50 | 1.28±0.52 | 1.20±0.48 | 0.001 |
| E deceleration time, ms | 203±56 | 205±56 | 202±55 | 0.31 |
| Isovolumic relaxation time, ms | 81±17 | 80±16 | 81±18 | 0.008 |
|
| ||||
| Speckle-tracking echocardiographic parameter | ||||
|
| ||||
| STe′, cm/s* | 3.7±1.3 | 3.8±1.4 | 3.6±1.3 | <0.001 |
|
| ||||
| E/STe′ ratio* | 22.7±10.1 | 22.5±10.1 | 22.9±10.0 | 0.41 |
| Circumferential strain, % | 20.3±5.3 | 20.3±5.2 | 20.3±5.4 | 0.90 |
| Longitudinal strain, % | 14.5±3.6 | 14.6±3.6 | 14.4±3.6 | 0.09 |
LV, left ventricular; STe′, speckle-tracking derived early diastolic tissue velocity. All strain values are reported as absolute values.
Tissue velocity values derived from speckle-tracking software are lower than values derived from tissue Doppler imaging. Thus, e′ is lower and E/e′ is higher in the present study compared to other studies that use conventional tissue Doppler imaging to measure tissue velocities.
Association of Estimated Sodium Intake with Adverse Strain and STe′
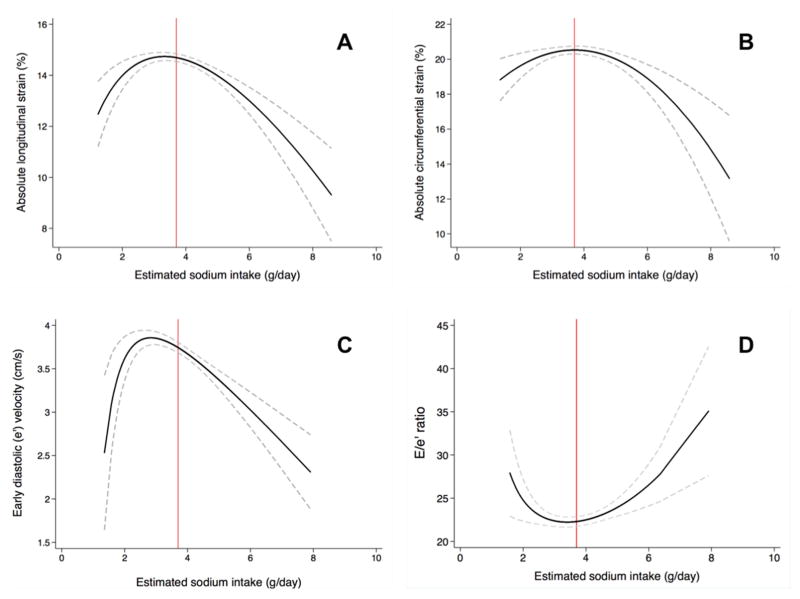
Figure 1 shows fractional polynomial plots of ESI and strain, STe′, and E/STe′ ratio, and a threshold cut-point for worse cardiac strain was identified at roughly 3.7 g/day of ESI. Results were similar when using restricted cubic splines (Online Figure 2). Therefore, 2 linear splines were created to model the subsequent relationships (spline 1 ≤3.7 g/day; spline 2 >3.7 g/day) as shown in Table 3. After adjusting for speckle-tracking analyst, image quality, study site, age, sex, smoking status, alcohol use, blocks walked per day, diuretic use, estimated glomerular filtration rate, LV mass, LVEF, and wall motion score index, ESI was associated with all strain parameters and STe′ in linear spline 2 (p <0.05 for all comparisons), but not spline 1 (p>0.05 for all comparisons). While an association was demonstrated between higher E/STe′ and increased ESI in spline 2 on univariate analysis, the relationship was non-significant after multivariable-adjustment. When analyzing the relationship using multivariable fractional polynomial regression for participants with ESI>3.7 g/day, results were similar (Online Table 2). Because the ESI threshold for STe′ occurred near 2.9 g/day per Figure 1 as opposed to 3.7 g/day observed in the indices of cardiac strain, we evaluated the association between ESI and STe′ using lower thresholds. No independent association between ESI and STe′ was observed until the cutpoint was increased (in increments of 0.10 g/day) from 2.9 g/day to 3.7 g/day.
Figure 1. Unadjusted fractional polynomial plots of estimated urinary sodium excretion and strain, e′ velocity, and E/e′ ratio.
(A) longitudinal strain, (B) circumferential strain, (C) early diastolic (e′) velocity, and (D) E/e′ ratio. A reference line is drawn at an estimated sodium intake value of 3.7 g/day. The 95% confidence intervals are noted by dashed lines. Plots are shown for the unadjusted relationship. Note: Tissue velocity values derived from speckle-tracking software are lower than values derived from tissue Doppler imaging. Thus, e′ is lower and E/e′ is higher in the present study compared to other studies that use conventional tissue Doppler imaging to measure tissue velocities.
Table 3.
Association of Urinary Sodium with Systolic Strain, Early Diastolic Tissue Velocity, and Estimated LV Filling Pressure on Crude and Multivariable-adjusted Analyses
| Dependent variable | Model | Estimate sodium intake ≤3.7 g/day (N=1457) | Estimate sodium intake >3.7 g/day (N=1539) | ||
|---|---|---|---|---|---|
|
| |||||
| β-Coefficient (95% CI) | P-value | β-Coefficient (95% CI) | P-value | ||
| Longitudin al strain, % | Crude | 0.28 (−0.16, 0.71) | 0.21 | −0.91 (−1.21, −0.60) | <0.001 |
| Multivariable-adjusted* | 0.11 (−0.30, 0.52) | 0.60 | −0.42 (−0.74, −0.11) | 0.009 | |
| Circumfere ntial strain, % | Crude | 0.44 (−0.19, 1.08) | 0.17 | −0.94 (1.42, 0.47) | <0.001 |
| Multivariable-adjusted* | 0.14 (−0.51, 0.80) | 0.67 | −0.52 (−1.03, −0.01) | 0.045 | |
| STe′ velocity, cm/s | Crude | −0.08 (−0.25, 0.10) | 0.41 | −0.32 (−0.45, −0.19) | <0.001 |
| Multivariable-adjusted* | −0.02 (−0.17, 0.13) | 0.77 | −0.15 (−0.27, −0.03) | 0.015 | |
| Estimated LV filling pressure (E/STe′ ratio) | Crude | −0.62 (−2.25, 1.01) | 0.46 | 1.55 (0.22, 2.88) | 0.023 |
| Multivariable-adjusted* | −0.76 (−2.62, 1.11) | 0.43 | 0.92 (−0.64, 2.49) | 0.25 | |
CI, confidence interval; STe′, speckle-tracking derived early diastolic tissue velocity. All strain parameters are reported as absolute values. Beta-coefficients reflect the change in the dependent variable per 1 gram/day increase in estimated sodium intake.
Adjusted for age, sex, smoking status, alcohol use, blocks walked per day, diuretic use, estimated glomerular filtration rate, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
On sensitivity analysis, after excluding participants on diuretics (instead of adjusting for diuretic use in the multivariable models), the association between ESI and circumferential strain for spline 2 remained present (Online Table 3). On interaction analysis, the associations between ESI and worse circumferential strain were only demonstrated in the presence of low estimated potassium intake. There were no other interactions observed by sex, race, or history of hypertension (Online Tables 4 and 5).
Additional models, which included further adjustment for systolic blood pressure (model 2) and serum aldosterone (model 3) generally weakened, but did not eliminate most of the observed associations observed for spline 2 and strain and STe′ (Table 4). Mediation analysis suggested that systolic blood pressure explained 14%, and 20% of the indirect effects between ESI and longitudinal strain and STe′, respectively, while serum aldosterone explained 19% of the indirect effects between ESI and longitudinal strain. We stratified the serum aldosterone analysis by race (Online Table 6). Serum aldosterone mediated a significant proportion of the relationship between ESI and both LS and STe′ in African Americans. However, no statistically significant relationship on mediation analysis was observed in white participants.
Table 4.
Association of Urinary Sodium with Systolic Strain and Early Diastolic Tissue Velocity in Participants with Estimate Sodium Intake >3.7 g/day on Mediation Analysis
| Dependent variable | Model 2† | Model 3‡ | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β-Coefficient (95% CI) | P-value | Proportion Explained by Systolic Blood Pressure | β-Coefficient (95% CI) | P-value | Proportion Explained by Serum Aldosterone | |
| Longitudinal strain, % | −0.36 (−0.68, −0.04) | 0.027 | 14%* | −0.34 (−0.66, −0.02) | 0.039 | 19%* |
| Circumferential strain, % | −0.49 (−1.00, 0.01) | 0.057 | 6% | −0.55 (−1.07, −0.04) | 0.035 | −6% |
| STe′, cm/s | −0.12 (−0.24, −0.00) | 0.048 | 20%* | −0.14 (−0.26, −0.02) | 0.025 | 7% |
CI, confidence interval, STe′, speckle-tracking derived early diastolic tissue velocity. All strain parameters are reported as absolute values. Beta-coefficients reflect the change in the dependent variable per 1 gram/day increase in estimated sodium intake.
All models adjusted for age, sex, smoking status, alcohol use, blocks walked per day, diuretic use, estimated glomerular filtration rate, left ventricular mass, wall motion abnormalities, ejection fraction, center, speckle-tracking analyst, and image quality.
Statistically significant change in model with addition of intermediary factor (p<0.05).
Additional adjustment for systolic blood pressure.
Additional adjustment for serum aldosterone.
Discussion
In an analysis of 2996 participants from the HyperGEN study, we found that ESI above 3.7 g/day was associated with reduced strain and STe′. Additionally, increased ESI was also associated with adverse left atrial and LV remodeling. Mediation analysis showed that systolic blood pressure and serum aldosterone explained a significant proportion of the indirect effects between ESI and several indices of strain and e′ velocity; however, adjustment for blood pressure or serum aldosterone did not eliminate these associations. A notable proportion of the relationship remained unexplained, and this may be due to a higher proportion of individuals with cardiometabolic risk factors in the elevated ESI group. To our knowledge, this is the first study of ESI and strain, and our data may offer mechanistic insight into why high sodium intake is associated with worse cardiovascular outcomes, including heart failure (7).
On interaction testing, we found that the association between ESI and worse circumferential strain was only apparent in participants with low estimated potassium intake. High potassium intake has been shown to counteract the effects of high sodium intake in spontaneously hypertensive rats (26). Additionally, in a randomized trial of potassium intake in veterans, potassium-enriched salt compared to regular salt decreased the risk of cardiovascular death (27). Notably, we did not observe other interactions by sex, race, or history of hypertension.
In our study, elevated ESI could either drive the association with adverse strain and STe′, or it may be a marker of a higher risk patient with poor dietary habits in general. If causal, elevated ESI may be multifactorially related to worse systolic strain and impaired diastolic relaxation (Central Illustration). In animal models, salt loading has been shown to increase not only blood pressure, but also myocardial fibrosis (28,29), which could lead to progressive decrement in strain and STe′. In addition, aldosterone excess acts synergistically with high sodium diets to accelerate myocardial fibrosis. As such, aldosterone may be a potent mediator of this relationship, as demonstrated in our study as well (30–32). Furthermore, human data from a crossover trial of young men randomized to salt tablets showed salt loading induced both (1) vascular endothelial dysfunction, demonstrated by impaired endothelium-dependent vasodilatory responses to acetylcholine, and (2) left ventricular diastolic dysfunction, possibly due to increased intracellular calcium with subsequent impaired myocardial relaxation (33). Notably, normal cardiac strain is dependent upon healthy endothelial function (34). Finally, high salt could result in increased arterial stiffness (35) which could lead to a larger reflected arterial waves that may progressively injure the myocardium. It is notable that ESI was not associated with E/e′ on multivariable analysis. We speculate that since E/e′ reflects LV filling pressures, the deleterious effects of sodium intake might first affect subclinical markers of myocardial dysfunction (strain and e′), while elevation in filling pressures may occur later in the trajectory toward HF development.
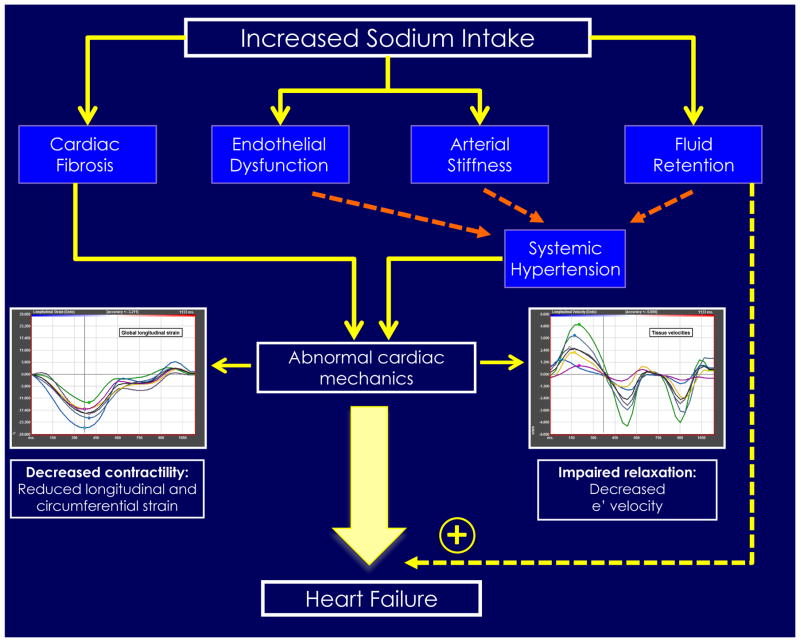
Central Illustration. Urinary Sodium and Cardiac Mechanics: Estimated Sodium Intake and Adverse Cardiac Structure and Function as well as heart failure.
Increased sodium intake has been associated with cardiac fibrosis, endothelial dysfunction, and arterial stiffness, which can lead to systemic hypertension. Together, these derangements may lead to adverse myocardial strain and e′ velocity as detected by speckle-tracking echocardiography in our study. In addition, increased sodium intake increases fluid retention, and when coupled with adverse strain and e′ velocity, may predispose individuals to heart failure.
Despite numerous observational studies and several clinical trials, the optimal level of sodium intake remains unclear. This is in part due to conflicting data depending on the endpoint used (i.e. blood pressure or cardiovascular events). The Prospective Urban Rural Epidemiology (PURE) study showed that, compared to a sodium intake <3 g/day, sodium intake >5g/day was associated with the highest risk of increasing systolic and diastolic blood pressure, while only modest effects were seen at sodium intake levels between 3 and 5 g/day. In the Dietary Approaches to Stop Hypertension (DASH) trial, participants were provided meals over 30 days to accomplish moderate (3.3 g/day), low (2.5 g/day), and very low (1.5 g/day) levels of sodium intake. Low and very low levels of sodium intake reduced blood pressure significantly, though the short-term duration of the study precluded data on long-term morbidity and mortality risk (36).
However, the competing risk of renin-angiotensin-aldosterone system (RAAS) activation has led to the belief that low levels of sodium intake (<3 g/day) are also likely detrimental (37). Indeed, the PURE study also demonstrated that low ESI was associated with higher risk for cardiovascular events and mortality, though observational follow-up data from the TOHP trials showed the effect between ESI and outcomes is linear. Therefore the optimal level of sodium remains unclear. To balance the potential risk of RAAS activation at low sodium intake levels with elevated blood pressure at high sodium intake levels, some have advocated a goal sodium intake of 3–4 g/day (8). We show in this analysis that strain appears to decrease roughly around 3.7 g/day, which supports this viewpoint. These findings are significant, since only a small fraction of patients reach more conservative goals. Practically, since the majority of sodium is consumed through packaged and processed foods, low sodium dietary intake has been difficult to achieve (8). For instance, in the InterMAP study, the mean sodium intake was >3 g/day for women and >4 g/day for men in the United States; globally, sodium intake averages roughly 4 g/day (38). Additionally, in TOHP-II, the intervention group (randomized to sodium intake <1.8 g/day) only achieved a mean ESI of 2.5 g/day by 6 months and 3.1 g/day by 36 months (39). Therefore, long-term reduction in sodium intake to low levels is difficult to achieve even in a clinical trial setting. Nevertheless, future clinical trials of lowering sodium intake could use speckle-tracking echocardiography as an intermediate endpoint to examine the causal relationship between lowering ESI to < 3–4 g/day and improvement in strain and STe′.
Strengths of our study include the large number of participants and comprehensive adjustment for several potential confounders, the inclusion of a large number of African Americans, and the novel measurement of myocardial strain with speckle-tracking echocardiography to understand the mechanism between ESI and adverse cardiovascular events. Certain limitations should be considered when interpreting our results. First, sodium intake was estimated using overnight urine samples, while 24-hour urine collection is considered the gold standard. However, such testing is not feasible in large epidemiologic studies, such as HyperGEN, given challenges with compliance (8). Next, due to the cross-sectional nature of our study, we were unable establish causality. Additionally, previous studies have suggested J-shaped relationships between ESI and cardiovascular events. Indeed, low sodium may lead to excess RAAS activation with resultant detrimental physiologic effects (37). However, very few participants had a low level of estimated urinary sodium excretion (<2 g/day), and thus we were underpowered to detect such a relationship.
Conclusions
In summary, in one of the largest speckle-tracking studies to date and the first to examine the relationship between ESI (derived from measured urinary sodium values) and strain, we found that an ESI of >3.7 g/day was associated with adverse strain and STe′. Systolic blood pressure and serum aldosterone explained a significant proportion of the indirect effects between ESI and strain and STe′. These data provide support for the adverse cardiovascular effects (including direct myocardial effects) of high sodium intake.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Daily sodium intake more than 3.7 g is associated with abnormal cardiac mechanics, but this relationship is mediated only partially by systolic blood pressure and serum aldosterone levels.
Translational Outlook
Future studies should investigate the mechanism by which dietary sodium impairs myocardial function and seek therapeutic interventions that favorably influence clinical outcomes.
Acknowledgments
Funding: This study was funded by grants from the National Institutes of Health (R01 HL107577 and R01 HL127028 to S.J.S., and R01 HL55673-19 to D.K.A.). The HyperGEN parent study was funded by cooperative agreements (U10) with the National Heart, Lung, and Blood Institute: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515
ABBREVIATIONS
- EF
Ejection Fraction
- ESI
Estimated Sodium Intake
- LV
Left Ventricle
- RAAS
Renin-Angiotensin-Aldosterone System
- STe′
Speckle-Tracking e′ Velocity
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mente A, O’Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens. 2014;32:1005–14. doi: 10.1097/HJH.0000000000000122. discussion 1015. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 4.Guideline: Sodium Intake for Adults and Children. Geneva: 2012. [PubMed] [Google Scholar]
- 5.Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–37. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 6.Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–11. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 7.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2014;16:394–402. doi: 10.1002/ejhf.56. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116:1046–57. doi: 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- 9.Shah SJ, Aistrup GL, Gupta DK, et al. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H88–100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar FG, Selvaraj S, Martinez EE, et al. Archeological Echocardiography: Digitization and Speckle Tracking Analysis of Archival Echocardiograms in the HyperGEN Study. Echocardiography. 2016;33:386–97. doi: 10.1111/echo.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraj S, Martinez EE, Aguilar FG, et al. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.115.004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvaraj S, Aguilar FG, Martinez EE, et al. Association of comorbidity burden with abnormal cardiac mechanics: findings from the HyperGEN study. J Am Heart Assoc. 2014;3:e000631. doi: 10.1161/JAHA.113.000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz DH, Selvaraj S, Aguilar FG, et al. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129:42–50. doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams RR, Rao DC, Ellison RC, et al. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan K, Devereux RB, Rao D, et al. Adducin 1 (alpha) Gly460Trp variant is associated with left ventricular geometry in Caucasians and African Americans: The HyperGEN Study. Int J Mol Epidemiol Genet. 2010;1:367–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri V, Dahlof B, DeQuattro V, et al. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–32. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Roman MJ, de Simone G, et al. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997;96:1416–23. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 21.Galema TW, Geleijnse ML, Yap SC, et al. Assessment of left ventricular ejection fraction after myocardial infarction using contrast echocardiography. Eur J Echocardiogr. 2008;9:250–4. doi: 10.1016/j.euje.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Peteiro J, Pinon P, Perez R, Monserrat L, Perez D, Castro-Beiras A. Comparison of 2- and 3-dimensional exercise echocardiography for the detection of coronary artery disease. J Am Soc Echocardiogr. 2007;20:959–67. doi: 10.1016/j.echo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Negishi K, Lucas S, Negishi T, Hamilton J, Marwick TH. What is the primary source of discordance in strain measurement between vendors: imaging or analysis? Ultrasound Med Biol. 2013;39:714–20. doi: 10.1016/j.ultrasmedbio.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Musch MW, Lucioni A, Chang EB. Aldosterone regulation of intestinal Na absorption involves SGK-mediated changes in NHE3 and Na+ pump activity. Am J Physiol Gastrointest Liver Physiol. 2008;295:G909–19. doi: 10.1152/ajpgi.90312.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djousse L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovascular disease and death in women: potential mediating mechanisms. Circulation. 2009;120:237–44. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobian L, Lange J, Ulm K, Wold L, Iwai J. Potassium reduces cerebral hemorrhage and death rate in hypertensive rats, even when blood pressure is not lowered. Hypertension. 1985;7:I110–4. doi: 10.1161/01.hyp.7.3_pt_2.i110. [DOI] [PubMed] [Google Scholar]
- 27.Chang HY, Hu YW, Yue CS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–96. doi: 10.1093/ajcn/83.6.1289. [DOI] [PubMed] [Google Scholar]
- 28.Boegehold MA. Microvascular changes associated with high salt intake and hypertension in Dahl rats. Int J Microcirc Clin Exp. 1993;12:143–56. [PubMed] [Google Scholar]
- 29.Frisbee JC, Lombard JH. Acute elevations in salt intake and reduced renal mass hypertension compromise arteriolar dilation in rat cremaster muscle. Microvasc Res. 1999;57:273–83. doi: 10.1006/mvre.1998.2138. [DOI] [PubMed] [Google Scholar]
- 30.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 31.Acelajado MC, Pimenta E, Calhoun DA. Salt and aldosterone: a concert of bad effects. Hypertension. 2010;56:804–5. doi: 10.1161/HYPERTENSIONAHA.110.160960. [DOI] [PubMed] [Google Scholar]
- 32.du Cailar G, Fesler P, Ribstein J, Mimran A. Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin-angiotensin system. Hypertension. 2010;56:865–70. doi: 10.1161/HYPERTENSIONAHA.110.159277. [DOI] [PubMed] [Google Scholar]
- 33.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 34.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. quiz 453–5. [DOI] [PubMed] [Google Scholar]
- 35.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 36.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 37.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011:CD004022. doi: 10.1002/14651858.CD004022.pub3. [DOI] [PubMed] [Google Scholar]
- 38.Zhou BF, Stamler J, Dennis B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17:623–30. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.