Abstract
The incidence of type 1 diabetes has risen considerably in the past 30 years due to changes in the environment that have been only partially identified. In this Series paper, we critically discuss candidate triggers of islet autoimmunity and factors thought to promote progression from autoimmunity to overt type 1 diabetes. We revisit previously proposed hypotheses to explain the growth in the incidence of type 1 diabetes in light of current data. Finally, we suggest a unified model in which immune tolerance to β cells can be broken by several environmental exposures that induce generation of hybrid peptides acting as neoautoantigens.
Introduction
The incidence of type 1 diabetes has increased by several times over the past 30 years.1 This increase can only be explained by changes in environment or lifestyle. Supporting the impact of environment or lifestyle on risk, migrants tend to acquire the same risk of type 1 diabetes as the population in their new area of residence.2, 3 In Europe, the risk of type 1 diabetes differs substantially in people who are genetically close but separated by socioeconomic borders.4 This risk has become more homogeneous within populations with free movement of people and trade.5 Improved understanding for the environmental determinants of type 1 diabetes could make it possible to prevent or delay the disease.
Type 1 diabetes is diagnosed after onset of overt hyperglycaemia;6 however, evidence is mounting that islet autoimmunity is the first stage of the disease.7 Islet autoimmunity is defined by the persistent presence of autoantibodies to pancreatic islet antigens. Islet immunity usually starts in early childhood, with incidence peaking in the second year of life,8–10 and can have a remitting-relapsing course before onset of diabetes.11 Development of two or more islet autoantibodies (to insulin, glutamic acid decarboxylase [GAD], insulinoma-associated antigen 2, or zinc transporter-8 [ZnT8]) marks a point of no return from which 70% of children progress to diabetes over the next 10 years.12 Prospective birth cohorts9, 10, 13–15 have helped to identify potential triggers of islet autoimmunity and the natural history of progression to diabetes. Candidate triggers include infections, diet, and toxins that affect children in utero, perinatally, or during early childhood. These triggers need to be recorded prospectively in studies rather than recalled retrospectively at the time of diabetes diagnosis, several years later.
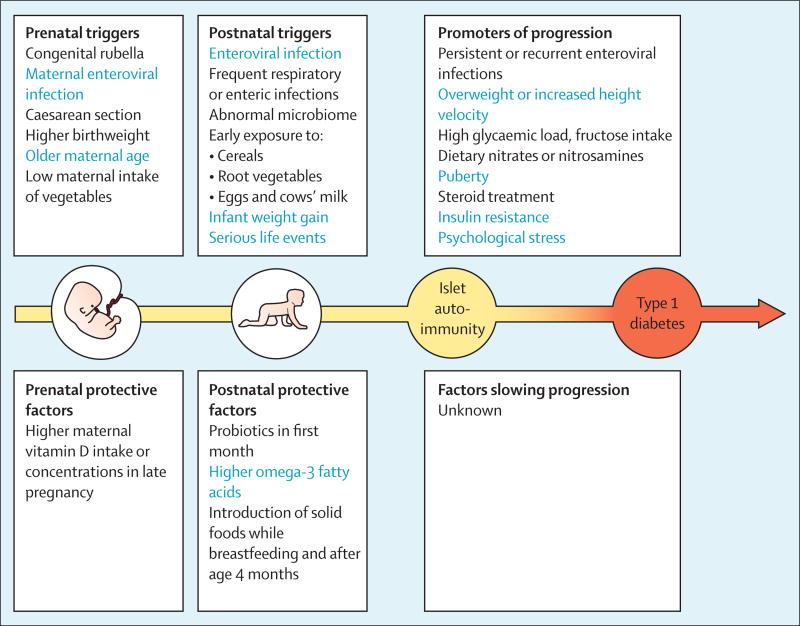
In paper one of this Series on development of type 1 diabetes, Pociot and Lernmark16 summarise factors for genetic susceptibility and resistance to type 1 diabetes. In this paper, we discuss candidate triggers of islet autoimmunity and factors thought to promote progression from autoimmunity to overt type 1 diabetes (figure 1). These factors seem to have their effect mainly in the genetically predisposed individuals. We also review data for candidate environmental factors that have not yet been confirmed to predict islet autoimmunity or type 1 diabetes or might have weak or confounding effect.
Figure 1.
Environmental triggers and protective factors for islet autoimmunity and promoters of progression to type 1 diabetes for which an association has been suggested
Triggers and factors with the strongest evidence base are shown in blue.
Infections
Early ecological reports,17 seroepidemiological studies,18 and case reports19 have drawn attention to viral infections as a potential cause of type 1 diabetes. Bacterial infections are rarely discussed, although bacteria as a cause of pancreatic lesions cannot be excluded. Several viruses have been implicated, with enteroviruses having the strongest evidence from studies in animal models20 and in human beings.21 These viruses have a tropism to human pancreatic islets in vivo and in vitro,19, 22 and have been detected in the pancreas of patients recently diagnosed with type 1 diabetes. Recent findings consistent with a persistent enteroviral infection in patients at diagnosis of type 1 diabetes include: more frequent detection of enteroviral VP1 protein immunoreactivity in the β cells of children with type 1 diabetes than in age-matched controls;23, 24 VP1 expression in β-cells that also produce elevated levels of PKR consistent with degradation of myeloid cell factor 1 (MCL1) and higher susceptibility of the cells to apoptosis;25 and that double-stranded RNA (dsRNA) and elevated levels of MDA5 in the β cells of patients with type 1 diabetes.26 An intriguing line of evidence suggests that enteroviral infections during pregnancy might result in persistent infection and islet autoimmunity in the mother27 and off spring.28, 29 A plausible mechanism for persistence of enteroviruses in the pancreatic islets has been proposed from studies of enteroviral myocarditis.30, 31 A spontaneous deletion of 22–36 nucleotides in the 5′ non-translated region of the virome resulted in lowgrade persistence of the defective virus that was unable to cause cytopathic damage, but was able to slowly replicate. Evidence for a similar pathomechanism in islet autoimmunity has yet to be reported. Whether enteroviruses act as triggers of islet autoimmunity, promoters of progression to type 1 diabetes, or non-specific precipitating stressors is an unsettled issue.32–37 Interestingly, an in-vitro study suggested that persistent enteroviral infection of human pancreatic ductal cells might diminish their ability to transdifferentiate into β cells, thus slowing down β-cell mass decline due to autoimmunity.38
Intestinal microbiota
Some of the candidate environmental factors for type 1 diabetes (eg, caesarean delivery, early childhood diet, and use of antibiotics) are intertwined with the development and function of the human microbiome. Gut microbes influence lipid and glucose metabolism, as well as immunity and systemic inflammation outside of the intestine.39–41 Commensal microbiota might modulate the risk of type 1 diabetes,39, 42–48 but studies so far have been underpowered and focused on taxa diversity. Some have reported lower microbial diversity in children with islet autoimmunity before progression to diabetes, compared with healthy controls.42, 43, 48 Larger studies are needed that use whole-genome sequencing of the microbiome at multiple timepoints before diagnosis and use carefully selected controls.
Vaccines
There has been speculation that vaccines might trigger autoimmunity, but no association has been detected with islet autoimmunity49, 50 or type 1 diabetes.51, 52 A recent meta-analysis of 23 studies investigating 16 vaccinations53 concluded that childhood vaccines do not increase the risk of type 1 diabetes. The BCG vaccine has attracted some interest as a potential immune-modulator that could theoretically reduce the incidence of autoimmunity. Clinical data have shown no association between BCG vaccination and type 1 diabetes or islet autoimmunity. A 20 year follow-up of the 1974 Canadian birth cohort, of which 45% were given BCG in the first year of life, showed no association with type 1 diabetes,54 nor did case-control studies from Canada55 and Sweden.56 The German BABYDIAB study57 reported no association between BCG vaccination and development of islet autoimmunity. In addition, in two clinical trials, vaccination with BCG at diagnosis of type 1 diabetes did not preserve β-cell function.58, 59
Hygiene hypothesis
The hygiene hypothesis posits that incidence of autoimmune diseases might be rising because of a decreasing frequency of childhood infections due to improved hygiene.60–62 However, a UK population-based study showed that infections in early life, routinely recorded by family doctors, were not associated with subsequent childhood type 1 diabetes.63 On the other hand, prospective studies have reported a significant increase in the risk of islet autoimmunity with more frequent respiratory infections during the first 6 months of life; the association was weaker for infections age 6–12 months and absent for those beyond 1 year of age.64 Similar results were reported from Norway,65 whereas the DAISY study37 in Colorado reported an association between islet autoimmunity and early childhood gastrointestinal infections, but not respiratory infections. In summary, prospective studies generally do not support the hygiene hypothesis for type 1 diabetes. It has been hypothesised that in countries with the highest incidence of type 1 diabetes, increased hygiene and sanitation resulted in a decline in herd immunity to enteroviruses among pregnant women, exposing fetuses and newborn babies to prenatal or infant enteroviral infections.66 Although direct evidence for this effect in human beings is not established, in animal models virus-induced diabetes can be prevented in off spring by infecting mothers with the same virus before pregnancy.67
Dietary factors
Breastfeeding
Although some retrospective studies showed a small reduction in the risk of type 1 diabetes with breastfeeding, all but one, ABIS in Sweden,68 of the prospective birth cohort studies failed to find a protective effect.13, 14, 69, 70 Nevertheless, children who were still breastfed at the time of introduction to cereals had a reduced risk of islet autoimmunity,13 and type 1 diabetes.71 These findings suggest that breastfeeding might play a protective role in the relationship between dietary factors and type 1 diabetes.
Cows’ milk
Most prospective birth cohort studies have not shown any link between early exposure to cows’ milk and either islet autoimmunity13, 14, 68, 69 or type 1 diabetes.71 In a double-blind, randomised trial (TRIGR Pilot II), 230 infants at genetically increased risk for type 1 diabetes who received a casein hydrolysate formula whenever breastmilk was not available had lower risk of islet autoimmunity than had those given cows’ milk-based formula during the first 6–8 months of life.72 Unfortunately, the larger phase 3 TRIGR study could not confirm this effect on islet autoimmunity;73 follow-up of the study participants for type 1 diabetes continues.
Studies exploring the role of cows’ milk consumption later in childhood on the risk for islet autoimmunity and type 1 diabetes have also produced contradictory results. Cows’ milk intake in childhood has been associated with both an increased risk of islet autoimmunity74, 75 and type 1 diabetes,76, 77 as well as a decreased risk of type 1 diabetes.78 A higher cows’ milk intake might promote progression to type 1 diabetes in children with islet autoimmunity,79 an effect that could be mediated by certain fatty acids present in cows’ milk and meats—eg, myristic, penta-decanoic, monounsaturated palmitoleic acid isomers 16:1 omega-7 and 16:1 omega-9, and conjugated linoleic acid.80 If confirmed, this observation could inform a new line of dietary interventions to prevent type 1 diabetes.
Solid foods and cereals
In DAISY, the timing of introduction of any type of cereal (gluten and non-gluten containing) was associated with an increased risk of islet autoimmunity in a U-shaped relationship with nadir at introduction at 4–6 months of life.13 BABYDIAB showed association only with early exposure to gluten,14 ABIS with gluten introduced late,74 and the Finnish DIPP study found no clear association with gluten.70 In Finland, DIPP reported that introduction of root vegetables by age 4 months doubled the risk of islet autoimmunity compared with later introduction and also that first exposure to egg before age 8 months was associated with an increased risk of islet autoimmunity.70 ABIS showed that less than daily consumption of vegetables (3–5 times per week) in the mothers’ diet was associated with increased risk of islet autoimmunity.81 Cross-study differences might be related to country differences in infant nutrition, and there is a risk of false positive associations caused by multiple comparisons. Therefore, results of these studies must be interpreted with caution.
Examination of dietary exposures and clinical type 1 diabetes also gives divergent results. In DAISY,71 young (<4 months) exposure to fruit and late (age ≥6 months) first exposure to any solid food, including rice, predicted development of type 1 diabetes. However, BABYDIAB82 found only weak associations to early gluten introduction. In aggregate, these studies lend support to the idea that general antigenic stimulation is more important than is the actual antigen in this disease process. This association might be due to immature immune response and the gut. The increased risk by late exposure to solid foods might be related to the larger amounts given at initial exposure to older children, nutrient deficiencies, or the cessation of breastfeeding before solid foods are introduced, resulting in a loss of the protective effect of breastmilk at the introduction of foreign food antigens. A randomised trial of gluten-free diet at 6–12 months of age did not reduce the development of islet autoimmunity in genetically high-risk infants83 and it did not decrease the levels of islet autoantibodies in children with established islet autoimmunity.84
Vitamin D
Vitamin D has been examined as a potentially protective factor because it has an active role in the regulation of the immune system, as well as metabolic pathways relevant to diabetes. Vitamin D has also been shown to shift the balance of the body’s T-cell response toward downregulation of the T-helper-1 immune response. The seasonality of birth in children with type 1 diabetes and the seasonal pattern at diagnosis of type 1 diabetes could be explained by seasonal variation in vitamin D production from exposure to the sun. In Belgium, the monthly averages of daily hours of sunshine were inversely related to the number of new patients with type 1 diabetes per month.85 However, meta-analysis of observational studies of vitamin D intake during pregnancy showed no effect on the incidence of type 1 diabetes.86 A Norwegian study found an association between higher serum 25-hydroxyvitamin D in late pregnancy and lower risk of type 1 diabetes in off spring,87 but a Finnish study found no such association between concentrations of the molecule in the first trimester of pregnancy and the risk of type 1 diabetes in babies.88
Two meta-analyses of retrospective studies showed that the risk of type 1 diabetes was lower in infants who were supplemented with vitamin D (calcitriol) compared with those who were not supplemented (pooled odds ratio 0·71).88, 89 DAISY examined plasma 25-hydroxyvitamin D concentrations in infancy and throughout childhood and found no association with islet autoimmunity or progression to type 1 diabetes.90 Dietary intake of vitamin D (from food and supplements) was also not associated with islet autoimmunity or progression to type 1 diabetes90 and the ABIS prospective study found no association between an intermediate dose of vitamin D supplementation during infancy and development of islet autoimmunity.78 Two clinical trials reported no effect of vitamin D supplementation on sustained insulin production in new-onset type 1 diabetes.91, 92 In summary, despite continuing interest in vitamin D supplementation as a potential intervention to prevent islet autoimmunity and type 1 diabetes, there is surprisingly little supporting evidence from prospective birth cohort studies.
Polyunsaturated fatty acids
Long-chain polyunsaturated fatty acids, specifically omega-3 fatty acids, affect inflammatory responses. A relative deficiency of omega-3 fatty acids, characteristic of many western diets, might predispose to inflammatory reactions. In Norway, a prospective study found no association between risk of type 1 diabetes in babies and docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and other fatty acid concentrations in the phospholipid fraction of maternal serum collected in late pregnancy.93 Among Finnish children, lower serum linoleic acid (an omega-6 fatty acid) concentrations, but not DHA or EPA concentrations, were associated with increased risk of islet autoimmunity.80 Interestingly, in a US study, higher omega-3 fatty acid intake during childhood and higher omega-3 fatty acid in the erythrocyte membrane predicted a lower risk of islet autoimmunity.94 However, it can be noted that the omega-3- fatty acid intake is even lower in the diet of certain areas of the world (eg, India), than in western diets, without any observed relation to development of type 1 diabetes.
Toxins and chemical compounds
Toxins in foods or water might activate autoimmune mechanisms in genetically susceptible individuals, and exposure to toxins might result in pancreatic islet cell death. The list of elements, man-made chemicals, and naturally occurring mycotoxins associated with type 1 diabetes is long and the evidence too preliminary to review here. Some circumstantial and ecological95 evidence suggests a connection between type 1 diabetes and water containing nitrates, nitrites, or nitrosamines, although other studies show no or contradictory associations.96–98 In a case-control study in Sweden, type 1 diabetes was associated with consuming higher amounts of foods containing nitrosamines and nitrates or nitrites99 and in the ABIS, water samples from families with a child with type 1 diabetes had higher concentrations of nitrate than did water samples from control families.100 However in Germany, water concentrations of nitrate and nitrite were not associated with risk of either islet autoimmunity or progression to type 1 diabetes.101
Birthweight and infant growth
Higher birthweight102, 103 and rapid weight gain during age 12–18 months104, 105 have been linked to type 1 diabetes. The magnitude of effect is modest and the associations have been noted in Scandinavian countries, but not in the USA or Germany.106, 107 The accelerator hypothesis proposes that excess weight gain leads to insulin resistance in early childhood and could initiate islet autoimmunity, eventually leading to type 1 diabetes.108 Although there is little evidence for the hypothesis,109 insulin resistance and rising blood glucose (glucotoxicity) might accelerate β-cell apoptosis directly or by inducing β-cell neoautoantigens in genetically predisposed people; rapid growth might increase insulin demand causing β-cell stress and increased presentation of autoantigens.
β-cell stress
Although the accelerator hypothesis mainly relates to rapid growth, the β-cell stress hypothesis proposes that factors causing increased insulin demand, such as rapid growth, overweight, puberty, low physical activity, trauma, infections, and glucose overload,110 might play an important role in development of type 1 diabetes.111 Serious life events (eg, divorce or death in the family) as shown in the ABIS study, might increase the risk of islet autoimmunity112, 113 and type 1 diabetes.15 Psychological stress not only increases insulin resistance leading to increase demand on the β cells, but stress (eg, via increased cortisol concentrations) might also directly influence the immune response.
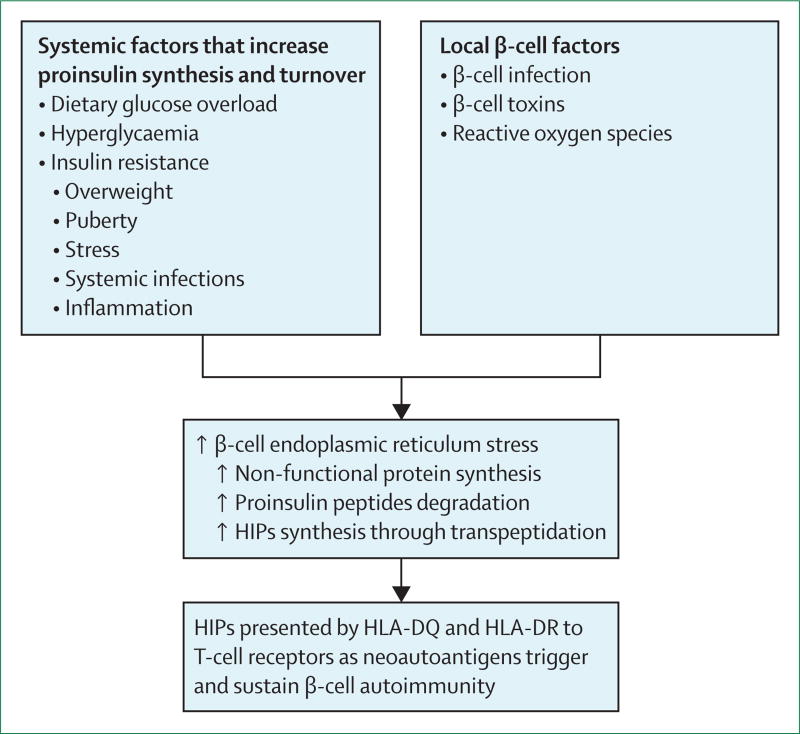
Prolonged endoplasmic reticulum stress impairs insulin synthesis and causes pancreatic β-cell apoptosis.114 In turn, reduced insulin production relieves endoplasmic reticulum stress and induces β-cell proliferation.115 Furthermore, endoplasmic reticulum stress might increase abnormal post-translational modification of endogenous β-cell proteins (figure 2).116 The findings summarised in the next section might provide the link between multiple environmental stressors of β cells and initiation of the autoimmune process.
Figure 2.
A unified model of the relationship between environmental factors, β-cell endoplasmic reticulum stress, generation of neoautoantigens (HIPs), and loss of immune tolerance that triggers islet autoimmunity
HIP=hybrid insulin peptide.
Post-translational modification and neoautoantigens
Physiological states related to oversecretion of insulin might promote generation of neoautoantigens via post-translational modification of islet proteins (eg, proinsulin, chromogranin A, islet amyloid polypeptide [IAPP], and GAD; figure 2). Although post-translational modification plays a role in other autoimmune diseases, such as coeliac disease, multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, or berylliosis,117 it has only recently become the subject of systematic studies of type 1 diabetes.118–124 One of the strongest lines of evidence for the primary role of post-translational modification in development of type 1 diabetes in non-obese diabetic (NOD) mice, as well as in human beings, comes from the discovery of a new class of naturally occurring autoantigens called hybrid insulin peptides (HIPs).121–124 HIPs are formed through transpeptidation of the C-terminal carbonyl groups of C-peptide fragments with N-terminal amino groups of peptides derived from chromogranin or IAPP, other β-cell prohormonal secretory granule proteins. HIP-reactive CD4 cells have been identified in both NOD123, 124 and human124 pancreas cells, suggesting that this mechanism might be responsible for the loss of self-tolerance in type 1 diabetes.
Molecular crowding of peptides derived from proinsulin, chromogranin A, and IAPP in secretory granules might promote formation of HIPs. In addition to the products of natural processing of these proteins by prohormone convertases and carboxypeptidase E, products generated by lysosomal proteases during granule turnover could also be involved.123 The role of IAPP in type 1 diabetes is unclear at present. Increased plasma concentrations of IAPP have been noted at diagnosis in some patients with type 1 diabetes;125 intra-β-cell amyloid formation commonly seen in type 2 diabetes can also be found in patients with type 1 diabetes.126
Heterogeneity of type 1 diabetes
The patterns of insulitis (ie, infiltration of the islets by CD8, CD4, and CD20 lymphocytes) in people with type 1 diabetes seem to differ by age at diagnosis. Insulitis shows high proportion of CD20 B cells in patients diagnosed before the age of 7 years, mixed pattern at age 7–13 years, and low CD20 content after age 13 years.127, 128 Importantly, patients diagnosed when older than 13 years retain about 40% of residual insulin-containing islets at diagnosis, consistent with the observed higher rates of partial remission of insulin dependency and the favourable results of immunomodulatory interventions. The reasons for spontaneous remission and reactivation of the autoimmune process are unclear. Reactivations of persistent viral infection or recurrent infections of the islets24, 26, 129 and of the exocrine pancreas38, 130 might be a plausible mechanism. Increases in insulin resistance due to puberty, weight gain, infections, or stress could also play a role.15, 109 In addition to age of diagnosis, HLA-DR-DQ genotype, pattern of islet autoantibodies development, and ethnic origin have been reported to be associated with variable diabetic phenotypes,8–10 although there seems to be a large overlap between potential categories. The process of systematic aetiological classification of subtypes of type 1 diabetes has just begun.131, 132
Discussion
There is no shortage of hypotheses to explain the rise in the incidence of type 1 diabetes in most of the world. The accelerator and β-cell stress hypotheses propose that several unspecific environmental factors, for example, overweight, fast growth, infections, dietary deficiencies or psychological stress, alone or in combination, might make pancreatic β cells exhausted and eventually fail due to a secondary autoimmune destruction. For a long time, these models lacked a convincing biological mechanism linking β cell stress to autoimmunity. The recent discovery of HIPs might provide such a link. The peptides are likely to be produced by exhausted β cells and can act as powerful neoantigens that initiate β-cell-specific autoimmunity in a susceptible host. There are similarities between this model and the aetiology of coeliac disease for which the immune-dominant peptides are also post-translationally modified. However, these peptides originate from among hundreds of proteins (gliadins, glutenins, hordein, secalins, and avenins) present in wheat, rye, barley, and oats, whereas HIPs are thought to be byproducts of degradation of insulin and other β-cell hormones and thus are truly neoautoantigens.
The hygiene hypothesis postulates that fewer early childhood infections and infestations and less diverse symbiotic gut flora deviate the immune system towards autoimmunity and atopy. A variant of the hygiene hypothesis specifically blames decreased herd immunity to enteroviruses for the rise in incidence of type 1 diabetes. The detection in pancreatic biopsies and post-mortem samples of material consistent with slowly-progressing persistent enteroviral infection of the β cells has provided new credence to this scenario.
The evidence is growing to support heterogeneity of type 1 diabetes by age, genotype, the autoimmune phenotype, and pathomorphology of the pancreatic islet. The combination or sequence of the causal environmental exposures might vary among individuals as well as over time within a population. This heterogeneity might explain inconsistent, sometimes even contradictory, observations from different populations. Importantly, environmental factors that trigger islet autoimmunity might differ from those that promote progression from autoimmunity to overt diabetes.
Continuing and future studies to define environmental causes of type 1 diabetes will benefit from wide representation of children from different areas of the world, with and without first-degree relatives with diabetes, and a large sample size that allows for stratified and pooled analyses to understand what local genetic and environmental factors are important and which are universal. In addition to the current focus on genomics and metagenomics, studies are beginning to evaluate metabolomics and proteomic markers that could reveal novel pathways involved in pathogenesis of type 1 diabetes and inform future interventions. The rise in type 1 diabetes is compatible with an epigenetic effect amplified over generations, but so far evidence is scant. Additional opportunities might lie in increased understanding of β-cell biology, including nerve supply, vascular supply, islet cell interplay, and the relationship to the exocrine pancreas.
Prevention of type 1 diabetes will be more feasible if modifiable checkpoints are shared by most pathways to diabetes. For instance, if β-cell stress-generated neoautoantigens are the major mechanism of loss of tolerance, intervention at this point might be more feasible than eradication of some causes of β-cell stress. If persistent viral infection of β cells is one of the common pathways, then vaccination might confer only partial benefit. Addition of antiviral or immune interventions might be needed to stop or delay the autoimmune process and preserve more β-cell function. Combined with modern devices and advanced insulins, these advances would lessen the heavy burden associated with type 1 diabetes.
Search strategy and selection criteria.
We identified papers through searches of PubMed with search terms “type 1 diabetes”, “autoimmunity”, “environmental”, “trigger”, and “promoter” and also from references from relevant articles. We did not include abstracts and reports from meetings. We included only articles published in English between 1960 and Jan 31, 2016.
Acknowledgments
This paper was supported by the National Institutes of Health grant DK32493 (MR), by the Juvenile Diabetes Research Foundation grant 17-2013-535 (MR), by Swedish Child Diabetes Foundation (Barndiabetesfonden; JL), by JDF-Wallenberg (K98-99JD-12813-01A; JL), the Swedish Medical Research Council (MFR) K99-72X-11242-05A (JL), and the Research Council of Southeast Sweden (JL)
Footnotes
Contributors
MR carried out literature search, provided figures and wrote parts of the Review. JL outlined the structure of the paper, added some references and contributed to data interpretation and writing.
Declaration of interests
We declare no competing interests.
Contributor Information
Marian Rewers, Barbara Davis Center for Diabetes, University of Colorado School of Medicine, Aurora, CO, USA.
Johnny Ludvigsson, Division of Pediatrics, Department of Clinical and Experimental Medicine, Medical Faculty, Linköping University and Linköping University Hospital, Linköping, Sweden.
References
- 1.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G for the EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 2.Söderström U, Aman J, Hjern A. Being born in Sweden increases the risk for type 1 diabetes—a study of migration of children to Sweden as a natural experiment. Acta Paediatr. 2012;101:73–77. doi: 10.1111/j.1651-2227.2011.02410.x. [DOI] [PubMed] [Google Scholar]
- 3.Oilinki T, Otonkoski T, Ilonen J, Knip M, Miettinen PJ. Prevalence and characteristics of diabetes among Somali children and adolescents living in Helsinki, Finland. Pediatr Diabetes. 2012;13:176–80. doi: 10.1111/j.1399-5448.2011.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Kondrashova A, Reunanen A, Romanov A, et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. 2005;37:67–72. doi: 10.1080/07853890410018952. [DOI] [PubMed] [Google Scholar]
- 5.Jarosz-Chobot P, Polanska J, Szadkowska A, et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia. 2011;54:508–15. doi: 10.1007/s00125-010-1993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. (2)Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl):S8–16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 7.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–74. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler AG, Bonifacio E for the BABYDIAB-BABYDIET Study Group. Age-related islet autoantibody incidence in off spring of patients with type 1 diabetes. Diabetologia. 2012;55:1937–43. doi: 10.1007/s00125-012-2472-x. [DOI] [PubMed] [Google Scholar]
- 9.Ilonen J, Hammais A, Laine AP, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62:3636–40. doi: 10.2337/db13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krischer JP, Lynch KF, Schatz DA, et al. for the TEDDY Study Group. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–87. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–94. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–79. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–20. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–28. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 15.Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. 2015;58:1188–97. doi: 10.1007/s00125-015-3555-2. [DOI] [PubMed] [Google Scholar]
- 16.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–39. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen E. Is diabetes of infectious origin? J Infect Dis. 1927;41:197–202. [Google Scholar]
- 18.Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. BMJ. 1969;3:627–30. doi: 10.1136/bmj.3.5671.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–79. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 20.Coppieters KT, Wiberg A, Tracy SM, von Herrath MG. Immunology in the clinic review series: focus on type 1 diabetes and viruses: the role of viruses in type 1 diabetes: a diffi cult dilemma. Clin Exp Immunol. 2012;168:5–11. doi: 10.1111/j.1365-2249.2011.04554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stene LC, Rewers M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin Exp Immunol. 2012;168:12–23. doi: 10.1111/j.1365-2249.2011.04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tracy S, Drescher KM, Chapman NM. Enteroviruses and type 1 diabetes. Diabetes Metab Res Rev. 2011;27:820–23. doi: 10.1002/dmrr.1255. [DOI] [PubMed] [Google Scholar]
- 23.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–51. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 24.Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64:1682–87. doi: 10.2337/db14-1370. [DOI] [PubMed] [Google Scholar]
- 25.Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56:185–93. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 26.Morgan NG, Richardson SJ. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol Metab. 2014;25:611–19. doi: 10.1016/j.tem.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 27.RešićLindehammer S, Honkanen H, Nix WA, et al. Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 2012;25:254–61. doi: 10.1089/vim.2012.0022. [DOI] [PubMed] [Google Scholar]
- 28.Dahlquist GG, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM A population-based case-control study. Diabetes. 1995;44:408–13. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 29.Viskari H, Knip M, Tauriainen S, et al. Maternal enterovirus infection as a risk factor for type 1 diabetes in the exposed off spring. Diabetes Care. 2012;35:1328–32. doi: 10.2337/dc11-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smithee S, Tracy S, Chapman NM. Mutational disruption of cis-acting replication element 2C in Coxsackievirus B3 leads to 5′-terminal genomic deletions. J Virol. 2015;89:11761–72. doi: 10.1128/JVI.01308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman NM, Kim KS, Drescher KM, Oka K, Tracy S. 5′ terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology. 2008;375:480–91. doi: 10.1016/j.virol.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and , other enterovirus infections in the pathogenesis of IDDM Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–57. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 33.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes. 2000;49:708–11. doi: 10.2337/diabetes.49.5.708. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz MS, Fine C, Ilic A, Sarvetnick N. Requirements for viral-mediated autoimmune diabetes: beta-cell damage and immune infi ltration. J Autoimmun. 2001;16:211–17. doi: 10.1006/jaut.2000.0486. [DOI] [PubMed] [Google Scholar]
- 35.Christen U, Edelmann KH, McGavern DB, et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–98. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329:381–94. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Stene LC, Oikarinen S, Hyöty H, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–80. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sane F, Caloone D, Gmyr V, et al. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell Mol Life Sci. 2013;70:4169–80. doi: 10.1007/s00018-013-1383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 41.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–55. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Goffau MC, Luopajärvi K, Knip M, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238–44. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murri M, Leiva I, Gomez-Zumaquero JM, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endesfelder D, zuCastell W, Ardissone A, et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. 2014;63:2006–14. doi: 10.2337/db13-1676. [DOI] [PubMed] [Google Scholar]
- 46.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gülden E, Wong FS, Wen L. The gut microbiota and Type 1 Diabetes. Clin Immunol. 2015;159:143–53. doi: 10.1016/j.clim.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kostic AD, Gevers D, Siljander H, et al. for the DIABIMMUNE Study Group. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–73. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graves PM, Barriga KJ, Norris JM, et al. Lack of association between early childhood immunizations and beta-cell autoimmunity. Diabetes Care. 1999;22:1694–97. doi: 10.2337/diacare.22.10.1694. [DOI] [PubMed] [Google Scholar]
- 50.Hummel M, Füchtenbusch M, Schenker M, Ziegler AG. No major association of breast-feeding, vaccinations, and childhood viral diseases with early islet autoimmunity in the German BABYDIAB Study. Diabetes Care. 2000;23:969–74. doi: 10.2337/diacare.23.7.969. [DOI] [PubMed] [Google Scholar]
- 51.Jefferson T, Demicheli V. No evidence that vaccines cause insulin dependent diabetes mellitus. J Epidemiol Community Health. 1998;52:674–75. doi: 10.1136/jech.52.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Offit PA, Hackett CJ. Addressing parents’ concerns: do vaccines cause allergic or autoimmune diseases? Pediatrics. 2003;111:653–59. doi: 10.1542/peds.111.3.653. [DOI] [PubMed] [Google Scholar]
- 53.Morgan E, Halliday SR, Campbell GR, Cardwell CR, Patterson CC. Vaccinations and childhood type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2016;59:237–43. doi: 10.1007/s00125-015-3800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rousseau MC, El-Zein M, Conus F, Legault L, Parent ME. Bacillus Calmette-Guérin (BCG) vaccination in infancy and risk of childhood diabetes. Paediatr Perinat Epidemiol. 2016;30:141–48. doi: 10.1111/ppe.12263. [DOI] [PubMed] [Google Scholar]
- 55.Parent ME, Siemiatycki J, Menzies R, Fritschi L, Colle E. Bacille Calmette-Guérin vaccination and incidence of IDDM in Montreal, Canada. Diabetes Care. 1997;20:767–72. doi: 10.2337/diacare.20.5.767. [DOI] [PubMed] [Google Scholar]
- 56.Dahlquist G, Gothefors L. The cumulative incidence of childhood diabetes mellitus in Sweden unaffected by BCG-vaccination. Diabetologia. 1995;38:873–74. doi: 10.1007/BF03035306. [DOI] [PubMed] [Google Scholar]
- 57.Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal Bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204–06. doi: 10.2337/diacare.28.5.1204. [DOI] [PubMed] [Google Scholar]
- 58.Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes A randomized clinical study. Diabetes Care. 1999;22:1703–07. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 59.Elliott JF, Marlin KL, Couch RM. Effect of Bacille Calmette-Guérin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21:1691–93. doi: 10.2337/diacare.21.10.1691. [DOI] [PubMed] [Google Scholar]
- 60.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 61.Gale EA. A missing link in the hygiene hypothesis? Diabetologia. 2002;45:588–94. doi: 10.1007/s00125-002-0801-1. [DOI] [PubMed] [Google Scholar]
- 62.Kolb H, Elliott RB. Increasing incidence of IDDM a consequence of improved hygiene? Diabetologia. 1994;37:729. doi: 10.1007/BF00417700. [DOI] [PubMed] [Google Scholar]
- 63.Cardwell CR, Carson DJ, Patterson CC. No association between routinely recorded infections in early life and subsequent risk of childhood-onset Type 1 diabetes: a matched case-control study using the UK General Practice Research Database. Diabet Med. 2008;25:261–67. doi: 10.1111/j.1464-5491.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 64.Beyerlein A, Chmiel R, Hummel S, Winkler C, Bonifacio E, Ziegler AG. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194–95. doi: 10.2337/dc14-1208. [DOI] [PubMed] [Google Scholar]
- 65.Rasmussen T, Witsø E, Tapia G, Stene LC, Rønningen KS. Self-reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high-risk HLA genotype: the MIDIA study. Diabetes Metab Res Rev. 2011;27:834–37. doi: 10.1002/dmrr.1258. [DOI] [PubMed] [Google Scholar]
- 66.Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48:1280–87. doi: 10.1007/s00125-005-1780-9. [DOI] [PubMed] [Google Scholar]
- 67.Larsson PG, Lakshmikanth T, Svedin E, King C, Flodström-Tullberg M. Previous maternal infection protects off spring from enterovirus infection and prevents experimental diabetes development in mice. Diabetologia. 2013;56:867–74. doi: 10.1007/s00125-013-2834-z. [DOI] [PubMed] [Google Scholar]
- 68.Holmberg H, Wahlberg J, Vaarala O, Ludvigsson J for the ABIS Study Group. Short duration of breast-feeding as a risk-factor for beta-cell autoantibodies in 5-year-old children from the general population. Br J Nutr. 2007;97:111–16. doi: 10.1017/S0007114507210189. [DOI] [PubMed] [Google Scholar]
- 69.Couper JJ, Steele C, Beresford S, et al. Lack of association between duration of breast-feeding or introduction of cow’s milk and development of islet autoimmunity. Diabetes. 1999;48:2145–49. doi: 10.2337/diabetes.48.11.2145. [DOI] [PubMed] [Google Scholar]
- 70.Virtanen SM, Takkinen HM, Nevalainen J, et al. Early introduction of root vegetables in infancy associated with advanced β-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to Type 1 diabetes. Diabet Med. 2011;28:965–71. doi: 10.1111/j.1464-5491.2011.03294.x. [DOI] [PubMed] [Google Scholar]
- 71.Frederiksen B, Kroehl M, Lamb MM, et al. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY) JAMA Pediatr. 2013;167:808–15. doi: 10.1001/jamapediatrics.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knip M, Virtanen SM, Seppä K, et al. the Finnish TRIGR Study Group. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–08. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knip M, Åkerblom HK, Becker D, et al. the TRIGR Study Group. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311:2279–87. doi: 10.1001/jama.2014.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahlberg J, Vaarala O, Ludvigsson J for the ABIS-study group. Dietary risk factors for the emergence of type 1 diabetes-related autoantibodies in 21/2 year-old Swedish children. Br J Nutr. 2006;95:603–08. doi: 10.1079/bjn20051676. [DOI] [PubMed] [Google Scholar]
- 75.Virtanen SM, Nevalainen J, Kronberg-Kippilä C, et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95:471–78. doi: 10.3945/ajcn.111.018879. [DOI] [PubMed] [Google Scholar]
- 76.Verge CF, Howard NJ, Irwig L, Simpson JM, Mackerras D, Silink M. Environmental factors in childhood IDDM A population-based, case-control study. Diabetes Care. 1994;17:1381–89. doi: 10.2337/diacare.17.12.1381. [DOI] [PubMed] [Google Scholar]
- 77.Virtanen SM, Läärä E, Hyppönen E, et al. Cow’s milk consumption HLA-DQB1 genotype type 1 diabetes: a nested case-control study of siblings of children with diabetes Childhood diabetes in Finland study group. Diabetes. 2000;49:912–17. doi: 10.2337/diabetes.49.6.912. [DOI] [PubMed] [Google Scholar]
- 78.Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus-a nationwide population-based case-control study in pre-school children. Diabetes Metab Res Rev. 2008;24:211–22. doi: 10.1002/dmrr.791. [DOI] [PubMed] [Google Scholar]
- 79.Lamb MM, Miller M, Seifert JA, et al. The eff ect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Pediatr Diabetes. 2015;16:31–38. doi: 10.1111/pedi.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virtanen SM, Niinistö S, Nevalainen J, et al. Serum fatty acids and risk of advanced beta-cell autoimmunity: a nested case-control study among children with HLA-conferred susceptibility to type I diabetes. Eur J Clin Nutr. 2010;64:792–99. doi: 10.1038/ejcn.2010.75. [DOI] [PubMed] [Google Scholar]
- 81.Brekke HK, Ludvigsson J. Daily vegetable intake during pregnancy negatively associated to islet autoimmunity in the off spring—the ABIS study. Pediatr Diabetes. 2010;11:244–50. doi: 10.1111/j.1399-5448.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 82.Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol. 2015;52:621–24. doi: 10.1007/s00592-014-0628-5. [DOI] [PubMed] [Google Scholar]
- 83.Hummel S, Pfl üger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–05. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25:1111–16. doi: 10.2337/diacare.25.7.1111. [DOI] [PubMed] [Google Scholar]
- 85.Weets I, Kaufman L, Van der Auwera B, et al. the Belgian Diabetes Registry. Seasonality in clinical onset of type 1 diabetes in Belgian patients above the age of 10 is restricted to HLA-DQ2/DQ8-negative males, which explains the male to female excess in incidence. Diabetologia. 2004;47:614–21. doi: 10.1007/s00125-004-1369-8. [DOI] [PubMed] [Google Scholar]
- 86.Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5:3551–62. doi: 10.3390/nu5093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the off spring. Diabetes. 2012;61:175–78. doi: 10.2337/db11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miettinen ME, Reinert L, Kinnunen L, et al. Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the off spring. Diabetologia. 2012;55:1291–94. doi: 10.1007/s00125-012-2458-8. [DOI] [PubMed] [Google Scholar]
- 89.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–17. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 90.Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 2011;54:2779–88. doi: 10.1007/s00125-011-2278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bizzarri C, Pitocco D, Napoli N, et al. for the IMDIAB Group. No protective eff ect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33:1962–63. doi: 10.2337/dc10-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha, 25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33:1443–48. doi: 10.2337/dc09-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sørensen IM, Joner G, Jenum PA, Eskild A, Stene LC. Serum long chain n-3 fatty acids (EPA and DHA) in the pregnant mother are independent of risk of type 1 diabetes in the off spring. Diabetes Metab Res Rev. 2012;28:431–38. doi: 10.1002/dmrr.2293. [DOI] [PubMed] [Google Scholar]
- 94.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–28. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 95.Benson VS, Vanleeuwen JA, Taylor J, Somers GS, McKinney PA, Van Til L. Type 1 diabetes mellitus and components in drinking water and diet: a population-based, case-control study in Prince Edward Island, Canada. J Am Coll Nutr. 2010;29:612–24. doi: 10.1080/07315724.2010.10719900. [DOI] [PubMed] [Google Scholar]
- 96.Moltchanova E, Rytkönen M, Kousa A, Taskinen O, Tuomilehto J, Karvonen M for the Spat Study Group and the Finnish Childhood Diabetes Registry Group. Zinc and nitrate in the ground water and the incidence of Type 1 diabetes in Finland. Diabet Med. 2004;21:256–61. doi: 10.1111/j.1464-5491.2004.01125.x. [DOI] [PubMed] [Google Scholar]
- 97.Muntoni S, Cocco P, Muntoni S, Aru G. Nitrate in community water supplies and risk of childhood type 1 diabetes in Sardinia, Italy. Eur J Epidemiol. 2006;21:245–47. doi: 10.1007/s10654-006-0014-x. [DOI] [PubMed] [Google Scholar]
- 98.Zhao HX, Mold MD, Stenhouse EA, et al. Drinking water composition and childhood-onset Type 1 diabetes mellitus in Devon and Cornwall, England. Diabet Med. 2001;18:709–17. doi: 10.1046/j.1464-5491.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- 99.Dahlquist GG, Blom LG, Persson LA, Sandström AI, Wall SG. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ. 1990;300:1302–06. doi: 10.1136/bmj.300.6735.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samuelsson U, Oikarinen S, Hyöty H, Ludvigsson J. Low zinc in drinking water is associated with the risk of type 1 diabetes in children. Pediatr Diabetes. 2011;12:156–64. doi: 10.1111/j.1399-5448.2010.00678.x. [DOI] [PubMed] [Google Scholar]
- 101.Winkler C, Mollenhauer U, Hummel S, Bonifacio E, Ziegler AG. Exposure to environmental factors in drinking water: risk of islet autoimmunity and type 1 diabetes--the BABYDIAB study. Horm Metab Res. 2008;40:566–71. doi: 10.1055/s-2008-1073165. [DOI] [PubMed] [Google Scholar]
- 102.Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta-analysis. Am J Epidemiol. 2009;169:1428–36. doi: 10.1093/aje/kwp065. [DOI] [PubMed] [Google Scholar]
- 103.Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53:641–51. doi: 10.1007/s00125-009-1648-5. [DOI] [PubMed] [Google Scholar]
- 104.Johansson C, Samuelsson U, Ludvigsson J. A high weight gain early in life is associated with an increased risk of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1994;37:91–94. doi: 10.1007/BF00428783. [DOI] [PubMed] [Google Scholar]
- 105.Hyppönen E, Kenward MG, Virtanen SM, et al. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care. 1999;22:1961–65. doi: 10.2337/diacare.22.12.1961. [DOI] [PubMed] [Google Scholar]
- 106.Magnus MC, Olsen SF, Granström C, et al. Infant growth and risk of childhood-onset type 1 diabetes in children from 2 Scandinavian birth cohorts. JAMA Pediatr. 2015;169:e153759. doi: 10.1001/jamapediatrics.2015.3759. [DOI] [PubMed] [Google Scholar]
- 107.Larsson HE, Vehik K, Haller MJ, et al. the TEDDY study. Growth and risk for islet autoimmunity and progression to type 1 diabetes in early childhood: The Environmental Determinants of Diabetes in the Young Study. Diabetes. 2016 doi: 10.2337/db15-1180. db151180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia. 2001;44:914–22. doi: 10.1007/s001250100548. [DOI] [PubMed] [Google Scholar]
- 109.Rewers M. The fallacy of reduction. Pediatr Diabetes. 2012;13:340–43. doi: 10.1111/j.1399-5448.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 110.Lamb MM, Yin X, Barriga K, et al. Dietary glycemic index, development of islet autoimmunity, and subsequent progression to type 1 diabetes in young children. J Clin Endocrinol Metab. 2008;93:3936–42. doi: 10.1210/jc.2008-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ludvigsson J. Why diabetes incidence increases--a unifying theory. Ann N Y Acad Sci. 2006;1079:374–82. doi: 10.1196/annals.1375.058. [DOI] [PubMed] [Google Scholar]
- 112.Sepa A, Wahlberg J, Vaarala O, Frodi A, Ludvigsson J. Psychological stress may induce diabetes-related autoimmunity in infancy. Diabetes Care. 2005;28:290–95. doi: 10.2337/diacare.28.2.290. [DOI] [PubMed] [Google Scholar]
- 113.Sepa A, Frodi A, Ludvigsson J. Mothers’ experiences of serious life events increase the risk of diabetes-related autoimmunity in their children. Diabetes Care. 2005;28:2394–99. doi: 10.2337/diacare.28.10.2394. [DOI] [PubMed] [Google Scholar]
- 114.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 115.Szabat M, Page MM, Panzhinskiy E, et al. Reduced insulin production relieves endoplasmic reticulum stress and induces β cell proliferation. Cell Metab. 2016;23:179–93. doi: 10.1016/j.cmet.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 116.Marré ML, James EA, Piganelli JD. β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol. 2015;3:67. doi: 10.3389/fcell.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doyle HA, Yang ML, Raycroft MT, Gee RJ, Mamula MJ. Autoantigens: novel forms and presentation to the immune system. Autoimmunity. 2014;47:220–33. doi: 10.3109/08916934.2013.850495. [DOI] [PubMed] [Google Scholar]
- 118.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modifi ed GAD65 epitopes in subjects with type 1 diabetes. Diabetes. 2014;63:3033–40. doi: 10.2337/db13-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Lummel M, van Veelen PA, de Ru AH, et al. Discovery of a selective islet peptidome presented by the highest-risk HLA-DQ8 trans molecule. Diabetes. 2016;65:732–41. doi: 10.2337/db15-1031. [DOI] [PubMed] [Google Scholar]
- 120.Crèvecoeur I, Rondas D, Mathieu C, Overbergh L. The beta-cell in type 1 diabetes: what have we learned from proteomic studies? Proteomics Clin Appl. 2015;9:755–66. doi: 10.1002/prca.201400135. [DOI] [PubMed] [Google Scholar]
- 121.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–31. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gottlieb PA, Delong T, Baker RL, et al. Chromogranin A is a T cell antigen in human type 1 diabetes. J Autoimmun. 2014;50:38–41. doi: 10.1016/j.jaut.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jin N, Wang Y, Crawford F, et al. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc Natl Acad Sci USA. 2015;112:13318–23. doi: 10.1073/pnas.1517862112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–14. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paulsson JF, Ludvigsson J, Carlsson A, et al. High plasma levels of islet amyloid polypeptide in young with new-onset of type 1 diabetes mellitus. PLoS One. 2014;9:e93053. doi: 10.1371/journal.pone.0093053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Bruce Verchere C. Metabolic stress, IAPP and islet amyloid. Diabetes Obes Metab. 2012;14(suppl 3):68–77. doi: 10.1111/j.1463-1326.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 127.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of beta cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65 doi: 10.2337/db15-1615. db151615. [DOI] [PubMed] [Google Scholar]
- 128.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63:3835–45. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1–9. doi: 10.1111/pedi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–90. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frederiksen BN, Steck AK, Kroehl M, et al. Evidence of stage- and age-related heterogeneity of non-HLA SNPs and risk of islet autoimmunity and type 1 diabetes: the diabetes autoimmunity study in the young. Clin Dev Immunol. 2013;2013:417657. doi: 10.1155/2013/417657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frederiksen BN, Kroehl M, Barón A, et al. Assessing age-related etiologic heterogeneity in the onset of islet autoimmunity. Biomed Res Int. 2015;2015:708289. doi: 10.1155/2015/708289. [DOI] [PMC free article] [PubMed] [Google Scholar]