Abstract
Driven by access to better drugs, on average, newly diagnosed multiple myeloma patients have over 10 years overall survival. Using modern combination therapies – with or without the addition of high-dose melphalan and autologous stem cell transplantation – up to 80% of patients reach a complete response. As a logical and necessary step forward, clinical studies have explored strategies to detect minimal residual disease (MRD) and its correlation with clinical outcomes. In this context, MRD has been proposed as a regulatory endpoint for drug approval in newly diagnosed multiple myeloma. To better define the role of MRD negativity in relation to clinical outcomes, we undertook a meta-analysis including published clinical trials of newly diagnosed multiple myeloma patients. We applied a random effects model which weighted studies using the inverse-variance method. Studies were combined on the scale of the logarithm of the hazard ratio (HR) and the corresponding standard error. We found that remaining MRD positive was associated with worse progression-free survival (HR=2.85; 95% confidence interval (CI) 2.17–3.74; P<0.001) and overall survival (HR=2.08; 95% CI 1.44–3.01; P<0.001). Our results show that MRD negativity is a strong predictor of clinical outcomes, supportive of MRD becoming a regulatory endpoint for drug approval in newly diagnosed multiple myeloma.
INTRODUCTION
At the start of the 21st Century, in the U.S., multiple myeloma had an average overall survival of about 3 years.1 Driven by access to better drugs, younger patients (<50 years) showed more than 10 years median overall survival by 2014.1 The U.S. Food and Drug Administration (FDA) approved 14 new drugs in the treatment of cancer in 2015; four of these were approved for the treatment of multiple myeloma (panobinostat, daratumumab, elotuzumab, and ixazomib).2 In 2015 and 2016, expanded label indications were FDA approved for lenalidomide and carfilzomib, respectively.2
A few years ago, using older drugs followed by high-dose melphalan (HDM) and autologous stem cell transplantation (ASCT), only a smaller fraction of patients obtained a complete response (CR).3 Today, using modern combination therapies, with or without the addition of HDM-ASCT, 100% of patients achieve a treatment response (overall response) with up to 80% of these patients reaching a CR.3 As a necessary and logical step forward, clinical studies have explored strategies to detect minimal residual disease (MRD) and its correlation with clinical outcomes. Both progression-free survival and overall survival have been associated with MRD in several studies3, suggesting that MRD negativity is a predictor of better clinical outcomes in multiple myeloma.4
Although individual smaller studies have suggested that MRD is associated with clinical outcomes3, to provide formal scientific evidence, we undertook a meta-analysis including published clinical trials of newly diagnosed multiple myeloma patients.
PATIENTS AND METHODS
On December 22, 2015, we conducted a systematic search for clinical trials of newly diagnosed multiple myeloma patients with information on MRD and clinical outcomes. We applied the following MEDLINE (via PubMed), EMBASE, and Cochrane’s Central Register of Controlled Trials (CENTRAL) search strategy: ((((Clinical trial[ptyp] OR random*[ti] OR (blinded OR blind) OR “randomized controlled trial” [tiab] OR rct[ti] OR “clinical trial”[ti] OR trial*[ti]))) AND (Flow cytometry[mh] OR flow[tiab] OR flow microfluoromet*[tiab] OR polymerase chain reaction[mh] OR pcr[tiab] OR “polymerase chain reaction”[tiab] OR gene expression profiling[mh] OR molecular[tw] OR sequence analysis[mh] OR “sequence analysis”[tiab] OR sequenc*[tiab] OR “minimal residual disease”[tiab] OR mrd[tiab])) AND (multiple myeloma[mh] OR “multiple myeloma”[ti] OR “plasma cell myeloma*”[ti]). Taking this approach, a total of 390 potential studies were first identified; however, after careful review of each individual abstract, we excluded 370 since they were not clinical trials with MRD assessment in multiple myeloma. Thus, 20 studies clinical trials of newly diagnosed multiple myeloma patients with information on MRD and clinical outcomes were identified and assessed for inclusion in this meta-analysis (Figure 1). Since all the 20 published studies did not include all details required for our pre-planned statistical analysis, we contacted the corresponding authors to obtain hazard ratio (HR) estimates and corresponding confidence intervals (CI) for the association between MRD and clinical outcomes.
FIGURE 1. SEARCH CRITERIA FOR SYSTEMATIC REVIEW.
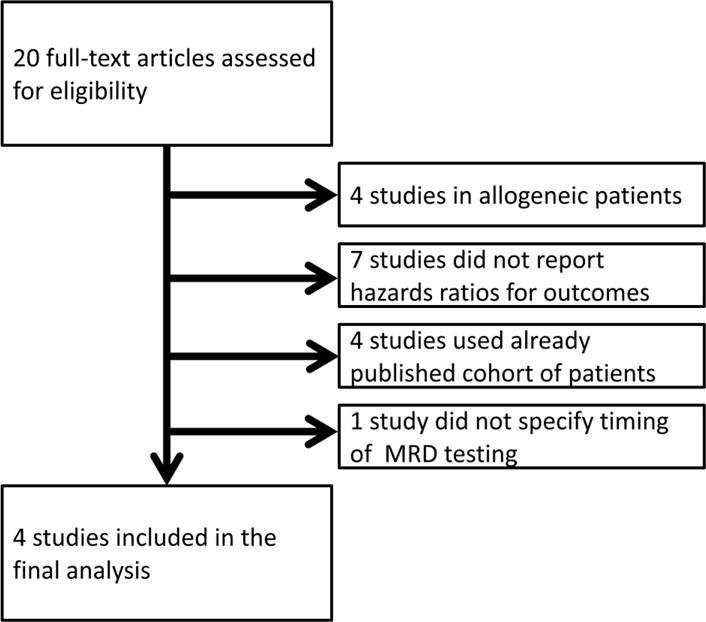
Footnote: On December 22, 2015; we applied a comprehensive MEDLINE (via PubMed), EMBASE, and Cochrane’s Central Register of Controlled Trials (CENTRAL) search strategy. For details see Methods Section. Upon careful review of the 20 identified studies5–8, 11–26, 4 studies were excluded because they reported on allogeneic transplantation11–14; 7 were excluded because they did not evaluate the association between MRD status and progression-free survival and/or overall survival15–21; 4 were excluded because they analyzed the same cohort of patients (duplicates)22–25; and 1 was excluded because the timing of MRD analysis was not specified26.
Statistical analysis
Four manuscripts were included in the meta-analysis for progression-free survival and two were included for overall survival. Each paper used a different landmark time point for MRD evaluation; these included: after eight cycles of therapy5, after six cycles of therapy6, at 100 days post-ASCT7, and 3–6 months post-ASCT8. Two patients in the original Korde et al.5 analysis were removed from the hazard ratio calculation due to either not having sufficient follow-up or progressing prior to the landmark time. In Silvennoinen et al.8, only patients achieving a CR or a near-(n)-CR were included in the HR calculation; for the other included studies, all patients were included. The meta-analysis was conducted using a random effects model, which weighted studies using the inverse-variance method. Studies were combined on the scale of the logarithm of the hazard ratio and the corresponding standard error. The analysis used the package ‘metafor’ in R Statistical Platform, v 3.2.3.9, 10
RESULTS
Upon careful review of the 20 identified studies5–8, 11–26, 4 studies were excluded because they reported on allogeneic transplantation11–14; 7 were excluded because they did not evaluate the association between MRD status and progression-free survival and/or overall survival15–21; 4 were excluded because they analyzed overlapping cohorts of patients (duplicates)22–25; and 1 was excluded because the timing of MRD analysis was not specified26 (Figure 1). Four studies with information on MRD status and HR for progression-free survival were included in the final analysis5–8; three studies had information on overall survival5–7 (however, one study had no deaths during the original follow-up window5) so two studies provided hazards ratios for overall survival.
Among the four studies included in the main analysis5–8, three used multiparameter flow-cytometry5–7 and one study used allele-specific quantitative polymerase chain reaction8, both with a sensitivity of at least 1 in 10,000 cells (10−4) to determine MRD status.3 In line with standard methodology9, 10, the random effects model weighted studies based on the inverse-variance method; the largest study (Paiva et al.)7 had the largest weight (W random = 60.4%) in the meta-analysis; and the smallest study (Korde et al.)5 had the smallest weight (W random = 2.2%) (Figure 2). Despite inherent differences across included studies (e.g., including eligibility criteria, use of drugs, application of MRD assays), all HRs were in the same direction favouring MRD negativity for a longer progression-free survival. Overall, the meta-analysis show that, compared to who achieved MRD negativity, patients who remained MRD positive had worse progression-free survival (HR=2.85; 95% CI 2.17–3.74; P<0.001) (Figure 2).
FIGURE 2. MRD STATUS AND CLINICAL OUTCOMES IN NEWLY DIAGNOSED MULTIPLE MYELOMA.
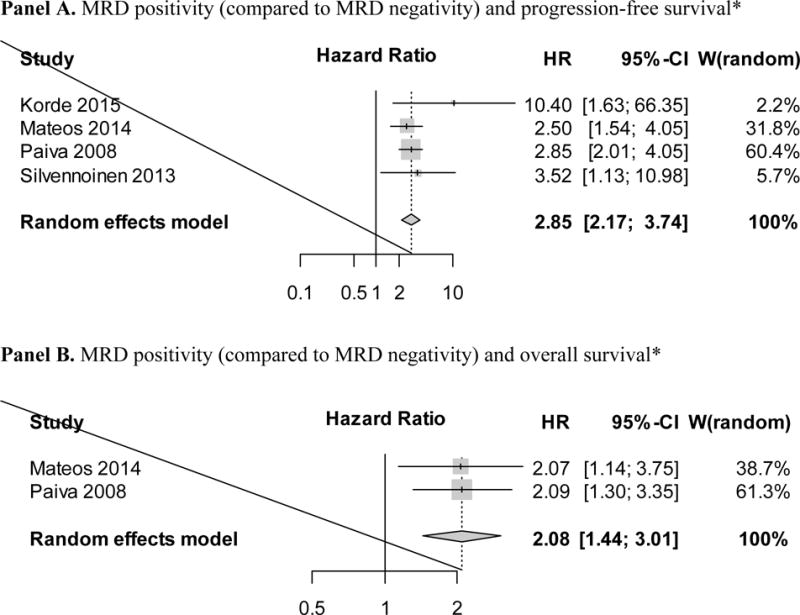
*A higher hazard ratio indicates increased risk for each survival endpoint.
Footnote: Four studies with information on MRD status and hazards ratios for progression-free survival were included in the final analysis5–8; three studies had information on overall survival5–7 (however, one study had no deaths during the original follow-up window5) so two studies provided hazards ratios for overall survival.
In addition to investigating MRD status in relation to progression-free survival, we were also interested in assessing the association between MRD negativity and overall survival. As described above, 4 studies with information on MRD status and hazards ratios for progression-free survival were included in the final analysis and 3 of these had information on overall survival5–7. However, due to the use of modern effective combination therapy, the study by Korde et al. had no deaths during the original follow-up window (up to 30 months).5 Therefore, the studies by Paiva et al. and Mateos at al. were the only two that provided hazards ratios for overall survival.6, 7 Based on these two studies, our meta-analysis showed that remaining MRD positive was associated with a higher risk of death (HR=2.08; 95% CI 1.44–3.01; P<0.001) (Figure 2).
CONCLUSIONS
For several decades, only limited progress was made in the field of drug development for multiple myeloma. Driven by access to new effective drugs, in 2016, newly diagnosed multiple myeloma patients – on average – have over 10 years overall survival in the U.S.3 Using modern combination therapies – with or without the addition of high-dose melphalan and autologous stem cell transplantation – up to 80% of patients reach a complete response.3 Consequently, clinical studies have explored strategies to detect MRD and its correlation with clinical outcomes. Furthermore, in this context, MRD has been proposed as a regulatory endpoint for drug approval in newly diagnosed multiple myeloma.27, 28 To fill important gaps in the literature, we were motivated to conduct a meta-analysis including all published clinical trials of newly diagnosed multiple myeloma patients. Using a robust random effects model, we found that MRD positivity is associated with worse progression-free survival (HR=2.85; P<0.001) and overall survival (HR=2.08; P<0.001). Our results are very important as they provide formal scientific evidence to the fact that MRD negativity is associated with better clinical outcomes in newly diagnosed multiple myeloma.
Although stringent criteria were applied to identify and include studies for our meta-analysis, inherently, similar to any meta-analysis, there were some differences between the included studies. For example, the studies by Paiva et al. and7 Silvennoinen et al.8 used various combinations including bortezomib and dexamethasone (with or without alkylating drugs) followed by high-dose melphalan and autologous stem cell transplantation, while the study by Mateos et al.6 used combinations including bortezomib and prednisone followed by maintenance therapy with either bortezomib/thalidomide or bortezomib/prednisone for up to 3 years.6 Given that there are differences in access to myeloma drugs across regions – and, indeed, these three studies were all conducted in Europe – it seems reasonable to conclude that these regimens are quite applicable to European treatment traditions. As illustrated in Figure 2, these three studies consistently showed that MRD positivity was associated with an HR between 2.5 and 3.5 (Figure 2). As a consequence of the rapid access to better myeloma drugs in the U.S. the myeloma treatment field has been moving fast forward and new combination therapies have been developed.2 The study by Korde et al.5 was a single arm phase 2 study open for newly diagnosed multiple myeloma patients of all ages (18 years and older). Per protocol, all patients received 8 cycles of carfilzomib, lenalidomide, and dexamethasone (CRd) combination therapy, followed by 2 years of lenalidomide maintenance therapy; and none of the enrolled patients underwent upfront high-dose melphalan and autologous stem cell transplantation; however, on a separate protocol, younger and older fit patients were offered stem cell collection for potential future use (i.e. delayed high-dose melphalan and autologous stem cell transplantation). Based on available literature, CRd combination therapy has the highest and deepest response rate for multiple myeloma patients to date5, 29, and, consequently, in the study by Korde et al.5 MRD negativity was associated with an HR of 10.4 (Figure 2). Per standard conventions9, 10, in our meta-analysis, the random effects model weighted studies based on the inverse-variance method, and, thus, the largest study (Paiva et al.)7 had the largest weight on the HR in the meta-analysis while and the smallest study (Korde et al.)5 had the smallest weight. It should be emphasized that although there are inherent differences across clinical trials, all HRs for the included studies were in the same direction favouring MRD negativity for a longer progression-free survival as well as overall survival.
Other aspects of difference across included studies include the actual MRD assays and the timing for MRD testing. As discussed above, among the four studies included in the main analysis5–8, three used multiparameter flow-cytometry5–7 and one study used allele-specific quantitative polymerase chain reaction8, both with a sensitivity of at least 1 in 10,000 cells (10−4) to determine MRD status.3 To harmonize procedures and to ensure that MRD status has the same meaning across studies, the International Myeloma Working Group (IMWG) recently revised the response criteria for myeloma and included MRD negativity as the highest degree of response to treatment30. Based on the 2016 IMWG criteria, MRD negativity can be defined by either multiparameter flow-cytometry-based or molecular-based assays; and MRD status shall only be determined in patients who have achieved a conventional CR30.
Moving forward, as better and better drugs become available for the treatment of multiple myeloma, ironically, traditional regulatory endpoints (i.e. overall survival and progression-free survival) will become key barriers for drug development. In fact, future registrational clinical trials will require very large sample sizes and very long followup (to be able to provide sufficient statistical power) to show that new drugs have an overall survival and/or progression-free survival benefit. Thus, due to these inherent limitations of traditional regulatory endpoints, future myeloma drugs will become majorly delayed and costs for drug development will increase significantly. Clearly, there is an urgent need for reliable surrogate regulatory endpoints for drug approval.27, 28 Indeed, Gormley et al, from the FDA, recently published a paper on regulatory perspectives on MRD testing in multiple myeloma.4 In their paper, the authors conclude that MRD assessment in multiple myeloma has the potential to become a surrogate clinical endpoint that could be used to support regulatory purposes for drug review.4 As pointed out by Gormley et al., standardization of MRD testing and consensus within the multiple myeloma community as to the role of MRD and possible incorporation into the response criteria will be integral steps towards this end.4 The International Myeloma Working Group (IMWG) has worked extensively to revise the response criteria for multiple myeloma and an updated version of the IMWG response criteria which will include guidelines for MRD testing is anticipated to be published in 2016. Also, concerted efforts aiming to standardize MRD testing and consensus within the multiple myeloma community are ongoing. The FDA has emphasized the importance of future meetings to facilitate a consensus process in the U.S. and expressed their interest in reviewing both testing protocols of MRD assays and clinical protocols incorporating MRD.4 In this context, the results from our meta-analysis play an important role as they provide scientific evidence on the impact of MRD negativity as a strong predictor of longer progression-free survival and overall survival in newly diagnosed multiple myeloma.
Based on the results from our meta-analysis, we conclude that MRD negativity is associated with better progression-free survival and overall survival in newly diagnosed multiple myeloma. Our findings are supportive of MRD assessment becoming a surrogate clinical endpoint that could be used to support regulatory purposes for drug review in multiple myeloma.4 In addition to the results from this meta-analysis; prospective clinical studies are ongoing and will to further confirm and expand on our findings.
Acknowledgments
We would like to thank the International Myeloma Foundation (IMF) in Los Angeles, USA, and we would like to thank our Core Grant (P30 CA008748) for grant support of this work. We also would like to thank authors who provided additional information regarding the included studies.
References
- 1.Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28(6):1346–1348. doi: 10.1038/leu.2014.23. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications. 2016 http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm.
- 3.Mailankody S, Korde N, Lesokhin AM, Lendvai N, Hassoun H, Stetler-Stevenson M, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nature reviews Clinical oncology. 2015;12(5):286–295. doi: 10.1038/nrclinonc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gormley NJ, Turley DM, Dickey JS, Farrell AT, Reaman GH, Stafford E, et al. Regulatory perspective on minimal residual disease flow cytometry testing in multiple myeloma. Cytometry Part B, Clinical cytometry. 2016;90(1):73–80. doi: 10.1002/cyto.b.21268. [DOI] [PubMed] [Google Scholar]
- 5.Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment With Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA oncology. 2015;1(6):746–754. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateos MV, Oriol A, Martinez-Lopez J, Teruel AI, Lopez de la Guia A, Lopez J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887–1893. doi: 10.1182/blood-2014-05-573733. [DOI] [PubMed] [Google Scholar]
- 7.Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–4023. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvennoinen R, Kairisto V, Pelliniemi TT, Putkonen M, Anttila P, Saily M, et al. Assessment of molecular remission rate after bortezomib plus dexamethasone induction treatment and autologous stem cell transplantation in newly diagnosed multiple myeloma patients. British journal of haematology. 2013;160(4):561–564. doi: 10.1111/bjh.12139. [DOI] [PubMed] [Google Scholar]
- 9.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2015. [Google Scholar]
- 10.Viechtbauer W. Conducting Meta-Analyses in R with the Metafor Package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 11.Cavo M, Terragna C, Martinelli G, Ronconi S, Zamagni E, Tosi P, et al. Molecular monitoring of minimal residual disease in patients in long-term complete remission after allogeneic stem cell transplantation for multiple myeloma. Blood. 2000;96(1):355–357. [PubMed] [Google Scholar]
- 12.Corradini P, Cavo M, Lokhorst H, Martinelli G, Terragna C, Majolino I, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102(5):1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 13.Galimberti S, Benedetti E, Morabito F, Papineschi F, Callea V, Fazzi R, et al. Prognostic role of minimal residual disease in multiple myeloma patients after non-myeloablative allogeneic transplantation. Leukemia Res. 2005;29(8):961–966. doi: 10.1016/j.leukres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Zagrivnaja M, Schwartz S, Badbaran A, Zabelina T, Lioznov M, et al. Kinetics of plasma-cell chimerism after allogeneic stem cell transplantation by highly sensitive real-time PCR based on sequence polymorphism and its value to quantify minimal residual disease in patients with multiple myeloma. Experimental hematology. 2006;34(5):688–694. doi: 10.1016/j.exphem.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(12):2077–2084. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig H, Viterbo L, Greil R, Masszi T, Spicka I, Shpilberg O, et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(2):247–255. doi: 10.1200/JCO.2011.39.5137. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli G, Terragna C, Lemoli RM, Cavo M, Benni M, Motta MR, et al. Clinical and molecular follow-up by amplification of the CDR-III IgH region in multiple myeloma patients after autologous transplantation of hematopoietic CD34+ stem cells. Haematologica. 1999;84(5):397–404. [PubMed] [Google Scholar]
- 18.Powles R, Sirohi B, Kulkarni S, Bhagwati N, Saso R, Raje N, et al. Acute lymphoblastic leukaemia-type intensive chemotherapy to eliminate minimal residual disease after high-dose melphalan and autologous transplantation in multiple myeloma – a phase I/II feasibility and tolerance study of 17 patients. Bone Marrow Transplant. 2000;25(9):949–956. doi: 10.1038/sj.bmt.1702379. [DOI] [PubMed] [Google Scholar]
- 19.Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(20):2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 20.Rawstron AC, Gregory WM, de Tute RM, Davies FE, Bell SE, Drayson MT, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932–1935. doi: 10.1182/blood-2014-07-590166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(25):2712–2717. doi: 10.1200/JCO.2013.54.8164. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 24.Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(12):1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 25.Silvennoinen R, Lundan T, Kairisto V, Pelliniemi TT, Putkonen M, Anttila P, et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood cancer journal. 2014;4:e250. doi: 10.1038/bcj.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrero S, Ladetto M, Drandi D, Cavallo F, Genuardi E, Urbano M, et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia. 2015;29(3):689–695. doi: 10.1038/leu.2014.219. [DOI] [PubMed] [Google Scholar]
- 27.Ahn IE, Mailankody S, Korde N, Landgren O. Dilemmas in treating smoldering multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(1):115–123. doi: 10.1200/JCO.2014.56.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landgren O, Gormley N, Turley D, Owen RG, Rawstron A, Paiva B, et al. Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium. Am J Hematol. 2014;89(12):1159–1160. doi: 10.1002/ajh.23831. [DOI] [PubMed] [Google Scholar]
- 29.Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. In press. [DOI] [PubMed] [Google Scholar]


