Abstract
Background
This study examined predictors and behaviors of pregnancy-related smoking among women who belonged to a private health maintenance organization and the recall accuracy of pregnancy-related smoking behaviors after 6-years.
Methods
A cohort of 725 pregnant women was followed for six years. Major predictors for smoking behavior before, during, and one-year following pregnancy were determined. In addition, accuracy of recall six years postpartum of smoking behavior at the time of pregnancy and one-year postpartum was tested.
Results
Mother’s education, asthma status, amount of pre-pregnancy smoking, gravidity, and father’s smoking status were important in the prediction of pregnancy associated smoking. Agreement for recall of smoking behavior during pregnancy (6 year recall) and one-year postpartum (5 year recall) were 90% and 91%, respectively.
Conclusions
Despite potentially adverse outcomes, a proportion of women continue to smoke throughout pregnancy. A number of variables proved to be important predictors of pregnancy associated smoking behavior. These factors should be considered by smoking cessation programs targeting women of reproductive age. Additionally, there was substantial agreement for maternal recall at six years postpartum of smoking behavior at the time of pregnancy and one-year postpartum. This should be considered in retrospective study designs that are primarily based on maternal recall of smoking behaviors before, during, and following pregnancy.
Keywords: Maternal smoking, Pregnancy, Maternal recall, Spontaneous quitting, Smoking predictors
Introduction
In 1995, the estimated smoking-associated costs of complicated pregnancies per year totaled nearly two billion dollars [1]. Although national estimates have reported that women’s smoking prevalence prior to pregnancy has decreased in recent years, some studies suggest this decline is not as all encompassing as otherwise believed. In 2004, for example, Cnattingius et al. [2] reported a 27% smoking prevalence rate among certain populations of women. This is the same rate that the Centers for Disease Control estimated in the early 1990s [1]. Maternal smoking has been associated with a number of risks including premature labor and birth, low birth weight, placenta previa, placenta abruption and sudden infant death syndrome [2–5]. It is of considerable interest to smoking cessation programs as well as national cessation goals that predictors of maternal smoking/cessation are established and considered valid [5, 6]. Our data suggests that work-related factors are important predictors of maternal smoking cessation and therefore work-based cessation and quitting maintenance programs may be effective for women within the occupational strata we identified as vulnerable to relapse.
Reporting on the phenomenon known as “spontaneous quitting,” the literature has shown that 11–65% of pre-pregnancy female smokers quit upon becoming aware of a pregnancy [7]. The large variation is mainly attributed to differences in sample populations according to socio-economic status [2, 6–17]. However, more years of education have also been associated with higher rates of spontaneous quitting as well as lower pre-pregnancy cigarette consumption, fewer miscarriages, being primigravid, high perceived risk to fetus, experience of morning sickness, and the absence of another smoker in the household [7]. The relationship of maternal asthma status and spontaneous quitting, to our knowledge, has never been studied.
The present study examined the pregnancy-related smoking behaviors of a prospective cohort who belonged to a private health maintenance organization (HMO) population in a two-county suburban area north of Detroit, Michigan. Major predictors for smoking behavior were assessed before, during and one-year following pregnancy. In addition, the accuracy of a 6-year recall of these behaviors (5-year recall for one-year postpartum behaviors) was investigated.
Methods
Subject Recruitment and Selection
The data used in this analysis were collected as part of an ongoing study to evaluate the environmental determinants of allergic diseases in a population of children followed from birth [18]. In order to be eligible, women had to be initiating prenatal care with one of seven facilities associated with Henry Ford Health System and belong to the HMO associated with the system. In addition they had to reside within one of two specified counties north of Detroit, have an estimated date of confinement between April 15, 1987 and August 31, 1989, and plan to deliver at one of three hospitals. Women younger than 18 years were excluded, as were subjects who anticipated placing their baby with adoptive parents or moving out of the study area. Only one child was enrolled per family. The Henry Ford Health System Internal Review Board approved all facets of this research.
Nurses recruited subjects during midterm prenatal visits (4–6th month of pregnancy) and consent was obtained. During these prenatal contacts women were interviewed by trained field staff about their demographics, allergy and pregnancy histories, and smoking habits. Enrolled subjects were contacted by telephone annually for six consecutive years at the time of the child’s birthday. During these annual contacts, extensive information was collected on the child’s health and indoor environmental exposures.
Data Collection
During the midterm prenatal visit maternal smoking at the time and one-year previously were recorded and categorized. Smoking behavior was self-reported by the mother for herself and for the father. Data was collected by the in-person interviewer. Later, during the first and sixth annual questionnaires, both maternal and paternal smoking behaviors were recorded and categorized as well. In addition to the standard annual questions, the sixth annual questionnaire asked the respondent to recall parental smoking behavior as the number of cigarettes smoked per week during pregnancy and for each year of the child’s life.
Mother’s age, occupation, education, gravidity, parity, asthma status, level of pre-pregnancy smoking consumption and father’s smoking status at the time of pregnancy were collected from the midterm prenatal questionnaire. Mother’s asthma status was assessed by asking each woman if a physician had ever diagnosed her with asthma. Mother’s age was calculated as of the time of the child’s birth. Occupation status was asked as an open-ended question and was coded using the 1977 Standard Occupational Classification codes and categorized based on the US Bureau of Labor Statistics’ 1975 Reference Edition of the “Handbook of Labor Statistics”. Father’s smoking status was categorized from questions included on the midterm prenatal questionnaire.
Statistical Methods
Bivariate analysis was used to calculate initial relationships between predictor variables and the outcomes of interest. Odds ratios (OR) for the outcomes of interest (smoking prior to pregnancy, prenatal smoking maintenance, and return to smoking one-year postpartum) were calculated for each variable individually, including 95% confidence intervals (CI). Odds ratios were adjusted for predictor variables and calculated using logistic regression. Since father’s smoking status, parity, and gravidity were measures collected at the time of pregnancy, they were not used in the analysis of pre-pregnancy smoking behavior.
Logistic regression models were used to predict smoking before pregnancy, prenatal smoking maintenance, and one-year postpartum relapse using Hosmer and Lemeshow’s Goodness-of-Fit tests. The c-statistic (calculated as the area under the ROC curve generated by logistic regression modeling) was used to discriminate between those models with comparable Goodness-of-Fit statistics. A c-statistic value of 0.60–0.79 was considered modestly predictive; a value of ≥0.80 was considered importantly predictive.
Overall percent agreement and kappa statistics were used to assess the accuracy of maternal recall of reported smoking behavior during pregnancy and one-year postpartum. A kappa between 0.61 and 0.80 represented substantial agreement [16]. At the first annual questionnaire, we only collected recall information on whether the subject had been a smoker or nonsmoker during pregnancy.
Results
Study Population
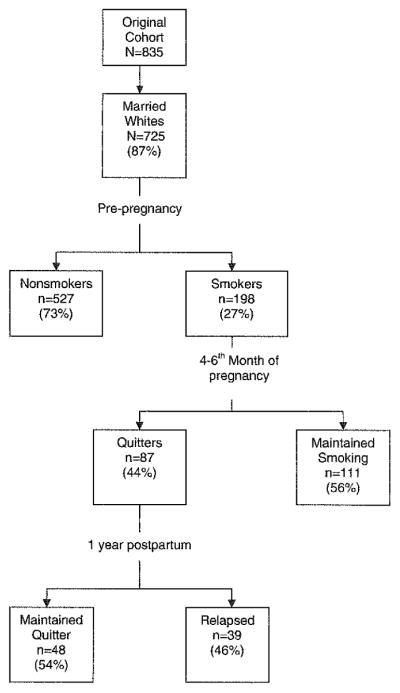
Of 1,184 eligible women, 940 pregnant women (79.4%) were contacted and consented to participate in the study. Ninety-nine of the 940 women were excluded due to fetal loss, birth complications, change in hospital of delivery, or loss of the cord blood specimen needed for the original study protocol. Those not enrolled in the study and those with missing cord blood did not differ from the 841 enrollees. Six additional subjects were excluded due to contaminated cord blood samples, leaving 835 potential study subjects. Because 93% of the women were married and 92% were white (non-Hispanic), analysis of the data was limited to this subset with a final cohort of 725 subjects (Fig. 1).
Fig. 1.
Flow diagram of study population from cohort initiation through 1-year postpartum
Pre-Pregnancy Smoking Behavior
The prevalence of pre-pregnancy smoking was 27 percent. As shown in Table 1, 42% (OR = 2.2, 95% CI 1.5–3.3) of women 25 years or younger were smokers one-year prior to the 4–6th month of pregnancy as compared to 24% of women older than 25 years. Compared to professionally employed women, the odds of being a pre-pregnancy smoker were higher for women in all other occupation categories; however, women in technical/sales/administrative support (TSAS) occupations behaved relatively similar to professional women (OR = 1.3 vs. 1.0, respectively). The greater the educational attainment of a woman the less likely she was to smoke prior to pregnancy. Women without asthma were 2.6 times more likely to smoke before a pregnancy than those with asthma. Overall, the strongest risk factors for smoking prior to pregnancy were having some college, having only a high school diploma or less, and maternal asthma status, with odds ratios of 3.7, 7.2, and 2.9, respectively (data not shown).
Table 1.
Demographic characteristics and mother’s asthma status in relation to smoking behavior one-year prior to the 4–6th month of pregnancy among pregnant women enrolled between 1987 and 1989 in a prospective cohort of women who belonged to an HMO population in a two-county area north of Detroit, MI (n = 725)
| N | % Smoking | Odds ratio | 95% CI | Adjusted ORa | 95% CI | |
|---|---|---|---|---|---|---|
| Age | ||||||
| >25 | 598 | 24 | 1.0b | 1.0b | ||
| ≤25 | 127 | 42 | 2.2 | 1.5–3.3 | 1.4 | 0.91–2.1 |
| Occupation | ||||||
| Professional | 209 | 19 | 1.0b | 1.0b | ||
| Technical, sales, and admin. support | 204 | 23 | 1.3 | 0.79–2.0 | 0.65 | 0.38–1.1 |
| Service | 56 | 57 | 5.6 | 3.0–10.6 | 2.1 | 1.0–4.2 |
| Production, craft, and farming | 24 | 54 | 5.0 | 2.1–12.0 | 2.3 | 0.87–6.0 |
| Not currently employed | 230 | 28 | 1.7 | 1.1–2.6 | 0.73 | 0.43–1.2 |
| Education | ||||||
| College degree(s) | 235 | 9 | 1.0b | 1.0b | ||
| Some college | 226 | 28 | 3.7 | 2.2–6.3 | 3.8 | 2.2–6.7 |
| High school diploma or less | 264 | 43 | 7.2 | 4.4–12.0 | 6.7 | 3.8–11.9 |
| Mother’s asthma status | ||||||
| Yes | 54 | 13 | 1.0b | 1.0b | ||
| No | 655 | 28 | 2.6 | 1.2–6.0 | 1.2 | 1.2–6.7 |
Odds ratios adjusted for all other variables on the table were calculated using logistic regression
Referent group
After adjustment for all other variables, the relationship of the association between smoking status and age, education, and mother’s asthma status remained inverse (Table 1). The OR for the two occupational categories TSAS and not currently employed, however, were no longer higher than professional woman after adjustment; instead these women had 0.65 and 0.73 likelihood, respectively, of smoking prior to pregnancy. When odds ratios were calculated for smoking status by occupation adjusted solely for education, they were similar to the fully adjusted estimates presented in Table 2 (data not shown).
Table 2.
Association between selected characteristics of pre-pregnancy smokers who maintained smoking at the 4–6th month of pregnancy (n = 198), and those who relapsed one-year postpartum having quit smoking by the 4–6th month of pregnancy (n = 87)
|
n = prepregnancy smokers (%) = maintained smoking n (%) |
Odds Ratio (95% CI) | Adjusted ORa (95% CI) |
n = quitters (%) = relapsed one-year postpartum n (%) |
Odds ratio (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤25 | 154 (55) | 1.0b | 1.0b | 65 (46) | 1.0b | 1.0b |
| >25 | 53 (58) | 1.1 (0.61–2.2) | 0.83 (0.34–2.0) | 22 (41) | 0.81 (0.30–2.1) | 1.2 (0.30–4.7) |
| Occupation | ||||||
| Professional | 40 (42) | 1.0b | 1.0b | 23 (22) | 1.0b | 1.0b |
| Technical, Sales, and admin. support | 47 (40) | 0.92 (0.39–2.2) | 1.2 (0.42–3.4) | 28 (61) | 5.6 (1.6–19.4) | 4.3 (1.1–16.6) |
| Service | 32 (69) | 3.0 (1.1–7.9) | 3.3 (0.96–11.4) | 10 (50) | 3.6 (0.74–17.6) | 3.4 (0.62–19.2) |
| Production, craft, and farming | 18 (85) | 7.4 (1.5–38.0) | 6.6 (0.69–62.0) | 2 (50) | 3.6 (0.19–68.3) | –c |
| Not currently employed | 65 (63) | 2.3 (1.0–5.2) | 2.0 (0.71–5.8) | 24 (46) | 3.0 (0.85–10.9) | 2.5 (0.62–10.3) |
| Education | ||||||
| College degree(s) | 22 (36) | 1.0b | 1.0b | 14 (29) | 1.0b | 1.0b |
| Some college | 63 (43) | 1.3 (0.5–3.6) | 0.85 (0.23–3.0) | 36 (56) | 3.1 (0.82–11.8) | 1.8 (0.39–7.9) |
| High school diploma or less | 113 (67) | 3.6 (1.4–9.3) | 2.4 (0.67–8.2) | 37 (41) | 1.7 (0.45–6.5) | 1.0 (0.22–5.0) |
| Mother’s asthma status | ||||||
| Yes | 7 (57) | 1.0b | 1.0b | 3 (67) | 1.0b | 1.0b |
| No | 185 (56) | 0.94 (0.21–4.3) | 0.36 (0.04–3.1) | 82 (45) | 0.41 (0.04–4.7) | –c |
| Gravidity | ||||||
| Primigravida | 51 (45) | 1.0b | 1.0b | 28 (50) | 1.0b | 1.0b |
| Multigravida | 121 (59) | 1.8 (0.92–3.5) | 2.4 (0.78–7.5) | 49 (43) | 0.75 (0.30–1.9) | 0.70 (0.15–3.3) |
| Parity | ||||||
| Primipara | 80 (51) | 1.0b | 1.0b | 39 (49) | 1.0b | 1.0b |
| Multipara | 93 (59) | 1.4 (0.75–2.5) | 0.75 (0.25–2.2) | 38 (42) | 0.77 (0.31–1.9) | 1.0 (0.21–5.0) |
| Pre-pregnancy smoking consumption | ||||||
| <1 pack/day | 72 (31) | 1.0b | 1.0b | 50 (40) | 1.0b | 1.0b |
| ≥1 pack/day | 126 (71) | 5.5 (2.9–10.3) | 6.3 (2.8–14.2) | 37 (51) | 1.6 (0.67–3.7) | 1.9 (0.69–5.3) |
| Father’s smoking status | ||||||
| Nonsmoker | 101 (49) | 1.0b | 1.0b | 52 (46) | 1.0b | 1.0b |
| Smoker | 97 (64) | 1.9 (1.1–3.3) | 1.2 (0.55–2.4) | 35 (43) | 0.88 (0.37–2.1) | 0.89 (0.32–2.4) |
Odds ratios adjusted for all other variables were calculated using logistic regression
Referent group
Cell size too small for adjustment calculation
Prenatal Smoking Maintenance
Spontaneous quitters represented 44% (n = 87) of our cohort. Results of the bivariate analysis are given in Table 2. Overall, paternal smoking status, gravidity, and maternal pre-pregnancy cigarette consumption were the most predictive models for prenatal smoking maintenance with odds ratios of 1.1, 2.1, and 6.0, respectively (data not shown). Women in TSAS occupations again behaved relatively similar to professional women (OR = 0.92 vs. 1.0, respectively). Mothers without a college degree were more likely to continue smoking (OR =1.3 for mothers with some college; OR = 3.6 for mothers with only a high school diploma or less). If the child’s father was a smoker then the mother was 1.9 times more likely to continue smoking, however, the OR fell to 1.2 (95% CI 0.55–2.4) after adjustment for all other variables. Mothers with an affirmative asthma status were more than two times more likely to maintain smoking after adjustment than mothers with a negative asthma status. Those women with higher pre-pregnancy smoking consumption were 5.5 times (adjusted OR = 6.3) more likely to continue smoking than lighter smokers.
One-Year Postpartum Smoking Relapse
Forty-six percent of the women who quit smoking during pregnancy relapsed one-year postpartum. After adjustment for all other variables, the women who relapsed after pregnancy were older, employed in non-professional occupations, and primiparous. They did not have a college degree and smoked more than one pack of cigarettes a day prior to pregnancy (Table 2). After adjustment, the direction of the association changed for age and parity. All other associations were maintained. Overall, the risk of having postpartum relapse in smoking was higher for those having completed only some college, having only a high school diploma or less, and maternal pre-pregnancy cigarette consumption, with odds ratios of 2.9, 1.6, and 1.6, respectively (data not shown).
Unlike in previous analyses of pre-pregnancy smoking and smoking maintenance, women in TSAS occupations did not behave similarly to professional women in the analysis of smoking relapse. Employment in TSAS occupations was the only significant association with one-year postpartum smoking relapse in the unadjusted and adjusted bivariate analysis. Women employed in this area were 4.3 times as likely to relapse as those employed professionally.
Accuracy of Smoking Behavior Recall
Data was available for 526 maternal respondents for the six year recall analysis of pregnancy associated smoking behavior and for 462 maternal respondents for the six year recall analysis of one-year postpartum smoking behavior. The percent agreement was 90% for recall of smoking during pregnancy and 91% for smoking one-year postpartum. The kappa statistic was 0.67 (95% CI 0.59–0.74) for recall of smoking during pregnancy and 0.74 (95% CI 0.67–0.82) for recall of one-year postpartum smoking behavior.
Discussion
The prevalence of pre-pregnancy smoking among the study population (27%) is in accordance with the CDC’s 1995 data on smoking prevalence rates among white women [1]. In addition, spontaneous quitting (44%) fell within the range reported in the literature (11–65%). Of these publications, only the Fingerhut et al. [15] study examined relapse rates one-year postpartum. Kahn, Certain and Whitaker looked at relapse rates at 17 ± 5 months and found an 80% relapse rate [13]. While this is higher than the reported relapse rate here (46%), their data was based on women who quit smoking for at least one week during pregnancy. Due to the low standards required to classify a woman as “quit”, this could have greatly increased the number of women who were counted as relapsed. The relapse rate (46%) was also lower than the 70% relapse rate reported by Fingerhut et al. [15]. This variation may be due to the several differences between our study and the Fingerhut study. The present study was prospective with a geographically and demographically limited population, while the Fingerhut study was a retrospective national telephone survey. As a national survey, the Fingerhut study population was more diverse than the population here and also had a lower average educational attainment. Although the Fingerhut study did not measure the women’s professional level, research has shown that professional level is directly related to education level. As both education and profession are associated with predicting postpartum smoking relapse this difference between studies is not entirely unexpected.
Five employment categories were used: professional, technical/sales/administrative support (TSAS), service, production/craft/farming, and not currently employed. The TSAS occupational group behaved similar to the professional occupational category for both the pre-pregnancy smoking and smoking maintenance analyses. However, the TSAS occupational group was the most divergent from the professional group when looking at spontaneous quitters who relapsed by one-year postpartum. Similarities in pre-pregnancy and prenatal findings between the two occupational groups may be due to similar levels of education. Many professional and TSAS jobs require an undergraduate degree for employment. This study and others have reported that education is an important predictor of pre-pregnancy smoking abstinence and prenatal spontaneous quitting [2, 6–8, 19, 20].
As anticipated, those women who reported physician-diagnosed asthma were much less likely to smoke. As a predictor of pre-pregnancy smoking, a negative maternal asthma status was a significant variable. Unexpectedly, however, among those women with asthma who did smoke, maternal asthma was associated with smoking maintenance. This may be due to the lack of power resulting from the small number of pre-pregnancy smokers with asthma (n = 7). Power decreased further in the relapse analysis and was thus dropped from the adjusted analysis. On the other hand, this result may indicate that women with asthma who smoke have higher levels of physiological addiction.
The level of pre-pregnancy smoking behavior was a major predictor for smoking maintenance and postpartum relapse in our study. Findings here support what has been previously demonstrated in the literature. Studies have found an association between lighter utilization prior to pregnancy and spontaneous quitting [6, 8, 9, 12, 15, 17, 21, 22]. The results in this study were very similar to those of Fingerhut despite the data collection differences in the two studies. A lighter level of pre-pregnancy smoking consumption could be related to a weaker physiological addiction.
Other studies have found the presence of another smoker in the home to be an important predictor of which mothers will quit [7, 12, 13, 17, 21–23]. Women in this study were more likely to maintain smoking if the child’s father was a smoker, however, relapse was less likely among spontaneous quitters when the child’s father smoked. Father’s smoking status was measured at the time of pregnancy and may not have appropriately measured whether the mother was married to or living with the child’s father one-year postpartum.
The scientific community relies heavily on subjects’ recollection of events and behaviors, making differential recall bias a serious threat to the use of retrospective data. The saliency of pregnancy as a life event and the social stigmatism associated with smoking during pregnancy put into question the generalizability of recall studies whose focus is not pregnancy associated smoking behavior. A number of studies have tested the accuracy of maternal recall of pregnancy associated events and exposures [24–30]. However, most of these studies use medical records to test recall, which relies on the physician to ask the question and the person’s truthful reply. Examining smoking-related deception and concealment, many studies have reported prevalence rates from 14–35%, adding greater uncertainty about self-reported quit rates [2, 6, 7, 10, 31, 32]. The Motherisk study conducted in Toronto used a research design similar to ours (i.e., prospective cohort); however, the study population was a self- or physician-referred sample [30]. This study provided the opportunity to assess the level of recall through a population-based, prospective longitudinal study design. The accuracy of recalled smoking behavior during pregnancy and one-year postpartum was substantial. Although women self-reporting as quit were not biochemically confirmed, the high levels of recall accuracy after five and six years indicate that overall rates of smoking concealment at baseline were low.
There were limitations to the present study analysis. First, the study population lacked variability in several demographic characteristics, particularly marital status and race. However, study demographics reflected the general population of the counties from which the participants were recruited. Even still, the generalizability of our results may be limited to groups included in our analysis. Second, several difficulties were associated with the classification of participants’ occupational status. The use of the 1977 Standard Occupational Classification code may not have offered the most appropriate method for classifying occupations held 1987–1989. Although the 1975 edition of the “Handbook of Labor Statistics” was the closest reference available to agree with the 1977 classification code, the labor groupings may not adequately reflect the socioeconomic grouping of occupations held in 1987–1989. A possible consequence is that the occupational analysis may be flawed by misclassification.
Strengths of this study include the prospective population based cohort study design, and the inclusion of the recall analysis. A limited number of studies have looked at accurate recall of smoking behavior through interviews over such a long period of time. These findings suggest that a reasonable amount of accurate recall is maintained and that overall rates of smoking concealment were limited.
In summary, the rates of pre-pregnancy and prenatal smoking in our study were similar to what has been previously reported in the literature. The one-year postpartum relapse rate among the spontaneous quitters in this study population was lower than what has been found in other studies. This is possibly due to the characteristics of the study population, representing a population skewed toward somewhat higher socio-economic status levels by virtue of HMO membership. Finally, six year postpartum maternal recall of pregnancy and one-year postpartum smoking behavior was substantial.
Acknowledgments
This work was supported by the National Institutes of Allergy and Infectious Diseases (Grant no. AI24156), the Fund for Henry Ford Hospital, and the National Institute of Environmental Health Sciences (Grant no. P03ES06639).
The authors acknowledge the work of the following persons who made this study possible: Shirley Blocki and Geraldine Birg, study nurses; Judith McCullough, Laboratory Research Coordinator; Cathey Boyer and Nonna Akkerman, data coordinators; Karen Wells, programmer; Deborah Greene, data keyer; and Susan McGuinness and Nancy Oja-Tebbe, who provided technical assistance in the preparation of the manuscript.
References
- 1.Centers for Disease Control. Medical-care expenditures attributable to cigarette smoking during pregnancy—United States, 1995. Morbidity and Mortality Weekly Report. 1997;46(44):1048–1050. [PubMed] [Google Scholar]
- 2.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6(Suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 3.Scheibmeir M, O’Connell KA. In harm’s way: Childbearing women and nicotine. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1997;26(4):477–484. doi: 10.1111/j.1552-6909.1997.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. Women and smoking: A report of the surgeon general. Executive summary. Morbidity and Mortality Weekly Report Recommendations and Reports. 2002;51(RR-12):i–iv. [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Smoking during pregnancy—United States, 1990–2002. Morbidity and Mortality Weekly Report. 2004;53(39):911–915. [PubMed] [Google Scholar]
- 6.Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking-cessation trial: When more isn’t better, what is enough? American Journal of Preventive Medicine. 1999;17(3):161–168. doi: 10.1016/S0749-3797(99)00071-9. [DOI] [PubMed] [Google Scholar]
- 7.Solomon L, Quinn V. Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine & Tobacco Research. 2004;6(Suppl 2):S203–S216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- 8.Mullen PD. How can more smoking suspension during pregnancy become lifelong abstinence? Lessons learned about predictors, interventions, and gaps in our accumulated knowledge. Nicotine & Tobacco Research. 2004;6(Suppl 2):S217–S238. doi: 10.1080/14622200410001669150. [DOI] [PubMed] [Google Scholar]
- 9.Pirie PL, Lando H, Curry SJ, McBride CM, Grothaus LC. Tobacco, alcohol, and caffeine use and cessation in early pregnancy. American Journal of Preventive Medicine. 2000;18(1):54–61. doi: 10.1016/S0749-3797(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 10.Mullen PD. Maternal smoking during pregnancy and evidence-based intervention to promote cessation. Primary Care. 1999;26(3):577–589. doi: 10.1016/S0095-4543(05)70118-4. [DOI] [PubMed] [Google Scholar]
- 11.LeClere FB, Wilson JB. Smoking behavior of recent mothers, 18–44 years of age, before and after pregnancy: United States, 1990. Advance Data. 1997;(288):1–11. [PubMed] [Google Scholar]
- 12.Giglia RC, Binns CW, Alfonso HS. Which women stop smoking during pregnancy and the effect on breastfeeding duration. BMC Public Health. 2006;6:195. doi: 10.1186/1471-2458-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. American Journal of Public Health. 2002;92(11):1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Campo P, Faden RR, Brown H, Gielen AC. The impact of pregnancy on women’s prenatal and postpartum smoking behavior. American Journal of Preventive Medicine. 1992;8(1):8–13. [PubMed] [Google Scholar]
- 15.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. American Journal of Public Health. 1990;80(5):541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen PD, Quinn VP, Ershoff DH. Maintenance of nonsmoking postpartum by women who stopped smoking during pregnancy. American Journal of Public Health. 1990;80(8):992–994. doi: 10.2105/ajph.80.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride CM, Pirie PL. Postpartum smoking relapse. Addictive Behaviors. 1990;15(2):165–168. doi: 10.1016/0306-4603(90)90020-X. [DOI] [PubMed] [Google Scholar]
- 18.Ownby DR, Johnson CC, Peterson EL. Maternal smoking does not influence cord serum IgE or IgD concentrations. The Journal of Allergy and Clinical Immunology. 1991;88(4):555–560. doi: 10.1016/0091-6749(91)90148-H. [DOI] [PubMed] [Google Scholar]
- 19.Ratner PA, Johnson JL, Bottorff JL. Smoking relapse and early weaning among postpartum women: is there an association? Birth (Berkeley, Calif) 1999;26(2):76–82. doi: 10.1046/j.1523-536x.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- 20.Visness CM, Kennedy KI. Maternal employment and breast-feeding: Findings from the 1988 National Maternal and Infant Health Survey. American Journal of Public Health. 1997;87(6):945–950. doi: 10.2105/ajph.87.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn VP, Mullen PD, Ershoff DH. Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addictive Behaviors. 1991;76(1–2):29–40. doi: 10.1016/0306-4603(91)90037-I. [DOI] [PubMed] [Google Scholar]
- 22.Cnattingius S. Smoking habits in early pregnancy. Addictive Behaviors. 1989;14(4):453–457. doi: 10.1016/0306-4603(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 23.Palma S, Perez-Iglesias R, Pardo-Crespo R, Llorca J, Mariscal M, gado-Rodriguez M. Smoking among pregnant women in Cantabria (Spain): Trend and determinants of smoking cessation. BMC Public Health. 2007;7:65. doi: 10.1186/1471-2458-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice F, Lewis A, Harold G, van den BM, Boivin J, Hay DF, Owen MJ, Thapar A. Agreement between maternal report and antenatal records for a range of pre and perinatal factors: The influence of maternal and child characteristics. Early Human Development. 2006;83:497–504. doi: 10.1016/j.earlhumdev.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology (Cambridge, Mass) 1999;10(6):774–777. doi: 10.1097/00001648-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. American Journal of Epidemiology. 1986;123(4):670–676. doi: 10.1093/oxfordjournals.aje.a114286. [DOI] [PubMed] [Google Scholar]
- 27.Githens PB, Glass CA, Sloan FA, Entman SS. Maternal recall and medical records: An examination of events during pregnancy, childbirth, and early infancy. Birth (Berkeley, Calif) 1993;20(3):136–141. doi: 10.1111/j.1523-536X.1993.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 28.Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. Journal of Clinical Epidemiology. 1998;51(5):399–405. doi: 10.1016/S0895-4356(97)00304-1. [DOI] [PubMed] [Google Scholar]
- 29.Bryant HE, Visser N, Love EJ. Records, recall loss, and recall bias in pregnancy: A comparison of interview and medical records data of pregnant and postnatal women. American Journal of Public Health. 1989;79(1):78–80. doi: 10.2105/ajph.79.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman Y, Koren G, Mattice K, Shear H, Pellegrini E, MacLeod SM. Determinants of recall and recall bias in studying drug and chemical exposure in pregnancy. Teratology. 1989;40(1):37–45. doi: 10.1002/tera.1420400106. [DOI] [PubMed] [Google Scholar]
- 31.Kendrick JS, Zahniser SC, Miller N, Salas N, Stine J, Gargiullo PM, et al. Integrating smoking cessation into routine public prenatal care: The smoking cessation in pregnancy project. American Journal of Public Health. 1995;85(2):217–222. doi: 10.2105/ajph.85.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell T, Crawford M, Woodby L. Measurements for active cigarette smoke exposure in prevalence and cessation studies: Why simply asking pregnant women isn’t enough. Nicotine & Tobacco Research. 2004;6(Suppl 2):S141–S151. doi: 10.1080/14622200410001669141. [DOI] [PubMed] [Google Scholar]