Abstract
Background
Netrin-1, a secreted laminin-related protein, is known to regulate not only axonal guidance and neuronal cell migration, but also blood–brain barrier integrity and inflammation. Two preliminary studies reported altered serum netrin-1 levels in multiple sclerosis; however, associations with longitudinal clinical and magnetic resonance imaging activity have not been investigated.
Objectives
We aimed to assess serum netrin-1 in multiple sclerosis and controls with respect to disease activity and its temporal dynamics.
Methods
Serum netrin-1 was assessed by enzyme-linked immunosorbent assay in 79 patients with clinically isolated syndrome or multiple sclerosis, and 30 non-inflammatory neurological disease controls. In patients, serum samples were collected immediately prior to gadolinium-enhanced 3 T magnetic resonance imaging at two time points (initial contrast-enhancing gadolinium+ n = 47, non-enhancing gadolinium– n = 32; reference gadolinium– n = 70; median time-lag 1.4, interquartile range 1.0–2.3 years).
Results
Serum netrin-1 levels were similar in clinically isolated syndrome, multiple sclerosis and controls, and gadolinium+ and gadolinium– patients. Among gadolinium+ patients, serum netrin-1 was decreased in clinically active (n = 8) vs non-active patients (n = 39; p = 0.041). Serum netrin-1 showed no temporal dynamics in multiple sclerosis and was unrelated to clinical data.
Conclusions
Serum netrin-1 levels show no multiple sclerosis specific changes and are not sensitive for detection of subclinical disease activity. Netrin-1 changes during relapses may deserve further examination.
Keywords: Multiple sclerosis, netrin-1, serum, disease activity, blood–brain barrier, magnetic resonance imaging
Introduction
Netrin-1 (NTN-1) is a secreted laminin-related protein with both attracting and repelling chemotropic effects, which regulate cell-migration and cell-cell or cell-substrate adhesion.1–3 In the central nervous system (CNS), NTN-1 is known to affect various processes required for tissue development and repair: axonal guidance, glial cell migration, neural growth, and the recruitment and differentiation of oligodendroglial precursor cells.1–6 A recent experimental study demonstrated, in cell culture and experimental autoimmune encephalomyelitis (EAE) models, that NTN-1 also acts as an important regulator of blood–brain barrier (BBB) integrity and inflammation.7 Furthermore, NTN-1 has been associated with multiple sclerosis (MS) pathology, as this protein has been reported to be upregulated in astrocytes and macrophages in human and murine demyelinated lesions.5,6,8,9 Conversely, NTN-1 levels appeared to be decreased in serum and cerebellum and spinal cord tissue of EAE versus control mice.10 Recently, two independent studies reported NTN-1 serum levels in MS in humans to be either elevated7 or decreased10 compared to controls, respectively. Moreover, the decrease in NTN-1 serum levels appeared to be more prominent in MS patients undergoing a clinical relapse, and 60 days post-relapse.10
In MS, active phases of the disease are associated with inflammation and BBB disruption. Magnetic resonance imaging (MRI)-based evidence of disease activity, indicated by the occurrence of new/enlarged T2 or T1 gadolinium (Gd)-enhancing lesions, provides a good surrogate for clinical measures like relapse rate and disability progression.11 Gd-enhancing lesions on MR images display ongoing inflammation, which is related to BBB disruption and immune cell infiltration at the site.12
The recently described results on NTN-1 in MS are partly contradictory, and the extent to which possible alterations of soluble NTN-1 levels may reflect BBB disruption, or even be implicated in pathophysiological processes of BBB breakdown in humans, has not yet been clarified. Therefore, we were prompted (a) to reassess possible differences in NTN-1 levels between MS patients and controls with non-inflammatory neurological diseases, and between patient subgroups (i.e. clinically isolated syndrome (CIS) and relapsing–remitting MS (RRMS)), and (b) to study the association of NTN-1 levels with disease activity, evidenced by Gd-enhanced lesions seen on MRI or by a clinical relapse. Furthermore, we wanted (c) to search for longitudinal changes of this protein in relation to MS.
Patients and methods
This study was approved by the ethics committee of the Medical University of Graz, Austria. All participants provided written informed consent.
Patients and controls
According to our study objectives we searched for patients (n = 79) who were seen at the MS outpatient clinic of the Department of Neurology, Medical University of Graz, between 2007–2015, and met the following criteria: (a) diagnosis of clinically isolated syndrome (CIS) suggestive of MS, or RRMS, according to available criteria13,14 at time of inclusion; (b) availability of an initial and optionally a reference Gd-enhanced MRI scan as described below, at two time-points with a minimum time interval of six months; (c) availability of serum samples at the time of both MRI scans; (d) no corticosteroid infusion within four weeks prior to sampling; and (e) availability of detailed clinical data.
Patients were considered to be in an active state of disease at the time of either examination if Gd-enhancement was present on MRI (i.e. subclinical activity), or if within 30 days prior to MRI and sampling a clinical attack had occurred (i.e. clinical activity).
As controls (n = 30), we included individuals who were seen at the outpatient clinic of the Department of Neurology, Medical University of Graz, between 2010–2012, and fulfilled the following criteria: (a) diagnosis of a neurological disease of non-inflammatory etiology (cranial/peripheral palsy – non-inflammatory neurological disease controls; headache or sensory disturbances – symptomatic controls);15 (b) availability of a diagnostic serum sample; (c) all routine-diagnostic variables measured in cerebrospinal fluid (CSF) and serum at diagnosis within normal range;15 (d) no immunomodulatory or immunosuppressive treatment prior to sampling.
Clinical assessment
The following demographic and clinical data were recorded at the time of, and in between, both MRI scans in the patients: age, gender, age at disease onset, occurrence of a relapse, and severity of disability assessed by the Expanded Disability Status Scale (EDSS).16 A relapse was defined as the appearance of at least one neurological symptom or the reappearance of a previous symptom attributed to MS with clinical worsening for at least 24 h succeeding a stable or improving neurological state during at least 30 days.14 Upon occurrence of a clinically documented relapse, patients usually obtained pulsed steroid therapy of either three- or five-day 1000 mg/day methylprednisolone (number of relapses in between both MRI scans: 0 n = 49, 1 n = 17, 2 n = 8, 3 n = 3, 4 n = 2). The time lag between the last corticosteroid infusion was at minimum four weeks prior to MRI and blood sampling (time interval last infusion prior to scan: at initial scan median 629 days, interquartile range (IQR) 258–1191 days; at reference scan median 679 days, IQR 379–1136 days). At the time of the initial scan, 36 out of 79 patients received long-term disease-modifying treatment (DMT) (interferon beta n = 16, glatiramer acetate n = 10, natalizumab n = 8, fingolimod n = 1, teriflunomide n = 1). At the time of the second MRI scan, 52 of 70 patients received DMT (interferon beta n = 24, glatiramer acetate n = 7, natalizumab n = 16, fingolimod n = 3, teriflunomide n = 1, dimethyl fumarate n = 1).
Serum sampling and analyses
Blood samples were drawn at the time of the MRI examinations in patients or as part of the diagnostic evaluation in controls. 8 ml of peripheral blood was obtained from each subject; an additional CSF sample was drawn by lumbar puncture in controls. Samples were aliquoted and stored at –80℃ immediately after collection for patients, or after routine diagnostic work-up for controls, until further analyses. Handling and storage of samples was performed according to international consensus guidelines.17 For serum and CSF sample pairs of controls a routine diagnostic work-up was performed.18 All of the control patients tested negative for inflammatory variables in serum and CSF.
Serum NTN-1 levels were measured by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (CSB-E11899h, Cusabio Biotech, Wuhan, China). For the evaluation of temporal dynamics of serum NTN-1 the relative concentration change from initial to the second MRI examination was calculated.
MRI
All patients underwent MRI examinations of the brain on a 3 Tesla Tim Trio system (Siemens Medical Systems, Erlangen, Germany) using a 12-element phased-array head coil. Intravenous Gd contrast (0.1 ml Gadovist per kg body weight) was administered subsequent to structural and T2-weighted imaging and blood sampling. Structural imaging was performed using a T2-weighted 2D fast fluid attenuated inversion recovery (FLAIR) sequence (repetition time/echo time/inversion time (TR/TE/TI) = 9000 ms/70 ms/2500 ms, in plane resolution = 0.9 × 0.9 mm2, slice thickness = 3 mm). Following Gd administration, an axial magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR/TE/TI = 1410 ms/2.29 ms/900 ms) matching the resolution and angulation of the FLAIR sequence was performed for the detection of active lesions. A second MRI examination, including blood sampling, was performed according to the identical protocol in 70 (88.6%) patients (time interval between both scans median 1.4 years, IQR 1.0–2.3 years).
Image analysis focused on the determination and counting of the number of Gd-enhancing lesions, and was performed by an experienced neurologist.
Statistical analyses
Statistical analyses were performed using SPSS Statistics (version 23.0, IBM Corp. Armonk, New York, USA) and GraphPad Prism (version 5.00, GraphPad Software, San Diego, USA).
All variables were tested for normal distribution using the Kolmogorov-Smirnov test. Group differences were determined by either chi-square test for categorical data or Mann–Whitney U test for continuous variables. Differences between more than two groups were defined by applying Kruskal–Wallis test, followed by post-hoc Dunn’s Multiple Comparison test. Longitudinal, paired samples were compared using the Wilcoxon signed rank test or sign test as appropriate. We performed Spearman correlations to determine the correlation coefficients for serum levels with demographic and clinical data.
Significance level was set at 5% (p < 0.05).
Results
Subject description
Demographic and clinical data of patients and controls included in this study are listed in Table 1. Patients (n = 79) and controls (n = 30) were comparable regarding age and gender, as were patient and control subgroups.
Table 1.
Demographic and clinical data of study subjects.
| CIS/MS n = 31/n = 48 | Controls n = 30 | p-Value | |
|---|---|---|---|
| n Female | 52 (65.8) | 19 (63.3) | n.s.a |
| Age (years) | 32.5 (26.7–41.0) | 36.5 (28.9–46.2) | n.s.b |
| Disease duration (years) | 3.2 (1.0–8.1) | N/A | |
| Time lag first-second MRI (years) | 1.4 (1.0–2.3) | N/A | |
| EDSS | 1.0 (0.0–2.3) | N/A | |
| EDSS at second MRI | 1.0 (0.0–2.0) | N/A | |
| n DMT | 36 (45.6) | N/A | |
| n DMT at second MRI | 52 (77.2) | N/A | |
| ARR | 0.72 (0.42–1.35) | N/A | |
| ARR at second MRI | 0.73 (0.41–1.13) | N/A | |
| Number of Gd+ lesionsc | 2 (1–4) | N/A |
ARR: annualized relapse rate for RRMS patients; CIS: clinically isolated syndrome; DMT: disease-modifying treatment; EDSS: Expanded Disability Status Scale; Gd+: gadolinium positive; MRI: magnetic resonance imaging; MS: multiple sclerosis; n: number of subjects; N/A: not applicable; n.s.: not significant; RRMS: relapsing–remitting multiple sclerosis.
Unless otherwise indicated, data are given for time at the first MRI scan. Values are given as number (%) or as median (interquartile range). Significance (p < 0.05) was assessed between subgroups by Chi-squared testa or Mann–Whitney U test.b
Data are given for Gd+ patients only (n = 13/n = 34).
At the time of the initial MRI scan, 47 of all patients showed Gd-enhancing lesions (Gd+) (70.2% female; age median 32.0, IQR 26.6–41.1 years; CIS n = 13, RRMS n = 33, secondary progressive MS n = 1) and 32 did not (Gd–) (59.4% female; age median 33.3, IQR 27.2–40.4 years; CIS n = 18, RRMS n = 14). Eight of the Gd+ but none of the Gd– patients had suffered from a clinical relapse within 30 days prior to the examination, i.e. were in an active disease stage as per our definition. In all patients with clinical activity blood sampling was performed prior to corticosteroid treatment. The control group consisted of non-inflammatory neurological disease controls (cranial/peripheral paresis n = 9) and symptomatic controls (headache n = 8, sensory disturbances n = 13).
Serum NTN-1 group differences
NTN-1 levels measured in serum from MS patients at time of the first MRI and from controls are given in Table 2.
Table 2.
Netrin-1 serum levels in clinically isolated syndrome (CIS)/multiple sclerosis (MS) patients and controls.
| CIS n = 31 | MS n = 48 | CIS/MS n = 31/n = 48 | Controls n = 30 | p-Value | |
|---|---|---|---|---|---|
| Gd+ CIS/MS n = 13/n = 34 | 440.3 (240.9–482.7) | 343.0 (267.1–537.0) | 344.1 (264.5–531.8) | n.s.a | |
| Gd– CIS/MS n = 18/n = 14 | 347.0 (290.4–429.8) | 292.1 (269.0–467.5) | 316.1 (279.4–438.5) | n.s.a | |
|
All
|
376.0 (280.0–471.1) |
335.8 (268.0–512.3) |
342.4 (272.8–482.7) |
404.5 (273.9–550.1) |
n.s.b |
| p-Value | n.s.a | n.s.a | n.s.a |
Gd+: gadolinium-positive; Gd–: gadolinium-negative; n: number of subjects; n.s.: not significant.
Values are given in pg/ml as median (interquartile range). Significance (p < 0.05) was assessed between subgroups by Mann–Whitney U testa or Kruskal–Wallis test.b
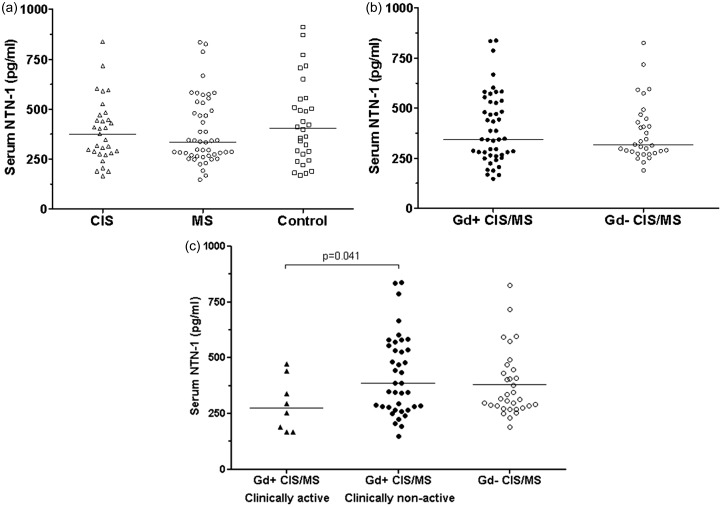
Serum NTN-1 levels did not significantly differ between CIS/MS patients and controls, or between CIS and clinically definite MS patients (Figure 1(a)).
Figure 1.
Serum netrin-1 (NTN-1) levels in gadolinium-positive (Gd+) and gadolinium-negative (Gd–) clinically isolated syndrome (CIS)/multiple sclerosis (MS) patients and controls.
Cross-sectional comparisons of serum NTN-1 levels were not significant between CIS patients (n = 13), MS patients (n = 34), and controls (n = 30) (a), and between CIS/MS patients with Gd-enhanced lesions at first magnetic resonance imaging (MRI) (Gd+ n = 47) and without Gd-active lesions (Gd– n = 32) (b). Only among Gd+ patients, those who were also in a clinically active state of disease (relapse within 30 days prior to examination, n = 8) had significantly lower serum NTN-1 levels compared to clinically non-active Gd+ patients (n = 39) (p = 0.041). NTN-1 levels in clinically active Gd+ were similar to NTN-1 levels in initially Gd– patients (n = 32) (c). Significance (p < 0.05) was assessed by applying Kruskal–Wallis test, or Mann–Whitney U test.
n: number of subjects.
No significant difference of serum NTN-1 levels was observed when comparing patients with initially MRI-based evidence of disease activity and non-active patients (Figure 1(b)). Within the subgroup of Gd+ patients, those patients who experienced an additional clinical attack within 30 days prior to examination (clinically active Gd+, n = 8) showed significantly decreased serum NTN-1 levels compared to patients who did not (clinically non-active Gd+, n = 39) (p = 0.041, Figure 1(c)). No differences were seen between clinically active Gd+ and initially Gd– patients (n = 32). Of the clinically active Gd+ patients, five underwent a second MRI scan, at which they were clinically inactive and showed no contrast enhancing lesions. At that time point their serum NTN-1 levels were not different from the initially Gd+ or Gd– patient groups any more. The subgroup of clinically active Gd+ patients did not differ from other patients (Gd+ and/or Gd-) with regard to any clinical or demographic data at time of either examination, or over time.
Serum NTN-1 – association with MRI data
At time of the initial scan, the Gd+ patients had a median of two Gd-enhanced lesions (IQR 1–4 Gd+ lesions). No associations between the number of Gd+ lesions and the NTN-1 levels at either time point were seen. Given the fact that serum NTN-1 levels did not associate with Gd+ lesions, and no serum NTN-1 changes were found for CIS/MS compared to controls, or longitudinally for both the Gd+ and Gd– patient groups, we did not perform any further MRI analyses.
Longitudinal changes of serum NTN-1 in MS
A second MRI was performed in 38 of the initially Gd+ patients (CIS n = 10, RRMS n = 28) and all of the initially Gd– patients (CIS n = 11, RRMS n = 21), and was Gd-negative in all patients. The time lag between the initial and second MRI scans was comparable between initially Gd+ (median 1.8 years, IQR 1.0–2.9 years) and Gd– (median 1.2 years, IQR 1.0–2.3 years) patient subgroups.
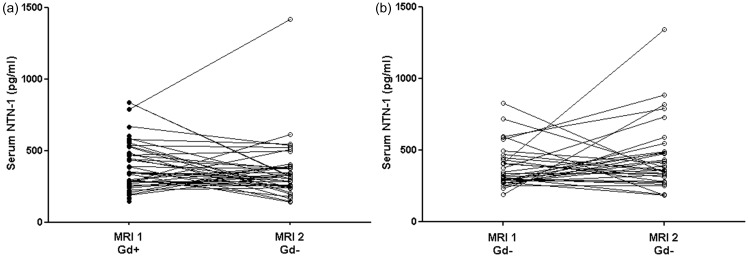
Serum NTN-1 levels showed no temporal dynamics in either initially Gd+ (serum NTN-1 at first MRI median 344.1, IQR 264.5–531.8 pg/ml; at second MRI median 318.5, IQR 252.0–389.7 pg/ml, Figure 2(a)) or Gd- subgroup (serum NTN-1 at first MRI median 316.1, IQR 279.4–438.5 pg/ml; at second MRI median 370.1, IQR 296.6–485.1 pg/ml, Figure 2(b)). Also, the ratio of the second to the initial serum NTN-1 level did not differ significantly between initially Gd+ (ratio second/initial NTN-1 median 0.89, IQR 0.53–1.32) and Gd– patients (ratio second/initial NTN-1 median 1.08, IQR 0.78–1.55). When considering only patients that showed either an increase or decrease in serum NTN-1 levels over time, no distinct clinical or demographic characteristics were notable between both groups.
Figure 2.
Temporal dynamics of serum netrin-1 (NTN-1) in gadolinium-positive (Gd+) and gadolinium-negative (Gd–) clinically isolated syndrome (CIS)/multiple sclerosis (MS) patients.
Longitudinal comparisons of serum NTN-1 between first and second scan time were not significant for either initially Gd+ (time interval magnetic resonance imaging (MRI)1–MRI2 median 1.8 (interquartile range (IQR) 1.0–2.9) years) (a) or Gd– CIS/MS patients (time interval MRI1–MRI2 median 1.2 (IQR 1.0–2.3) years) (b). Significance (p < 0.05) was assessed by applying Wilcoxon signed rank test.
Serum NTN-1 – association with demographic and clinical data
Serum NTN-1 levels or the longitudinal change in time (delta NTN-1) were similar for both genders. DMT usage, type, or change over time did not alter NTN-1 variables. Also, no correlations were found for NTN-1 variables with age at sampling, age at disease onset, disease duration, physical disability as determined by EDSS at sampling, EDSS change over time, annual relapse rate at time of either time point of examination (for RRMS patients: at initial scan median 0.72, IQR 0.42–1.35; at reference scan median 0.73, IQR 0.41–1.13), and the number or rate of relapses between both examinations. The presence of relapses between both examinations (n = 29/70) did not affect the NTN-1 serum levels.
Discussion
We aimed to investigate the role of serum NTN-1 in early MS and its relation to BBB disruption, i.e. disease activity evidenced by contrast-enhanced MRI or clinical relapse. We found no evidence for altered serum NTN-1 levels in patients with CIS or MS compared to controls, nor did NTN-1 levels differ between Gd+ and Gd– patients. Moreover, we did not find any temporal dynamics for NTN-1 in longitudinal serum samples in relation to disease activity assessed by Gd-enhancing lesions on MRI. Gd+ patients with additional clinical disease activity at the time of blood sampling had significantly lower serum NTN-1 levels compared to clinically stable patients in this subgroup.
NTN-1 is a protein known to have a varied and complex functionality within the nervous system,1–7 and intercellular upregulation was seen at site of lesions in MS.5,6,8,9 The functionality of NTN-1 was associated with inflammation,7,8,19 endothelial and in specific BBB integrity,7,20,21 and remyelination.5,6,9,22 Along these lines, two studies have also reported on NTN-1 serum levels in MS patients with contradictory results. In a primarily experimental study, a two-fold increase was reported in MS patients (n = 7) compared to controls (n = 9) (p ≤ 0.05).7 NTN-1 levels of the patients in that study were up to 15 times as high as in our patient groups.
Another study analyzed serum samples from a larger cohort of MS patients (n = 90) and found decreased NTN-1 levels compared to controls (n = 30) (p ≤ 0.001). This decrease appeared to be more prominent in patients with RRMS during and until 60 days after a clinical relapse (n = 10), compared to non-active patients (p ≤ 0.001), whereas solely CIS patients did not differ from controls.10 Notably, serum NTN-1 levels of MS patients in this study were comparable to the serum levels found in both our patients and controls. However, their control group (mean 51.9 ± standard deviation (SD) 10.5 years) seemed to be considerably older than their MS patient group (mean 42.2, range 22–76 years).10 Experimental studies have suggested that NTN-1 expression may increase with age, because of a decreased NTN-1 responsiveness with older age.23,24 Furthermore, we included only patients with CIS and early RRMS, whereas the previous study also investigated progressive forms of MS.10 Notably, the same ELISA kit was used in both previous and our studies.
We tested if soluble NTN-1 would be differently regulated during active phases of the disease, but could not find any significant differences between Gd+ and Gd– patients. Only among patients with MRI-based signs of disease activity, those who additionally experienced a clinical attack within 30 days prior to sampling had decreased serum NTN-1 levels compared to those who did not. As these results are based on a very small number of subjects, the possible association of serum NTN-1 alterations with clinical relapses should be investigated in further studies including a higher number of patients.
Recently, the effect of NTN-1 on BBB regulation has been experimentally investigated in human in vitro7 and murine in vivo models.7,25 NTN-1 appeared to regulate junctional protein levels,7,25 to reduce diffusion of blood-derived plasma proteins across the BBB, and to counteract inflammatory effects at the BBB.7 Also, treatment with NTN-1 in EAE mice improved disease severity and BBB breakdown.7 We did not find any associations of NTN-1 levels with BBB dysfunction in the human situation by correlating the serum levels with the number of Gd-enhanced lesions or by comparing patients with and without Gd-enhancing lesions on MRI. Moreover, we did not find any changes in serum NTN-1 levels when following the dynamics thereafter, in either initially Gd+ or Gd– patients.
MS lesions with only subtle breakdown of the BBB can show delayed enhancement or enhancement at higher doses only.26 It therefore cannot be excluded that some patients with disease activity have been missed. However, to obtain consistent sensitivity, a five-minute post-injection delay was included in all examinations, which was considered to be sufficient to detect most of the active lesions.27
Still, the effect of BBB disruption, as evidenced by Gd-enhanced MRI in MS patients, on serum NTN-1 might have been too small to detect here. This could partially explain the fact that clinically active patients did show a significant decrease in NTN-1 serum levels in comparison to solely sub-clinically active patients. Possibly, correlations between serum NTN-1 and MRI-based evidence for disease activity might arise when considering patients with more extensive or tumefactive contrast-enhancing lesions.
Apart from BBB regulation, other functions of NTN-1 might play a role in MS as well. Multiple functions of NTN-1 have been shown not only in the CNS, but also in the periphery: anti- or pro-inflammation, cell adhesion, motility, proliferation and differentiation during development of several epithelial and endothelial tissues, and apoptosis and tumor growth mediation.4,28–35 We further searched for a correlation of serum NTN-1 with demographic and clinical data, including disease duration, physical disability, etc. However, we found no correlations of NTN-1 levels with any clinical or demographic data.
Here, we focused on patients in early phases of the disease with relatively short disease duration and low grade of disability, which needs to be acknowledged as a limitation of the study. We therefore cannot exclude the possibility that serum NTN-1 might be altered in more advanced phases of the disease, including patients with a higher degree of disability, as well as progressive MS forms. Nevertheless, with the present study design with simultaneous collection of blood samples and MRI examinations we can conclude that at least in the early phase of the disease BBB breakdown as evidenced by MRI contrast-enhancing lesions is not reflected by altered NTN-1 levels in the serum.
In summary, serum NTN-1 is unrelated to MS-specific changes and not sensitive to subclinical disease activity. The possible association of serum NTN-1 changes with clinical relapses may deserve further examination.
Acknowledgements
The authors would like to thank Kerstin Kröll, Annemarie Ferstl-Rohrbacher and Roberta Bichler for excellent technical assistance. M Voortman received funding from the Austrian Federal Ministry of Science, Research and Economics and was trained within the frame of the PhD Program Molecular Medicine of the Medical University of Graz.
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M Voortman, T Pekar, D Bachmayer, J-J Archelos, T Stojakovic, H Scharnagl, S Ropele and A Pichler declare that there is no conflict of interest.
C Enzinger has received funding for travel and speaker honoraria from Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis Genzyme and Teva Pharmaceutical Industries Ltd./Sanofi Aventis; research support from Merck Serono, Biogen Idec., and Teva Pharmaceutical Industries Ltd./Sanofi Aventis; serving on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva Pharmaceutical Industries Ltd./Sanofi Aventis; academic editor for PLOS One.
S Fuchs serves on scientific advisory boards for Biogen Idec, Novartis, Genzyme, Merck Serono, Teva Pharmaceutical Industries Ltd. and has received funding for travel and speaker honoraria from Bayer Schering Pharma, Merck Serono, Biogen Idec and Sanofi Aventis.
F Fazekas serves on scientific advisory boards for Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd.; serves on the editorial boards of Multiple Sclerosis, the Polish Journal of Neurology and Neurosurgery, and the Swiss Archives of Neurology and Psychiatry; provides services for Actelion and Parexel and has received speaker honoraria and support from Almirall, Merck Serono, Novartis, Pfizer, Roche, Shire and Teva Pharmaceutical Industries Ltd.
T Seifert-Held received speaker honoraria and support from Teva Pharmaceutical Industries Ltd, Novartis, Biogen Idec, Eisai.
M Khalil has received funding for travel and speaker honoraria from Bayer Schering Pharma, Novartis Genzyme, Merck Serono, Biogen Idec, and Teva Pharmaceutical Industries Ltd.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This study represents a sub-study supported by the Austrian Federal Ministry of Science, Research and Economics (core-study named ‘BIG-WIG MS’ (Bildgebung, Immunpathogenese, Gesundungsfaktoren – Wien, Innsbruck, Graz – bei Multiple Sklerose’; ‘Neuroimaging, immunopathogenesis and salutogenic factors in MS – a collaborative effort of the universities of Vienna, Innsbruck and Graz’)). This study was further supported by an unrestricted research grant from TEVA Pharmaceutical Industries Ltd.
References
- 1.De Castro F. Chemotropic molecules: Guides for axonal pathfinding and cell migration during CNS development. News Physiol Sci 2003; 18: 130–136. [DOI] [PubMed] [Google Scholar]
- 2.Shekarabi M. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci 2005; 25: 3132–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarjour A, Bull S-J, Almasieh M, et al. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J Neurosci 2008; 28: 11003–11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: Versatile extracellular cues with diverse functions. Development 2011; 138: 2153–2169. [DOI] [PubMed] [Google Scholar]
- 5.Cayre M, Courtès S, Martineau F, et al. Netrin 1 contributes to vascular remodeling in the subventricular zone and promotes progenitor emigration after demyelination. Development 2013; 140: 3107–3117. [DOI] [PubMed] [Google Scholar]
- 6.Tepavčević V, Kerninon C, Aigrot MS, et al. Early netrin-1 expression impairs central nervous system remyelination. Ann Neurol 2014; 76: 252–268. [DOI] [PubMed] [Google Scholar]
- 7.Podjaski C, Alvarez JI, Bourbonniere L, et al. Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain 2015; 138: 1598–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon C, Ahn M, Jeong C, et al. Immunohistochemical study of netrin-1 in the spinal cord with rat experimental autoimmune encephalomyelitis. Immunol Invest 2011; 40: 160–171. [DOI] [PubMed] [Google Scholar]
- 9.Bin JM, Rajasekharan S, Kuhlmann T, et al. Full-length and fragmented netrin-1 in multiple sclerosis plaques are inhibitors of oligodendrocyte precursor cell migration. Am J Pathol 2013; 183: 673–680. [DOI] [PubMed] [Google Scholar]
- 10.Mulero P, Córdova C, Hernández M, et al. Netrin-1 and multiple sclerosis: A new biomarker for neuroinflammation? Eur J Neurol. Epub ahead of print 5 July 2017. DOI: 10.1111/ene.13340. [DOI] [PubMed]
- 11.Filippi M, Preziosa P, Rocca M. Magnetic resonance outcome measures in multiple sclerosis trials: Time to rethink? Curr Opin Neurol 2014; 27: 290–299. [DOI] [PubMed] [Google Scholar]
- 12.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett 2011; 585: 3770–3780. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 Revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler J 2013; 19: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 17.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009; 73: 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson M, Alvarez-Cermeño J, Bernardi G, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J Neurol Neurosurg Psychiatry 1994; 57: 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ly NP, Komatsuzaki K, Fraser IP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A 2005; 102: 14729–14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Noble F, Klein C, Tintu A, et al. Neural guidance molecules, tip cells, and mechanical factors in vascular development. Cardiovasc Res 2008; 78: 232–241. [DOI] [PubMed] [Google Scholar]
- 21.Wilson B, Ii M, Park K, et al. Netrins promote developmental and therapeutic angiogenesis. Science (80–) 2006; 313: 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Li Y, Lu H, et al. Netrin-1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2013; 33: 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birey F, Aguirre A. Age-dependent netrin-1 signaling regulates NG2+ glial cell spatial homeostasis in normal adult gray matter. J Neurosci 2015; 35: 6946–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shewan D, Dwivedy A, Anderson R, et al. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci 2002; 5: 955–962. [DOI] [PubMed] [Google Scholar]
- 25.Wen J, Qian S, Yang Q, et al. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp Ther Med 2014; 8: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver NC, Good CD, Barker GJ, et al. Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 1997; 120: 1149–1161. [DOI] [PubMed] [Google Scholar]
- 27.Uysal E, Erturk SM, Yildirim H, et al. Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 H gadolinium chelates for detecting multiple sclerosis lesions. Am J Roentgenol 2007; 188: 697–702. [DOI] [PubMed] [Google Scholar]
- 28.Yıldırım ME, Kefeli U, Aydın D, et al. The value of plasma netrin-1 in non-small cell lung cancer patients as diagnostic and prognostic biomarker. Tumor Biol 2016; 37: 11903–11907. [DOI] [PubMed] [Google Scholar]
- 29.Ko SY, Dass CR, Nurgali K. Netrin-1 in the developing enteric nervous system and colorectal cancer. Trends Mol Med 2012; 18: 544–554. [DOI] [PubMed] [Google Scholar]
- 30.Ramesh G, Krawczeski CD, Woo JG, et al. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2010; 5: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol 2008; 294: F731–F738. [DOI] [PubMed] [Google Scholar]
- 32.Obermüller N, Geiger H, Weipert C, et al. Current developments in early diagnosis of acute kidney injury. Int Urol Nephrol 2014; 46: 1–7. [DOI] [PubMed] [Google Scholar]
- 33.Lu X, Le Noble F, Yuan L, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 2004; 432: 179–186. [DOI] [PubMed] [Google Scholar]
- 34.Çekmez Y, Garip Ş, Ulu İ, et al. Maternal serum Netrin-1 levels as a new biomarker of preeclampsia. J Matern Neonatal Med 2016; 7058: 1–3. [DOI] [PubMed] [Google Scholar]
- 35.Ramesh G, Berg A, Jayakumar C. Plasma netrin-1 is a diagnostic biomarker of human cancers. Biomarkers 2011; 16: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]