ABSTRACT
Adherens junctions (AJs) are molecular complexes that mediate cell-cell adhesive interactions and play pivotal roles in maintenance of tissue organization in adult organisms and at various stages of development. AJs consist of cadherin adhesion receptors, providing homophilic ligation with cadherins on adjacent cells, and members of the catenin protein family: p120, β- and α-catenin. α-catenin's linkage with the actin cytoskeleton defines the linear or punctate organization of AJs in different cell types. Myosin II-dependent tension drives vinculin recruitment by α-catenin and stabilizes the linkage of the cadherin/catenin complex to F-actin. Neoplastic transformation leads to prominent changes in the organization, regulation and stability of AJs. Epithelial-mesenchymal transition (EMT) whereby epithelial cells lose stable cell-cell adhesion, and reorganize their cytoskeleton to acquire migratory activity, plays the central role in cancer cell invasion and metastasis. Recent data demonstrated that a partial EMT resulting in a hybrid epithelial/mesenchymal phenotype with retention of E-cadherin is essential for cancer cell dissemination. E-cadherin and E-cadherin-based AJs are required for collective invasion and migration, survival in circulation, and metastatic outgrowth.
KEYWORDS: actin, adherens junctions, cancer cell dissemination, cell-cell adhesion, E-cadherin, EMT
Introduction
Cell-cell adhesive interactions play a key role in morphogenetic processes in development and in tissue organization. Adherens junctions (AJs)— specialized cell structures that mediate cell-cell adhesion—regulate cytoskeleton reorganization, intracellular signaling and transcriptional regulation. AJs are highly conservative structures consisting of cadherin cell-cell adhesion receptors, which are responsible for the homophilic interactions between adjacent cells, and a cytoplasmic multiprotein complex that links the AJs with the actin filaments.1,2 In epithelial cells, endothelial cells, neural cells, fibroblasts, intercalated disks of cardiac myocytes, different subtypes of the classical cadherins (e.g., E-, N-, P-, VE-cadherin) were identified in AJs. Depending on how AJs associate with actin filaments, AJs may be classified into 2 forms: linear (tangential), typical of epithelial cells, and punctate (radial), typical of fibroblasts.3 The actin cytoskeleton regulatesthe assembly, organization, stability and remodeling of AJs.
Structure and organization of AJs, functional connection of AJs with the actin cytoskeleton
In epithelial tissues, E-cadherin is a key mediator of stable cell–cell adhesion. In epithelial cells, E-cadherin-based AJs form a continuous, linear circumferential belt (zonula adherens) around the apical part of the cell. Junction-associated actin filaments run parallel to the cell-cell border and form a compact belt-like structure called a circumferential bundle.3 The AJs are located between tight junctions and desmosomes, and these 3 junction systems form the so called epithelial junctional complex4 that maintains structural and functional integrity of epithelia. Tight junction assembly and maturation are closely linked to the assembly of adherens junctions. In epithelial junctional complex, tight junctions ensure selective and regulated paracellular diffusion to maintain homeostasis in organs and tissues.5-7 Tight junctions also form the border between the apical and basolateral plasma membrane domains that restricts the exchange of membrane components between the apical and basolateral cell domains. It had been previously found that formation of tight junctions is necessary for apico-basal polarization. Actin bundles underlying tight junctions and AJs drive compaction of epithelial cells.8,9 Mechanical stability in epithelial tissues is provided by another specialized adhesive structures, desmosomes, which anchor intermediate filaments to the plasma membrane and thus mediate strong adhesion between cells. The composition, molecular architecture and functions of cell-cell junctions have been described in several reviews.1,2,7,10,11
Classical cadherins are transmembrane glycoproteins consisting of an amino-terminal extracellular domain subdivided into 5 repetitive extracellular subdomains (EC1-EC5), a transmembrane domain, and a C-terminal cytoplasmic domain.1,2 The extracellular domain mediates Ca2+-dependent homophilic interaction with the cadherin molecule on the surface of a neighboring cell. Adhesion is provided by trans interaction of the EC1 domains of cadherins of neighboring cells. Upon establishment of adhesive interaction between cells, cis interactions of the EC1 domain of one cadherin molecule with the EC2 domain of an adjacent cadherin molecule cause cadherins to cluster.12,13 Through cis interactions of the EC domains adhesive clusters are formed, however, AJs can still assemble even in the absence of E-cadherin cis oligomerization.14 The extreme importance of association of E-cadherin cytoplasmic domains with F-actin under tension for formation of adhesive clusters and their maturation into AJs has been reviewed in detail.2,15 The cytoplasmic domain of E-cadherin binds to members of the catenin protein family, such as β-catenin and p120-catenin. p120-catenin regulates the stability of cell-cell adhesion by controlling the retention of E-cadherin at the cell surface.16-18 α-Catenin, whose N-terminal domain interacts with β-catenin, plays a key role in linking of AJs with actin cytoskeleton.19-22 α-Catenin's C-terminal domain binds actin filaments, and its central part contains both the vinculin-binding domain MI and the MII and MIII domains that inhibit the binding of vinculin.22,23 The binding of α-catenin to F-actin through its actin-binding domain stabilizes adhesive clusters24 and initiates vinculin recruitment by α-catenin. In a great number of studies it has been demonstrated that tension generated by myosin II is indispensable for AJ assembly.22,25-27 Recent studies showed that α-catenin recruits vinculin through a force-dependent conformational change in α-catenin.26,28,29 Application of a force to an α-catenin molecule induces unfolding of α-catenin and hence, destabilization of the interactions between the MI vinculin binding and MII and MIII inhibitory domains,23,30 and opening of the MI domain, resulting in an apparent 1000-fold increase in affinity for vinculin. The force threshold of this transition (∼5 pN) is comparable to combined forces of a few myosin II motors (2–3 pN), therefore, tension generated by myosin II is capable of inducing force-dependent intramolecular unfolding of α-catenin and vinculin recruitment that stabilize the cadherin/catenin complex providing additional linkages to F-actin.26
Recent super-resolution microscopy studies of the nanoscale protein organization in adhesion complexes using a planar cadherin-coated substrate have provided new insights into molecular architecture and protein-protein interactions in AJs and the role of force-dependent conformational changes of vinculin in triggering actin polymerization.31 It was discovered that plasma-membrane proximal cadherin–catenin compartment was segregated from the actin cytoskeletal compartment by an intermediate zone containing vinculin, zyxin, and VASP. In all cases, vinculin position was determined by α-catenin. In MDCK cells, vinculin is recruited to E-cadherin adhesions while in a relatively compact, low tension state. However, in C2C12 myoblasts that form N-cadherin-based adhesions containing vinculin in high tension state, molecules of vinculin are extended up to 30 nm. Besides tension, conformational activation of vinculin is regulated by the Abl kinase and PTP1B phosphatase. Vinculin activation changes the position of VASP, moving it down into the actin cytoskeletal compartment where VASP promotes further actin assembly. It was also found that actin cytoskeletal compartment of adhesion complexes also contained other actin-binding proteins, such as EPLIN, myosin II, palladin, and α-actinin.
EPLIN can additionally stabilize the circumferential actin belt by inhibiting actin depolymerization and crosslinking actin filaments.32 Depletion of EPLIN disrupted cell-cell adhesion converting linear AJs into punctate AJs associated with straight actin bundles.33 Another actin-binding protein, afadin, is recruited to the AJs via α-catenin. Afadin, through binding to JAM and nectins, is also involved in the establishment of apico-basal polarity. The activated afadin interacts with p120 catenin and strengthens its binding to E-cadherin, which results in reduced E-cadherin endocytosis.34-36 Myosin IIA is involved in the formation and disassembly of the AJs in epithelial cells.37-39 Actomyosin-based contractility maintains shape and function of AJs and supports structural integrity in epithelial tissues. Treatment with myosin ATPase inhibitor blebbistatin resulted in wavy appearance of the AJs. In fibroblasts, radial (punctate) AJs are associated with straight actin bundles oriented perpendicular to the cell-cell boundary and formation and maintenance of these AJs critically depends on myosin II-mediated contractility and the function of Rho effector, ROCK.40,41
It was shown that tension-sensitive actin assembly supports actomyosin contractility at AJs and junctional integrity.42 Several years ago de novo actin nucleation was detected at cell-cell junctions.43-46 Actin polymerization at cell-cell junctions is mediated by 2 types of actin nucleators, Arp2/3 and formins. It was shown that the Arp2/3 complex colocalizes with E-cadherin at AJs and inhibition of Arp2/3-mediated actin assembly strongly affects AJ formation.46 Arp2 or Arp3 RNAi reduces actin nucleation at AJs and junctional F-actin content.43,47 De novo Arp2/3-dependent polymerization of actin filaments was also detected in vitro at cadherin-enriched junctions on purified membranes; assembly of junctional actin critically depended on the presence of α-actinin-4.48
Arp2/3-mediated actin assembly at AJs is regulated by the Arp2/3 activator WAVE2. WAVE2 has been identified at cadherin-based cell–cell junctions and depletion of WAVE2 dramatically alters junctional actin assembly at the new cell-cell junctions and the integrity of the AJs in epithelial cells.44,45 Another Arp2/3 activator, N-WASP, colocalized with E-cadherin at cell-cell contacts.9 N-WASP, however, does not activate Arp2/3 at AJs but instead stabilizes the junctional cytoskeleton at a postnucleation stage.47 Recently it has been demonstrated that E-cadherin in AJs can directly interact with cortactin, which in the presence of N-WASP can recruit WAVE2 and Arp2/3, thus promoting actin nucleation at the AJs.49
The role of members of the formin family in the actin assembly at the AJs remains incompletely understood. Dia1 (Diaphanous-related formin 1), a major downstream effector of RhoA, was enriched at the cell-cell contacts in MCF7 breast cancer cells and depletion of Dia1 by RNAi reduced E-cadherin and actin accumulation at cell-cell contacts.50 FMNL2 (Formin-like 2) was shown to be required for Rac1-dependent actin assembly and turnover at AJs in MCF10A mammary epithelial cells cultured in 3D Matrigel.51 In Eph4 mammary epithelial cells the formin inhibitor SMIFH2 reduced F-actin and E-cadherin accumulation at the AJs. Fmnl3 (Formin-like 3) colocalized with E-cadherin at the AJs and overexpression of Fmnl3 increased junctional F-actin accumulation.52
Regulation of AJs by Rho and Rap GTPases
Rho GTPases are the main regulators of the assembly, dynamics, reorganization, and contractility of the actin cytoskeleton. Several years ago it was found that Rho, Rac and Cdc42 are required for the establishment of E-cadherin-mediated adhesion.53 Active Rac1 and Arp2/3 are initially localized in the zone of early contacts between 2 epithelial cells but their levels rapidly decrease as E-cadherin accumulates.54 As the newly formed contact begins to expand, the marginal actin bundle directly underneath it disappears; however, the actin bundles at the contact edges remain intact.55,56 RhoA activity and actomyosin contractility at the contact edges are required for expansion of the contact.54 Rho effector Dia1 also contributes to the formation of linear AJs. Depletion of Dia1 disrupted linear AJs50 but did not affect the assembly of radial AJs.57 In epithelial cells, the activities of the 2 Rho effectors Dia1 and ROCK must be precisely balanced for AJ maintenance.3 Of particular importance for the AJ integrity is the centralspindlin complex regulating Rho GEF/GAP balance. In MCF-7 cells, centralspindlin recruits RhoGEF Ect2, thus supporting junctional integrity through myosin IIA. Centralspindlin also inhibits the junctional localization of p190RhoGAP.58
The spatial organization of VE-cadherin-based AJs and the cytoskeletal networks in endothelial cells is pivotal for endothelial barrier function and vascular permeability. Endothelial AJs are highly dynamic and morphologically heterogeneous. It was found that in a monolayer of HUVECs, VEGF, TNF-α, or thrombin induce a transformation of linear continuous AJs aligned with circumferential actin bundles into fragmented radial AJs (“focal AJs”) oriented perpendicular to the cell-cell boundary and attached to straight actin bundles. In contrast to linear AJs, focal AJs contain vinculin. Inhibition of actomyosin contractility prevented this junction remodeling.59 The small GTPases of Rho family and Rap GTPases control the endothelial barrier function by regulating the spatial organization and contractility of junction-associated actin cytoskeleton.60-62 RhoA can activate various effector proteins in endothelial cells that may affect junction integrity. ROCK is one of the key regulators of linear junction disassembly. ROCK-dependent actomyosin contractility of the radial actin bundles destabilizes the endothelial barrier function. However, Rho-Dia1 signaling activated by a RhoGEF Syx promotes junction stability.63 Junctional strengthening is also effected by Rac1 activating WAVE2 which promotes actin polymerization44,45 and by Cdc42 activating WASP which may strengthen the circumferential actin bundle.47 Rap1 supports endothelial barrier function by spatially controlling myosin II activity in circumferential actin bundle through activation of the Cdc42–MRCK pathway and suppression of the Rho–ROCK pathway inhibiting formation of straight actin bundles connected to radial AJs.64
The role of cadherin-mediated adhesion in development
Cadherins play important roles at various stages of development. During blastocyst stage of early embryonic development, cells undergo compaction and apico–basal polarization. In embryos lacking both maternal and zygotic E-cadherin, during blastocyst stage of development, cells fail to compact and form a trophectodermal epithelium.65 (Cdh1−/−) mouse embryos develop normally until the blastocyst stage then they die.66,67 During development, AJs help maintain tissue integrity while epithelial cells undergo dramatic changes in shape, relative position and motility. The important morphogenetic processes such as tissue invagination and tissue extension are provided by actomyosin bundles and networks connected with cadherin-based AJs.3,68 Apical constriction that transforms columnar-shaped epithelial cells into wedge-shaped cells initiates invagination of the neuroectoderm during the neural tube closure. It has been found that Shroom 3 is the key player in various morphogenetic processes that require apical constriction.69,70 Shroom 3 recruits ROCK to the AJs that leads to contraction of the circumferential actomyosin belt and apical constriction.71,72 During neural tube morphogenesis, cells within the neural plate intercalate toward the midline, extending the tissue along the anterior-posterior axis in a process known as convergent extension. Tissue extension that was described in the chick epiblast, in the mouse endoderm and other tissues is also driven by cell intercalation and convergent extension movement. During neural crest cell delamination, cells undergo a partial EMT and migrate as a group of cells held together by N-cadherin-based AJs.73 Recent studies demonstrated that in Xenopus, E-cadherin is also required for proper migration of cranial neural crest cells in vivo, as knockdown of E-cadherin inhibits migration of cranial neural crest cells.74 Recent data suggest a novel role for E-cadherin in cell migration in embryos. In the developing zebrafish embryo, migration of primordial germ cells within the embryo requires E-cadherin-mediated cell-cell adhesion between germ cells and somatic cells. Dominant-negative mutant of E-cadherin lacking the extracellular domains inhibited cell motility.75
E-cadherin plays an important role in differentiation of epithelial cells. Studies of tissue-specific disruption of Cdh1 in mouse demonstrated that E-cadherin is required for morphogenesis of intestinal epithelium45,76 and for terminal differentiation of alveolar secretory cells of the mammary gland during lactation.44,77 Thus the studies summarized here provide important insights into the functions of AJs in development.
EMT, a driving force in cancer
The changes in cadherin-mediated cell-cell interactions play a key role in the tumor progression. Cancer cell dissemination from the primary tumor with formation of distant metastases remains the major cause of mortality of patients with cancers. Cancer cell dissemination is a result of the invasion-metastasis cascade that includes: 1) escape of cancer cells from the primary tumor and invasion of the basement membrane; 2) migration in the tissue stroma; 3) intravasation into blood and lymph vessels; 4) spreading through circulation; 5) arrest at distant organ sites and extravasation; 6) survival in distant organs; 7) metastatic outgrowth.78,79 The program of epithelial-mesenchymal transition (EMT) whereby epithelial cells lose apico-basal polarity and stable cell-cell adhesion and gain mesenchymal-like properties, such as migratory activity, is considered a central driving force of cancer cell dissemination.80 EMT is the first step of the invasion-metastasis cascade where in the primary tumor neoplastic cells disrupt stable cell-cell contacts and migrate away from the tumor, invading the basement membrane. Among the EMT-inducing factors are: aberrant activation or deregulation of oncogenic signaling pathways, such as TGFβ, EGF, HGF, Notch, FGF, Wnt, and IGF, extracellular signals from the tumor microenvironment (e.g., cancer associated macrophages or fibroblasts), and hypoxia.81,82 These signals usually activate the EMT-inducing transcription factors (EMT-TFs) – TWIST1, SNAI1, SNAI2, ZEB1, ZEB2, or PRRX1 that repress E-cadherin.83 EMT is also characterized by appearance of mesenchymal markers such as N-cadherin, vimentin and fibronectin.80 N-cadherin can drive migration of transformed cells through interaction with fibroblast growth factor receptor (FGFR) and enhancement of FGF signaling.84
For a long time downregulation of E-cadherin was considered a crucial step in carcinoma progression promoting invasion and metastasis.85-87 The studies of cell lines derived from human carcinomas demonstrated that cells expressing E-cadherin had an epithelioid phenotype and were noninvasive in vitro while carcinoma cells that lost E-cadherin expression had a fibroblastic phenotype and were invasive.88,89 Down-regulation of E-cadherin expression was found in esophageal, gastric, colon, prostate cancer, and hepatocarcinoma.90-95 In many carcinomas expression of E-cadherin is downregulated by either hypermethylation of the CDH1 gene promoter or an increase in expression of EMT-TFs, while bona fide mutations in the CDH1 gene were described only in a few types of cancers, such as diffuse-type gastric cancers and breast carcinomas.87,96 It has been generally accepted that in human malignancies, reduced expression of E-cadherin was associated with infiltrative growth, metastasis and poor prognosis for the patient.86 The impact of downregulation of E-cadherin expression on tumor progression is mostly associated with weakening of cell-cell adhesion; however, E-cadherin in AJs can also interact with receptor tyrosin kinases (EGFR, IGFR, and c-Met) to inhibit their signaling.97-99
Many invasive carcinomas, however, retain expression of E-cadherin. Immunohistochemical studies found accumulation of E-cadherin at the plasma membrane and its colocalization with α-catenin and p120 in ductal breast carcinomas, colorectal carcinomas, prostate carcinomas, pancreas carcinomas and oral squamous cell carcinomas.100-106 This suggests that invasion of carcinoma cells into the adjacent tissues is not prevented by the presence of E-cadherin. A few years ago the classic view of EMT in tumor as the transformation of epithelial cells into mesenchymal cells evolved into a concept of plasticity and transitional states.107-109 Recent data demonstrated that carcinoma cells often undergo a partial EMT where cells acquire mesenchymal traits but retain epithelial markers.110,111 In cancer, EMT is not a binary switch between epithelial and mesenchymal states, but rather a spectrum of intermediate states that are determined by the microenvironment. It is considered that a hybrid epithelial/mesenchymal phenotype may be metastable and epithelial-mesenchymal plasticity allows cells to adopt functional behaviors and quickly change their phenotype depending on external signals.112
AJs in neoplastic cells
For tumor cells, E-cadherin and E-cadherin-based AJs play an important role in survival, growth, invasion and metastasis.113 Many invasive carcinomas infiltrate surrounding tissues as multicellular clusters in which tumor cells remain connected to the neighboring tumor cells. This process, known as collective invasion,114 is mediated by E-cadherin-based AJs. Collective invasion has been described for carcinomas of breast, prostate, and colon, squamous cell carcinomas.115-118
Invasive tumors can maintain well-differentiated epithelial morphology and apico-basal polarity.119 In samples of mammary ductal carcinoma, adherens junctions, tight junctions, and desmosomes were visualized by electron microscopy.120 It has been shown that in squamous cell carcinomas derived from the esophagus, oral epithelium, lung, and cervix, E-cadherin and tight junction proteins (occludin, claudins, ZO-1, and cingulin) are expressed.121 Cell lines established from highly differentiated carcinomas can also maintain polarized epithelial phenotype (e.g., MCF-7, and T-47D breast carcinomas, A-549 non-small cell lung carcinoma, Caco-2 and T854 colorectal carcinomas). In the cells of these lines stable linear (tangential) AJs associated with circumferential actin bundles were observed.42,58 The assembly and regulation of integrity of linear AJs in well-differentiated transformed cells are similar to those in normal epithelial cells.9,58
In transformed epithelial cells that undergo partial EMT, AJs are often morphologically distinct from linear AJs in normal epithelial cells. In IAR epithelial cells, transformed by mutated Ras or chemical carcinogens, we described radial (punctate) E-cadherin-based AJs associated with straight actin bundles (Fig. 1). Unlike stable linear AJs in normal epithelial cells, the punctate AJs in transformed IAR cells were very dynamic and unstable, similar to AJs of fibroblasts. Why AJs assemble into 2 different types, linear and punctate, is far from clear. We consider that the changes in the AJ organization may be due to the significant reorganization of actin cytoskeleton and disappearance of circumferential actin bundle as a result of activation of EMT in transformed cells leading to alterations of directions of tension in the area of cell-cell contacts.57 In these cells, the deficiency of tangential tension and increased centripetal tension in the zone of cell-cell contact leads to the assembly of straight actin bundles and to maturation of punctate AJs. The link with straight actin bundles is essential for the formation of punctate AJs. As our experiments with the ROCK inhibitor Y-27632 and the myosin II ATPase inhibitor blebbistatin have shown, the formation and maintenance of punctate AJs critically depend on myosin II-mediated contractility.57 In the study of punctate AJs at the edge of epithelial colonies it was shown that after laser ablation of perpendicular actin filaments linked with the AJs, the punctate AJs converted into linear AJs.122 Eplin (whose one or 2 isoforms may be lost in certain neoplasms) is considered as a key player in the formation of linear AJs.33 EPLIN is lost in punctate AJs at the edge of epithelial colonies.122 During laser ablation and conversion of the punctate AJs into linear AJs EPLIN was recruited to the junctions. As suggested by Takeichi,3 a tensile force that is exerted on punctate AJs by perpendicular actin filaments disturbs binding of EPLIN to the AJs, and EPLIN-free punctate AJs are more dynamic than linear AJs. Our data, however, does not support this hypothesis because immunofluorescent staining revealed that both stable linear AJs in normal IAR-2 cells and unstable punctate AJs in transformed IAR cells accumulate EPLIN (unpublished).
Figure 1.
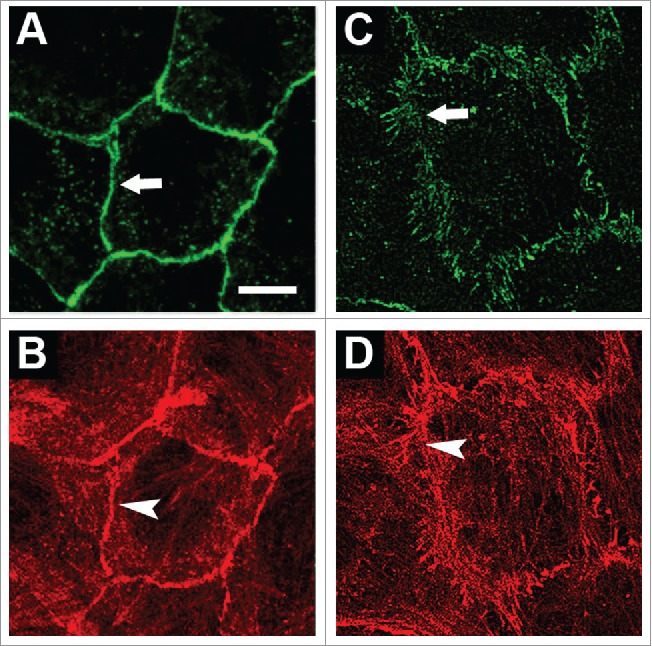
Organization of AJs and the actin cytoskeleton in normal and transformed IAR cells. Cells were stained for E-cadherin (A, C) and actin (B, D). (A-B) In normal IAR-2 epithelial cells, AJs organized as adhesion belts encircling each cell and co-localizing with circumferential actin bundles. (C-D) In transformed IAR-6–1 epithelial cells, AJs organized into clusters (radial AJs) associated with thin actin bundles. © PLoS One. Reproduced by permission of Natalya Gloushankova. Permission to reuse must be obtained from the rightsholder.57
Our studies demonstrated the differences in cell behavior during the establishment of cell-cell contact by normal IAR-2 and transformed IAR-6–1 cells retaining expression of E-cadherin and forming punctate AJs. Cell-cell interactions of normal epithelial cells resulted in the establishment of a stable cell-cell contact followed by contact paralysis at the site of the contact and at the free edges. In the culture of transformed cells, no stable cell-cell contacts were observed but rather, only transitory E-cadherin-mediated contacts between cells. These transitory contacts did not inhibit lamellipodia formation at the site of the contact. Eventually, the migrating cells changed the direction of their migration.40 In more detail the process of the cell-cell interactions was reviewed by B. Stramer and R. Mayor.123
We found that E-cadherin-based but not N-cadherin-based AJs were instrumental in effective collective migration of transformed epithelial cells over planar substrates and in migration chambers.124 Transformed IAR cells retaining E-cadherin, could migrate both collectively and individually. In contrast, neoplastic IAR cells that lost E-cadherin expression and formed only N-cadherin-based AJs migrated only individually. The recent studies of tumor budding at the cancer-host interface in human pancreatic, colorectal, lung and breast carcinoma demonstrated that collective cell migration but not single cell migration promotes cancer cell invasion.125 The experimental studies of mouse model of mammary cancer and pancreatic cancer containing a mixture of transformed cells that express different fluorescent proteins demonstrated multicolored polyclonal metastases arising from tumor cell clusters. It was established that these clusters formed at the early stages of invasion but not in distant tissues.126-128
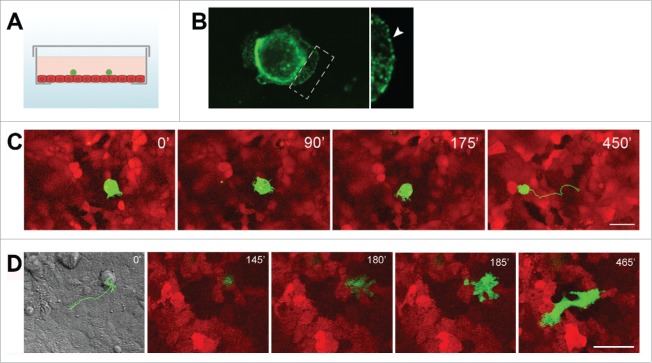
Recently our laboratory performed a comparative study of the behavior of transformed IAR cells that either did or did not express E-cadherin on the confluent monolayer of normal epithelial cells (Fig. 2). We demonstrated that transformed epithelial cells that retain expression of E-cadherin can establish E-cadherin-based adhesions with normal epithelial cells: dot-like dynamic E-cadherin-based adhesions in protrusions and large AJs at the cell sides and rear.129 Live-imaging studies revealed that unlike transformed cells that did not express E-cadherin, transformed cells (4 lines) retaining expression of E-cadherin were able to migrate over and invade the monolayer of normal epithelial cells (transepithelial assay). The cells expressing a dominant-negative mutant form of E-cadherin with the mutation in the first extracellular domain practically lost the ability to adhere to normal cells and invade the epithelial monolayer. Analyzing the invasive behavior of transformed cells that expressed both E-and N-cadherin in transepithelial assay, we compared the effect of selective depletion of E-cadherin or N-cadherin. Depletion of N-cadherin with RNAi did not affect the invasive behavior of the cells, while depletion of E-cadherin led to a reduction in the number of cells that had invaded the epithelial monolayer. Thus, the ability of cancer cells to form E-cadherin-based AJs with the surrounding normal epithelial cells may play an important role in driving cancer cell dissemination in the body. It has been established earlier that cancer-associated fibroblasts (CAFs) may also be involved in collective cancer cell migration by remodeling the extracellular matrix and creating tracks for cancer cells.130 Recent data demonstrated that CAFs can also actively drive collective migration of cancer cells with the help of heterophilic E-N AJs.131
Figure 2.
Interactions between transformed IAR-6–1 epithelial cells and normal IAR-2 epithelial cells. (A) A scheme of the experimental design: a glass bottom culture dish with a confluent IAR-2 monolayer (red) and transformed IAR cells (green) seeded sparsely onto the monolayer. (B) Transformed IAR-6–1 cells form E-cadherin-based AJs with underlying normal IAR-2 cells. E-cadherin accumulates in dot-like adhesions at the leading edge and in prominent AJs encircling the IAR-6–1 cell. Right - boxed region is enlarged. (C) Transformed IAR-6–1 epithelial cells migrate over the monolayer of normal IAR-2 epithelial cells. IAR-6–1 cells express GFP, IAR-2 cells express mKate2. Selected frames from a live imaging sequence. A corresponding track of the migrating IAR-6–1 cell is shown on Frame 4. (D). Transformed IAR-6–1 cells invade the monolayer of normal IAR-2 cells. IAR-6–1 cells express GFP, IAR-2 cells express mKate2. Frames from a live imaging sequence, substrate level. Frame 1 is a DIC image of the corresponding field taken at t = 0′, with the overlaid track of the migrating IAR-6–1 cell. The IAR-6–1 cell is on top of the monolayer at 145′; a pseudopod invades the monolayer and spreads at 180′; the entire cell migrates across the monolayer and spreads on the underlying substrate at 185′, and the cell acquires an elongated shape and migrates underneath the monolayer up until 465′. © PLoS One. Reproduced by permission of Natalya Gloushankova. Permission to reuse must be obtained from the rightsholder.129
The next stage of the invasion-metastasis cascade is intravasation of cancer cells into pre-existing or newly formed vessels and systemic circulation. A live-imaging study in animals using intravital high-resolution 2-photon microscopy has recently investigated in great detail the process of entering the vessels by tumor cells. Tumor cell intravasation occurred exclusively at the sites that consist of tumor-associated macrophages contacting with a tumor cell and an endothelial cell. VEGFA signaling from tumor-associated perivascular macrophages led to destruction of endothelial AJs, transiently increased local vascular permeability and resulted in tumor cell intravasation at these sites.132 Over the past few years the presence of circulating tumor cells (CTCs) and circulating tumor cell aggregates in the blood of patients with cancer was confirmed in many studies.133-138 In many cancers high CTC numbers are associated with disease progression.139 CTCs often have hybrid epithelial-mesenchymal phenotype expressing both epithelial and mesenchymal markers.111,140 Cadherin-mediated interactions favor survival of CTC in the clusters within the bloodstream. Clusters of tumor cells are also more efficient than single CTCs in forming experimental metastases.128,129 Clusters of ≤ 20 cells can rapidly and reversibly reorganize into single-file chains and transit through capillary-sized vessels.141
Ectopic expression of E-cadherin early in tumor progression plays an important role in the intraperitoneal dissemination of epithelial ovarian carcinoma cells. The early steps of epithelial ovarian carcinoma metastasis involve shedding of the cells from the primary tumor to form floating cells or multi-cellular clusters in ascites. E-cadherin expression promotes aggregation of tumor cells into clusters142 that may suppress ovarian cancer cell anoikis and have a positive effect on survival of tumor cells.143
E-cadherin-based cell-cell interactions have a pivotal role in metastatic colonization of distant tissues. Re-expression of E-cadherin in metastases from primary tumors where E-cadherin was downregulated, was described earlier.144,145 Mesenchymal-epithelial transition (MET) is required for metastatic outgrowth of tumor cells in distant tissues. Several studies showed that EMT inducers, such as Twist and Paired Related Homeobox 1 (PRRX1) that are necessary for cancer cell migratory and invasive properties, need to be repressed for successful metastatic outgrowth.146-148
Conclusion
Cadherin-mediated cell-cell interactions are essential for tissue organization and morphogenetic processes. In epithelial tissues, E-cadherin-based AJs provide stable cell-cell adhesion. Neoplastic transformation may lead to reorganization of actin cytoskeleton and rearrangement of stable linear AJs into dynamic punctate (radial) AJs, which modulates adhesive function of E-cadherin and migratory behavior of tumor cells. The contemporary concept of the partial EMT takes into account the importance of retaining of E-cadherin-based AJs by cancer cells for their collective migration, survival and metastatic outgrowth (Fig. 3). Transformed epithelial cells can also establish E-cadherin-based adhesions with normal epithelial cells, to migrate over and invade the monolayer of normal epithelial cells. Thus, the ability of cancer cells to form E-cadherin-based AJs may play an important role in driving cancer cell dissemination in the body.
Figure 3.
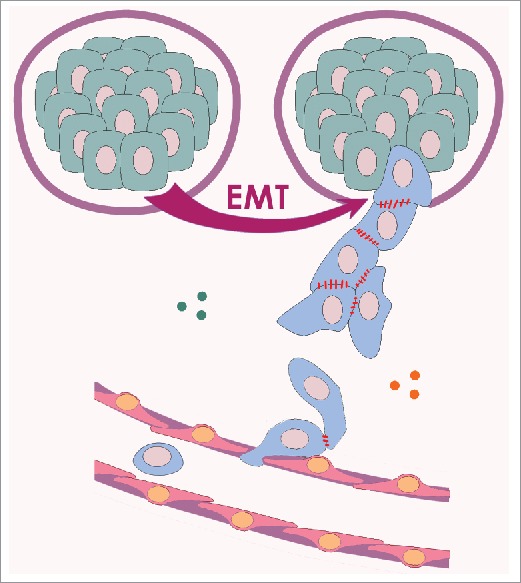
Initial stages of cancer cell dissemination. Cells that have undergone partial EMT (blue; note the presence of Ecad AJs), break through the basement membrane and commence the invasion-metastasis cascade. They cross the stroma by collective migration, enter into a blood vessel and begin to spread in circulation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This paper is dedicated to the memory of our teacher, Prof. J.M. Vasiliev, who recently passed away.
Funding
The Russian Science Foundation (Grant 16–15–10288) is acknowledged for financial support.
References
- [1].Takeichi M. Self-organization of animal tissues: Cadherin-mediated processes. Dev Cell. 2011;21(1):24-6. https://doi.org/ 10.1016/j.devcel.2011.06.002. PMID:21763603. [DOI] [PubMed] [Google Scholar]
- [2].Mège RM, Ishiyama N. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb Perspect Biol. 2017;9(5):a028738. https://doi.org/ 10.1101/cshperspect.a028738. PMID:28096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takeichi M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15(6):397-410. https://doi.org/ 10.1038/nrm3802. PMID:24824068. [DOI] [PubMed] [Google Scholar]
- [4].Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375-412. https://doi.org/ 10.1038/nrm.2016.80. PMID:13944428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zihni C, Mills C, Matter K, Balda MS. Tight junctions: From simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564-80. PMID:27353478. [DOI] [PubMed] [Google Scholar]
- [6].Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132(3):451-63. https://doi.org/ 10.1242/jcs.126565. PMID:8636221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maiers JL, Peng X, Fanning AS, DeMali KA. ZO-1 recruitment to α-catenin–a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci. 2013;126(Pt 17):3904-15. PMID:23813953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142(4):1105-19. https://doi.org/ 10.1091/mbc.E05-01-0043. PMID:9722621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16(6):2636-50. PMID:15800060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hartsock A, Nelson WJ. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660-9. https://doi.org/ 10.1016/j.bbamem.2007.07.012. PMID:17854762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778(3):572-87. https://doi.org/ 10.1016/j.yexcr.2007.07.035. PMID:17854763 https://doi.org/. [DOI] [PubMed] [Google Scholar]
- [12].Lambert M, Thoumine O, Brevier J, Choquet D, Riveline D, Mège RM. Nucleation and growth of cadherin adhesions. Exp Cell Res. 2007;313(19):4025-40. PMID:17765222. [DOI] [PubMed] [Google Scholar]
- [13].Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, et al.. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19(2):244-56. https://doi.org/ 10.1016/j.str.2010.11.016. PMID:21300292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Strale PO, Duchesne L, Peyret G, Montel L, Nguyen T, Png E, Tampé R, Troyanovsky S, Hénon S, Ladoux B, et al.. The formation of ordered nanoclusters controls cadherin anchoring to actin and cell-cell contact fluidity. J Cell Biol. 2015;210(2):333-46. https://doi.org/ 10.1083/jcb.201410111. PMID:26195669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yap AS, Gomez GA, Parton RG. Adherens junctions revisualized: Organizing cadherins as nanoassemblies. Dev Cell. 2015;35(1):12-20. https://doi.org/ 10.1016/j.devcel.2015.09.012. PMID:26460944. [DOI] [PubMed] [Google Scholar]
- [16].Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163(3):525-34. https://doi.org/ 10.1083/jcb.200307111. PMID:14610055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163(3):535-45. https://doi.org/ 10.1083/jcb.200306001. PMID:14610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reynolds AB. Exposing p120 catenin's most intimate affair. Cell. 2010;141(1):20-2. https://doi.org/ 10.1016/j.cell.2010.03.022. PMID:20371340. [DOI] [PubMed] [Google Scholar]
- [19].Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92(19):8813-7. https://doi.org/ 10.1038/ncb2055. PMID:7568023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142(3):847-57. PMID:9700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144(6):1311-22. PMID:10087272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533-42. PMID:20453849. [DOI] [PubMed] [Google Scholar]
- [23].Ishiyama N, Tanaka N, Abe K, Yang YJ, Abbas YM, Umitsu M, Nagar B, Bueler SA, Rubinstein JL, Takeichi M, et al.. An autoinhibited structure of α-catenin and its implications for vinculin recruitment to adherens junctions. J Biol Chem. 2013;288(22):15913-25. https://doi.org/ 10.1074/jbc.M113.453928. PMID:23589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hong S, Troyanovsky RB, Troyanovsky SM. Binding to F-actin guides cadherin cluster assembly, stability, and movement. J Cell Biol. 2013;201(1):131-43. https://doi.org/ 10.1083/jcb.201211054. PMID:23547031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189(7):1107-15. https://doi.org/ 10.1083/jcb.201001149. PMID:20584916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mège RM, et al.. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat Commun. 2014;5:4525. https://doi.org/ 10.1038/ncomms5525. PMID:25077739. [DOI] [PubMed] [Google Scholar]
- [27].Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346(6209):1254211. https://doi.org/ 10.1126/science.1254211. PMID:25359979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rangarajan ES, Izard T. The cytoskeletal protein α-catenin unfurls upon binding to vinculin. J Biol Chem. 2012;287(22):18492-9. https://doi.org/ 10.1074/jbc.M112.351023. PMID:22493458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Choi HJ, Pokutta S, Cadwell GW, Bobkov AA, Bankston LA, Liddington RC, Weis WI. αE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci U S A. 2012;109(22):8576-81. https://doi.org/ 10.1073/pnas.1203906109. PMID:22586082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maki K, Han SW, Hirano Y, Yonemura S, Hakoshima T, Adachi T. Mechano-adaptive sensory mechanism of α-catenin under tension. Sci Rep. 2016;6:24878. https://doi.org/ 10.1038/srep24878. PMID:27109499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bertocchi C, Wang Y, Ravasio A, Hara Y, Wu Y, Sailov T, Baird MA, Davidson MW, Zaidel-Bar R, Toyama Y. Nanoscale architecture of cadherin-based cell adhesions. Nat Cell Biol. 2017;19(1):28-37. https://doi.org/ 10.1038/ncb3456. PMID:27992406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol. 2003;160(3):399-407. https://doi.org/ 10.1083/jcb.200212057. PMID:12566430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105(1):13-9. https://doi.org/ 10.1073/pnas.0710504105. PMID:18093941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al.. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139(2):517-28. https://doi.org/ 10.1074/jbc.M414447200. PMID:9334353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150(5):1161-76. PMID:10974003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280(25):24095-103. PMID:15857834. [DOI] [PubMed] [Google Scholar]
- [37].Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2(7):e658. https://doi.org/ 10.1371/journal.pone.0000658. PMID:17668046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16(10):4531-42. https://doi.org/ 10.1091/mbc.E05-04-0330. PMID:16030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12(7):696-702. https://doi.org/ 10.1038/ncb2072. PMID:20543839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gloushankova NA, Krendel MF, Alieva NO, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Dynamics of contacts between lamellae of fibroblasts: Essential role of the actin cytoskeleton. Proc Natl Acad Sci U S A. 1998;95(8):4362-7. https://doi.org/ 10.1016/j.yexcr.2006.01.031. PMID:9539742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312(9):1637-50. PMID:16519885. [DOI] [PubMed] [Google Scholar]
- [42].Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol. 2014;24(15):1689-99. https://doi.org/ 10.1016/j.cub.2014.06.028. PMID:25065757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209-19. https://doi.org/ 10.1242/jcs.03311. PMID:10660044. [DOI] [PubMed] [Google Scholar]
- [44].Yamazaki D, Oikawa T, Takenawa T. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci. 2007;120(Pt 1):86-100. PMID:17164293. [DOI] [PubMed] [Google Scholar]
- [45].Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell. 2012;23(23):4601-10. https://doi.org/ 10.1091/mbc.E12-08-0574. PMID:23051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem. 2004;279(32):34062-70. https://doi.org/ 10.1074/jbc.M404814200. PMID:15159390. [DOI] [PubMed] [Google Scholar]
- [47].Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol. 2011;13(8):934-43. https://doi.org/ 10.1038/ncb2290. PMID:21785420. [DOI] [PubMed] [Google Scholar]
- [48].Tang VW, Brieher WM. α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol. 2012;196(1):115-30. https://doi.org/ 10.1083/jcb.201103116. PMID:22232703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Han SP, Gambin Y, Gomez GA, Verma S, Giles N, Michael M, Wu SK, Guo Z, Johnston W, Sierecki E, et al.. Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. J Biol Chem. 2014;289(11):7764-75. https://doi.org/ 10.1074/jbc.M113.544478. PMID:24469447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120(Pt 21):3870-82. https://doi.org/ 10.1242/jcs.014365. PMID:17940061. [DOI] [PubMed] [Google Scholar]
- [51].Grikscheit K, Frank T, Wang Y, Grosse R. Junctional actin assembly is mediated by Formin-like 2 downstream of Rac1. J Cell Biol. 2015;209(3):367-76. https://doi.org/ 10.1083/jcb.201412015. PMID:25963818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rao MV, Zaidel-Bar R. Formin-mediated actin polymerization at cell-cell junctions stabilizes E-cadherin and maintains monolayer integrity during wound repair. Mol Biol Cell. 2016;27(18):2844-56. https://doi.org/ 10.1091/mbc.E16-06-0429. PMID:27440924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137(6):1421-31. https://doi.org/ 10.1002/(SICI)1097-0169(1999)43:4. PMID:9182672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178(3):517-27. PMID:17646397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gloushankova NA, Alieva NA, Krendel MF, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Cell-cell contact changes the dynamics of lamellar activity in nontransformed epitheliocytes but not in their ras-transformed descendants. Proc Natl Acad Sci U S A. 1997;94(3):879-83. PMID:9023350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Krendel MF, Bonder EM. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil Cytoskeleton. 1999;43(4):296-309. PMID:10423271<296::AID-CM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [57].Ayollo DV, Zhitnyak IY, Vasiliev JM, Gloushankova NA. Rearrangements of the actin cytoskeleton and E-cadherin-based adherens junctions caused by neoplasic transformation change cell-cell interactions. PLoS One. 2009;4(11):e8027. https://doi.org/ 10.1371/journal.pone.0008027. PMID:19956566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14(8):818-28. https://doi.org/ 10.1038/ncb2532. PMID:22750944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196(5):641-52. https://doi.org/ 10.1083/jcb.201108120. PMID:22391038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87(2):243-53. https://doi.org/ 10.1093/cvr/cvq086. PMID:20299335. [DOI] [PubMed] [Google Scholar]
- [61].Oldenburg J, de Rooij J. Mechanical control of the endothelial barrier. Cell Tissue Res. 2014;355(3):545-55. https://doi.org/ 10.1007/s00441-013-1792-6. PMID:24519624. [DOI] [PubMed] [Google Scholar]
- [62].Ngok SP, Anastasiadis PZ. Rho GEFs in endothelial junctions: Effector selectivity and signaling integration determine junctional response. Tissue Barriers. 2013;1(5):e27132. https://doi.org/ 10.4161/tisb.27132. PMID:24790803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al.. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol. 2012;199(7):1103-15. https://doi.org/ 10.1083/jcb.201207009. PMID:23253477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol. 2013;202(6):901-16. https://doi.org/ 10.1083/jcb.201301115. PMID:24019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stephenson RO, Yamanaka Y, Rossant J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development. 2010;137(20):3383-91. https://doi.org/ 10.1242/dev.050195. PMID:20826529. [DOI] [PubMed] [Google Scholar]
- [66].Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91(17):8263-7. https://doi.org/ 10.1038/ncb3136. PMID:8058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A. 1995;92(3):855-9. PMID:7846066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17(5):533-9. PMID:25925582. [DOI] [PubMed] [Google Scholar]
- [69].Ernst S, Liu K, Agarwala S, Moratscheck N, Avci ME, Dalle Nogare D, Chitnis AB, Ronneberger O, Lecaudey V. Shroom3 is required downstream of FGF signalling to mediate proneuromast assembly in zebrafish. Development. 2012;139(24):4571-81. https://doi.org/ 10.1242/dev.083253. PMID:23136387. [DOI] [PubMed] [Google Scholar]
- [70].Chung MI, Nascone-Yoder NM, Grover SA, Drysdale TA, Wallingford JB. Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development. 2010;137(8):1339-49. https://doi.org/ 10.1242/dev.044610. PMID:20332151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13(24):2125-37. https://doi.org/ 10.1242/jcs.02626. PMID:14680628. [DOI] [PubMed] [Google Scholar]
- [72].Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118(Pt 22):5191-203. PMID:16249236. [DOI] [PubMed] [Google Scholar]
- [73].Kuriyama S, Theveneau E, Benedetto A, Parsons M, Tanaka M, Charras G, Kabla A, Mayor R. In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity. J Cell Biol. 2014;206(1):113-27. https://doi.org/ 10.1083/jcb.201402093. PMID:25002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huang C, Kratzer MC, Wedlich D, Kashef J. E-cadherin is required for cranial neural crest migration in Xenopus laevis. Dev Biol. 2016;411(2):159-71. https://doi.org/ 10.1016/j.ydbio.2016.02.007. PMID:26879760. [DOI] [PubMed] [Google Scholar]
- [75].Kardash E, Reichman-Fried M, Maître JL, Boldajipour B, Papusheva E, Messerschmidt EM, Heisenberg CP, Raz E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12(1):47-53; sup pp 1–11. https://doi.org/ 10.1038/ncb2003. PMID:20010816. [DOI] [PubMed] [Google Scholar]
- [76].Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol. 2012;371(1):1-12. https://doi.org/ 10.1016/j.ydbio.2012.06.005. PMID:22766025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115(1-2):53-62. https://doi.org/ 10.1038/nrc1098. PMID:12049767. [DOI] [PubMed] [Google Scholar]
- [78].Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453-8. PMID:12778135. [DOI] [PubMed] [Google Scholar]
- [79].Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147(2):275-92. https://doi.org/ 10.1016/j.cell.2011.09.024. PMID:22000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871-90. https://doi.org/ 10.1016/j.cell.2009.11.007. PMID:19945376. [DOI] [PubMed] [Google Scholar]
- [81].Lindsey S, Langhans SA. Crosstalk of oncogenic signaling pathways during epithelial-mesenchymal transition. Front Oncol. 2014;4:358. https://doi.org/ 10.3389/fonc.2014.00358. PMID:25566498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72(19):4883-9. https://doi.org/ 10.1158/0008-5472.CAN-12-1223. PMID:23002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415-28. https://doi.org/ 10.1038/nrc2131. PMID:17508028. [DOI] [PubMed] [Google Scholar]
- [84].Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727-35. https://doi.org/ 10.1242/jcs.000455. PMID:18322269. [DOI] [PubMed] [Google Scholar]
- [85].Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190-3. https://doi.org/ 10.1038/32433. PMID:9515965. [DOI] [PubMed] [Google Scholar]
- [86].Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1(6):a003129. https://doi.org/ 10.1101/cshperspect.a003129. PMID:20457567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Vasioukhin V. Adherens junctions and cancer. In: Harris TJ, editor. An introduction to adherens junctions: From molecular mechanisms to tissue development and disease. Subcell Biochem. 2012;60:1-5. https://doi.org/ 10.1007/978-94-007-4186-7_1. PMID:22674065. [DOI] [PubMed] [Google Scholar]
- [88].Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66(1):107-19. https://doi.org/ 10.3892/ijmm.2011.640. PMID:2070412. [DOI] [PubMed] [Google Scholar]
- [89].Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113(1):173-85. PMID:2007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ling ZQ, Li P, Ge MH, Zhao X, Hu FJ, Fang XH, Dong ZM, Mao WM. Hypermethylation-modulated down-regulation of CDH1 expression contributes to the progression of esophageal cancer. Int J Mol Med. 2011;27(5):625-35. PMID:21373750. [DOI] [PubMed] [Google Scholar]
- [91].Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: Down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53(7):1690-5. https://doi.org/ 10.1091/mbc.E06-04-0348. PMID:8453643. [DOI] [PubMed] [Google Scholar]
- [92].Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of E-cadherin/catenin complex in hepatocellular carcinomas. World J Gastroenterol. 2008;14(37):5665-73. PMID:18837082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schipper JH, Frixen UH, Behrens J, Unger A, Jahnke K, Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: Inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991;51(23 Pt 1):6328-37. PMID:1933895. [PubMed] [Google Scholar]
- [94].Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52(18):5104-9. PMID:1516067. [PubMed] [Google Scholar]
- [95].Schuhmacher C, Becker I, Oswald S, Atkinson MJ, Nekarda H, Becker KF, Mueller J, Siewert JR, Höfler H. Loss of immunohistochemical E-cadherin expression in colon cancer is not due to structural gene alterations. Virchows Arch. 1999;434(6):489-95. PMID:10394882. [DOI] [PubMed] [Google Scholar]
- [96].Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871-90. PMID:19945376 https://doi.org/ 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [97].Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127(5):1375-80. PMID:7962096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18(6):2013-25. PMID:17392517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739-48. https://doi.org/ 10.1038/sj.emboj.7600136. PMID:15057284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hashizume R, Koizumi H, Ihara A, Ohta T, Uchikoshi T. Expression of beta-catenin in normal breast tissue and breast carcinoma: A comparative study with epithelial cadherin and alpha-catenin. Histopathology. 1996August;29(2):139-46. https://doi.org/ 10.1038/modpathol.3880334. PMID:8872147. [DOI] [PubMed] [Google Scholar]
- [101].Kleer CG, van Golen KL, Braun T, Merajver SD. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2001;14(5):458-64. PMID:11353057. [DOI] [PubMed] [Google Scholar]
- [102].Kartenbeck J, Haselmann U, Gassler N. Synthesis of junctional proteins in metastasizing colon cancer cells. Eur J Cell Biol. 2005;84(2–3):417-30. https://doi.org/ 10.1016/j.ejcb.2005.01.005. PMID:15819418. [DOI] [PubMed] [Google Scholar]
- [103].Hollestelle A, Peeters JK, Smid M, Timmermans M, Verhoog LC, Westenend PJ, Heine AA, Chan A, Sieuwerts AM, Wiemer EA, et al.. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res Treat. 2013;138(1):47-57. https://doi.org/ 10.1007/s10549-013-2415-3. PMID:23338761. [DOI] [PubMed] [Google Scholar]
- [104].Whittle MC, Hingorani SR1. Disconnect between EMT and metastasis in pancreas cancer. Oncotarget. 2015October 13;6(31):30445-6. https://doi.org/ 10.18632/oncotarget.5720. PMID:26387140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: A broad survey using high-density tissue microarray technology. Hum Pathol. 2001;32(7):690-7. https://doi.org/ 10.1053/hupa.2001.25902. PMID:11486167. [DOI] [PubMed] [Google Scholar]
- [106].Vered M, Allon I, Buchner A, Dayan D. E-cadherin in oral SCC: An analysis of the confusing literature and new insights related to its immunohistochemical expression. Histol Histopathol. 2012;27(2):141-50. https://doi.org/ 10.14670/HH-27.141. PMID:22207548. [DOI] [PubMed] [Google Scholar]
- [107].Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: A cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174(5):1588-93. https://doi.org/ 10.2353/ajpath.2009.080545. PMID:19342369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015;25(11):675-86. https://doi.org/ 10.1016/j.tcb.2015.07.012. PMID:26437589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21-45. https://doi.org/ 10.1016/j.cell.2016.06.028. PMID:27368099. [DOI] [PubMed] [Google Scholar]
- [110].Lecharpentier A, Vielh P, Perez-Moreno P, Planchard D, Soria JC, Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105(9):1338-41. https://doi.org/ 10.1038/bjc.2011.405. PMID:21970878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al.. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580-4. https://doi.org/ 10.1126/science.1228522. PMID:23372014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438-49. https://doi.org/ 10.1038/nm.3336. PMID:4202396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin's dark side: Possible role in tumor progression. Biochim Biophys Acta. 2012;1826(1):23-31. https://doi.org/ 10.1016/j.bbcan.2012.03.002. PMID:22440943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14(8):777-83. https://doi.org/ 10.1038/ncb2548. PMID:22854810. [DOI] [PubMed] [Google Scholar]
- [115].Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9(4):261-72. https://doi.org/ 10.1016/j.ccr.2006.03.010. PMID:16616332. [DOI] [PubMed] [Google Scholar]
- [116].Gavert N, Vivanti A, Hazin J, Brabletz T, Ben-Ze'ev A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9(1):14-24. https://doi.org/ 10.1158/1541-7786.MCR-10-0406. PMID:21123622. [DOI] [PubMed] [Google Scholar]
- [117].Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155(7):1639-51. https://doi.org/ 10.1016/j.cell.2013.11.029. PMID:24332913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer–ready for diagnostic practice? Hum Pathol. 2016;47(1):4-19. https://doi.org/ 10.1016/j.humpath.2015.08.007. PMID:26476568. [DOI] [PubMed] [Google Scholar]
- [119].Tan DS, Potts HW, Leong AC, Gillett CE, Skilton D, Harris WH, Liebmann RD, Hanby AM. The biological and prognostic significance of cell polarity and E-cadherin in grade I infiltrating ductal carcinoma of the breast. J Pathol. 1999;189(1):20-7. https://doi.org/ 10.1002/(SICI)1096-9896(199909)189:1. PMID:10451483<20::AID-PATH394>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [120].Ng WK. Fine-needle aspiration cytology findings of an uncommon micropapillary variant of pure mucinous carcinoma of the breast: Review of patients over an 8-year period. Cancer. 2002;96(5):280-8. https://doi.org/ 10.1002/cncr.10747. PMID:12378595. [DOI] [PubMed] [Google Scholar]
- [121].Langbein L, Pape UF, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW. Tight junction-related structures in the absence of a lumen: Occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol. 2003;82(8):385-400. https://doi.org/ 10.1078/0171-9335-00330. PMID:14533737. [DOI] [PubMed] [Google Scholar]
- [122].Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol. 2011;194(4):643-56. https://doi.org/ 10.1083/jcb.201104124. PMID:21844208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Stramer B, Mayor R. Mechanisms and in vivo functions of contact inhibition of locomotion. Nat Rev Mol Cell Biol. 2017;18(1):43-55. https://doi.org/ 10.1038/nrm.2016.118. PMID:27677859 [DOI] [PubMed] [Google Scholar]
- [124].Zhitniak IIu, Glushankova NA. Morphology, cell-cell interactions, and migratory activity of IAR-2 epithelial cells transformed with the RAS oncogene: Contribution of cell adhesion protein E-cadherin. Ontogenez. 2011;42(6):453-64. https://doi.org/ 10.1002/path.4416. PMID:22288108. [DOI] [PubMed] [Google Scholar]
- [125].Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, et al.. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. J Pathol. 2014;234(3):410-22. PMID:25081610. [DOI] [PubMed] [Google Scholar]
- [126].Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al.. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110-22. https://doi.org/ 10.1016/j.cell.2014.07.013. PMID:25171411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Maddipati R, Stanger BZ. Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov. 2015;5(10):1086-97. https://doi.org/ 10.1158/2159-8290.CD-15-0120. PMID:26209539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, et al.. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A. 2016;113(7):E854-63. https://doi.org/ 10.1073/pnas.1508541113. PMID:26831077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rubtsova SN, Zhitnyak IY, Gloushankova NA. A Novel Role of E-Cadherin-Based Adherens Junctions in Neoplastic Cell Dissemination. PLoS One. 2015;10(7):e0133578. https://doi.org/ 10.1371/journal.pone.0133578. PMID:26207916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392-400. https://doi.org/ 10.1038/ncb1658. PMID:18037882. [DOI] [PubMed] [Google Scholar]
- [131].Labernadie A, Kato T, Brugués A, Serra-Picamal X, Derzsi S, Arwert E, Weston A, González-Tarragó V, Elosegui-Artola A, Albertazzi L, et al.. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19(3):224-237. https://doi.org/ 10.1038/ncb3478. PMID:28218910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5(9):932-43. https://doi.org/ 10.1158/2159-8290.CD-15-0012. PMID:26269515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178(3):989-96. https://doi.org/ 10.1016/j.ajpath.2010.12.003. PMID:21356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9(8):997-1007. https://doi.org/ 10.1158/1541-7786.MCR-10-0490. PMID:21665936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, Schram E, Nieva J, Bazhenova L, Morgan A, et al.. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9(1):016001. https://doi.org/ 10.1088/1478-3975/9/1/016001. PMID:22306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al.. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010;107(43):18392-7. https://doi.org/ 10.1073/pnas.1012539107. PMID:20930119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ignatiadis M, Rothé F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, Metallo J, Kheddoumi N, Singhal SK, Michiels S, et al.. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6(1):e15624. https://doi.org/ 10.1371/journal.pone.0015624. PMID:21264346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H, Fasching PA, et al.. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5):dju066. https://doi.org/ 10.1093/jnci/dju066. PMID:24832787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].McInnes LM, Jacobson N, Redfern A, Dowling A, Thompson EW, Saunders CM. Clinical implications of circulating tumor cells of breast cancer patients: Role of epithelial-mesenchymal plasticity. Front Oncol. 2015;5:42. https://doi.org/ 10.3389/fonc.2015.00042. PMID:25767772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SG, Nandi S, Lim CT, Thiery JP. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget. 2015;6(17):15578-93. https://doi.org/ 10.18632/oncotarget.3903. PMID:26008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O'Keefe R, et al.. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A. 2016;113(18):4947-52. https://doi.org/ 10.1073/pnas.1524448113. PMID:27091969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wu C, Cipollone J, Maines-Bandiera S, Tan C, Karsan A, Auersperg N, Roskelley CD. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation. 2008;76(2):193-205. https://doi.org/ 10.1111/j.1432-0436.2007.00193.x. PMID:17608733. [DOI] [PubMed] [Google Scholar]
- [143].Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: Unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25(6):643-55. https://doi.org/ 10.1007/s10585-008-9171-5. PMID:18398687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217-22. https://doi.org/ 10.1186/bcr651. PMID:14580257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Bukholm IK, Nesland JM, Børresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients. J Pathol. 2000;190(1):15-9. https://doi.org/ 10.1002/(SICI)1096-9896(200001)190:1. PMID:10640987<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [146].Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725-36. https://doi.org/ 10.1016/j.ccr.2012.09.022. PMID:23201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ocaña OH, Córcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709-24. https://doi.org/ 10.1016/j.ccr.2012.10.012. PMID:23201163. [DOI] [PubMed] [Google Scholar]
- [148].Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massagué J, Benezra R. TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 2013;5(5):1228-42. https://doi.org/ 10.1016/j.celrep.2013.11.014. PMID:24332369. [DOI] [PMC free article] [PubMed] [Google Scholar]