Abstract
Despite the large number of animal complete mitochondrial genomes currently available in public databases, knowledge about mitochondrial genomics in invertebrates is uneven. This paper reports, for the first time, the complete mitochondrial genome of the grooved carpet shell, Ruditapes decussatus, also known as the European clam. Ruditapes decussatus is morphologically and ecologically similar to the Manila clam Ruditapes philippinarum, which has been recently introduced for aquaculture in the very same habitats of Ruditapes decussatus, and that is replacing the native species. Currently the production of the European clam is almost insignificant, nonetheless it is considered a high value product, and therefore it is an economically important species, especially in Portugal, Spain and Italy. In this work we: (i) assembled Ruditapes decussatus mitochondrial genome from RNA-Seq data, and validated it by Sanger sequencing; (ii) analyzed and characterized the Ruditapes decussatus mitochondrial genome, comparing its features with those of other venerid bivalves; (iii) assessed mitochondrial sequence polymorphism (SP) and copy number variation (CNV) of tandem repeats across 26 samples. Despite using high-throughput approaches we did not find evidence for the presence of two sex-linked mitochondrial genomes, typical of the doubly uniparental inheritance of mitochondria, a phenomenon known in ∼100 bivalve species. According to our analyses, Ruditapes decussatus is more genetically similar to species of the Genus Paphia than to the congeneric Ruditapes philippinarum, a finding that bolsters the already-proposed need of a taxonomic revision. We also found a quite low genetic variability across the examined samples, with few SPs and little variability of the sequences flanking the control region (Largest Unassigned Regions (LURs). Strikingly, although we found low nucleotide variability along the entire mitochondrial genome, we observed high levels of length polymorphism in the LUR due to CNV of tandem repeats, and even a LUR length heteroplasmy in two samples. It is not clear if the lack of genetic variability in the mitochondrial genome of Ruditapes decussatus is a cause or an effect of the ongoing replacement of Ruditapes decussatus with the invasive Ruditapes philippinarum, and more analyses, especially on nuclear sequences, are required to assess this point.
Keywords: Complete mitochondrial genome, Mitochondrial length polymorphism, Mitochondrial repeats, Codon usage, Bivalve molluscs, European clam, Comparative mitochondrial genomics, mtDNA de novo assembly, RNA-Seq, Doubly uniparental inheritance
Introduction
Despite a large number of animal complete mitochondrial genomes (mtDNAs) being available in public databases (>55,000 in GenBank), up to now sequencing has been focused mostly on vertebrates (∼50,000 in GenBank), and the current knowledge about mitochondrial genomics in invertebrates—with the notable exception of few model organisms (e.g., Drosophila and Caenorhabditis elegans)—is uneven. To better understand invertebrate mitochondrial biology—and, most importantly, mitochondrial biology and evolution in general—it is necessary to adopt a more widespread approach in gathering and analyzing data. Failing to do so would bias our knowledge toward a few taxonomic groups, with the risk of losing a big part of the molecular and functional diversity of mitochondria. Actually, despite maintaining its core features in terms of genetic content, mtDNA in Metazoa shows a wide range of variation in some other traits such as, for example, genome architecture, abundance of unassigned regions (URs)—namely regions with no assigned product (protein, RNA)—repeat content, gene duplications, introns, UTRs, and even additional coding genes (see Breton et al., 2014 for a review) or genetic elements (e.g., small RNAs, see Pozzi et al., 2017). All this emerging diversity is in sharp contrast with the—at this point outdated—textbook notion about mtDNAs role being limited to the production of a few subunits of the protein complexes involved in oxidative phosphorylation.
This paper reports, for the first time, the complete mitochondrial genome of the grooved carpet shell, Ruditapes decussatus (Linnaeus, 1758). Ruditapes decussatus—also known as the European clam—is distributed all over the Mediterranean coasts, as well as on the Atlantic shores, from Lofoten Islands (Norway) to Mauritania, including the British Isles. Ruditapes decussatus lives in warm coastal waters, especially in lagoons, and it is morphologically and ecologically similar to the Manila clam Ruditapes philippinarum, which has been recently introduced for aquaculture in the very same habitats of Ruditapes decussatus. Ruditapes philippinarum, native from the Philippines, Korea, and Japan, was accidentally introduced into North America in the 1930s, and from there it was purposely introduced in France (1972), UK (1980), and Ireland (1982) for aquaculture purposes (Gosling, 2003). According to historical records, Ruditapes decussatus was one of the most important species for aquaculture in Europe, but overfishing, irregular yields, recruitment failure, and outbreaks of bacterial infection pushed the producers to introduce Ruditapes philippinarum; Italy imported large quantities of Ruditapes philippinarum seed from UK in 1983 and 1984. Compared to the European clam, the Manila clam turned out to be faster growing, more resistant to disease, to have a more extended breeding period and a greater number of spawning events, and to begin sexual maturation earlier (i.e., at a smaller size). Upon introduction of the more robust Ruditapes philippinarum, Ruditapes decussatus suffered a population decline in the Southwestern Europe (Arias-Pérez et al., 2016), and currently the production of the European clam is almost insignificant. Nonetheless the grooved carpet shell is considered a high value product, and therefore it is an economically important species, especially in Portugal, Spain and Italy (Gosling, 2003; Leite et al., 2013; de Sousa et al., 2014).
Molluscs in general, and bivalves in particular, exhibit an extraordinary degree of mtDNA variability and unusual features, such as: large mitochondrial genomes (up to ∼47Kb), high proportion of URs (i.e., number of base pairs annotated as URs over the total mtDNA length), novel protein coding genes with unknown function, frequent and extensive gene rearrangement, and differences in strand usage (Gissi, Iannelli & Pesole, 2008; Breton et al., 2011; Ghiselli et al., 2013; Milani et al., 2014b; Plazzi, Puccio & Passamonti, 2016). Moreover, mitochondrial genome size varies among bivalves because of gene duplications and losses (Serb & Lydeard, 2003; Passamonti et al., 2011; Ghiselli et al., 2013), and sometimes genes are fragmented as in the case of ribosomal genes in oysters (Milbury et al., 2010). The most notable feature of bivalve mtDNA is the doubly uniparental inheritance (DUI) system of transmission (Skibinski, Gallagher & Beynon, 1994a, 1994b; Zouros et al., 1994a, 1994b). Under DUI, two different mitochondrial lineages (and their respective genomes) are transmitted to the progeny: one is inherited from the egg (female-transmitted or F-type mtDNA), the other is inherited from the spermatozoon (male-transmitted or M-type mtDNA). Following fertilization, the early embryo is heteroplasmic, but the type of mitochondria present in the adult is tightly linked to its sex. Females are commonly homoplasmic for F, while males are heteroplasmic with the following distribution of mtDNA types: the germ line is homoplasmic for the M-type (which will be transmitted via sperm to male progeny), the soma is heteroplasmic to various degrees, depending on tissue type and/or species (Ghiselli, Milani & Passamonti, 2011; Zouros, 2013). To date, the only known animals exhibiting DUI are about 100 species of bivalve molluscs (Gusman et al., 2016). This natural and evolutionarily stable heteroplasmic system can be extremely useful to investigate several aspects of mitochondrial biology (see Passamonti & Ghiselli, 2009; Breton et al., 2014; Milani & Ghiselli, 2015; Milani, Ghiselli & Passamonti, 2016). Indeed, despite the fact that many aspects of DUI are still unknown, there is evidence that DUI evolved from a strictly maternal inheritance (SMI) system (Milani & Ghiselli, 2015; Milani, Ghiselli & Passamonti, 2016), by modifications of the molecular machinery involved in mitochondrial inheritance, through as-yet-unknown specific factors (see Diz, Dudley & Skibinski, 2012; Zouros, 2013 for proposed models). The detection of DUI is not a straightforward process, especially using PCR-based approaches: given that the divergence between F and M genomes is often comparable to the distance between mtDNAs of different classes of Vertebrates, primers may fail to amplify one of the two mtDNAs, yielding a false-negative result. Moreover, M-type mtDNA can be rare in somatic tissues, so it may be difficult to amplify from animals sampled outside of the reproductive season, when gonads are absent (thoroughly discussed in Theologidis et al., 2008). High-throughput sequencing (HTS) approaches can overcome such problems, because a prior knowledge of the mtDNA sequence is not needed, and low-copy variants can be easily unveiled (see Ju et al., 2011; King et al., 2014). Until now, HTS has been scarcely utilized to study mitochondrial transcriptomes and genomes (Pesole et al., 2012; Smith, 2013), even if it showed very good potential (Lubośny et al., 2017/2; Yuan et al., 2016). In this work we: (i) assembled Ruditapes decussatus mitochondrial genome from RNA-Seq data, and validated it by Sanger sequencing, (ii) analyzed and characterized Ruditapes decussatus mitochondrial genome, comparing its features with those of other venerid bivalves; (iii) assessed mitochondrial sequence polymorphism (SP) and structural variants—copy number variation (CNV) of tandem repeats—among the sampled animals.
Materials and Methods
Sampling
The 26 Ruditapes decussatus specimens used in this study were collected from the Northern Adriatic Sea, in the river Po Delta region (Sacca di Goro, approximate GPS coordinates: 44°50′06″N, 12°17′55″E) at the end of July 2011, during the spawning season. Each individual was dissected, and gonadal liquid collected with a glass capillary tube. All the samples showed ripe gonads, consistently with the time of the year when the sampling occurred. The gonadal liquid was checked under a light microscope to assess the sex of the individual, and to make sure that the sample consisted of mature gametes. Both the gamete samples and the clam bodies were flash-frozen in liquid nitrogen, and stored at −80 °C, until nucleic acid extraction. Table S1 shows the sample list, and details about data availability.
RNA-Seq
In total, 12 samples (six males and six females) were used for RNA-Seq. Total RNA extraction and library preparation were performed following the protocol described in Mortazavi et al. (2008), with the modifications specified in Ghiselli et al. (2012). The 12 samples were indexed, pooled and sequenced in two lanes (two technical replicates) of Illumina GA IIx, using 76 bp paired-end reads.
De novo assembly
The mitochondrial genome of Ruditapes decussatus was not available in the databases, so we used the transcriptome data to generate a draft to be used as a guide for Sanger sequencing. Illumina reads from all 12 samples were pooled and compared to a set of 20 Bivalvia mitochondrial genomes to identify reads with mitochondrial origin. Alignment was done using BLASTN. All reads with similarity yielding E-value < 1E-5 were then assembled into contigs using the A5 pipeline (version 2013032; Tritt et al., 2012) and joined into scaffolds using CAP3 (Huang & Madan, 1999). For the quality check step, we applied a PHRED Q-score cutoff threshold of 33; the other A5 parameters were set as default. CAP3 was run with default settings as well.
Sanger validation
In total, 14 Ruditapes decussatus samples from the same collection campaign—sexed, and stored at −80 °C—were used for DNA extraction. DNA from the gonadic tissue was extracted using the Qiagen DNeasy kit. Primers for mtDNA amplification were designed based on contigs obtained from RNA-Seq matching venerid mtDNA sequences, then the “primer walking” method was used to Sanger-sequence the complete mitochondrial genome of Ruditapes decussatus. The primers were designed with the software Primer3 (Rozen & Skaletsky, 2000) and tested on several samples, then a female was chosen as reference sample for Sanger validation of mtDNA de novo assembly. In addition, we amplified the largest unassigned region (LUR) of 13 females to assess its variability (see Results and Discussion). The list of the primers and their sequences are reported in Table S2. PCR reactions were performed in a final volume of 50 μl using the GoTaq Flexi DNA Polymerase Kit (Promega, Madison, WI, USA), on a 2720 Thermal Cycler (Applied Biosystem, Foster City, CA, USA). The PCR reactions were set as follows: initial denaturation 95 °C for 1 min, then 30 cycles of amplification (denaturation 95 °C for 1 min, annealing 48–60 °C for 1 min, extension 72 °C for 1 min/kb), then the final extension at 72 °C for 5 min. PCR products were checked by electrophoretic run on 1% agarose gel, and then purified using the DNA Clean & Concentrator-25 kit (Zymo Research, Irvine, CA, USA).
Sanger sequencing was performed by Macrogen Inc. (http://www.macrogen.com).
Sequences were aligned with the software MEGA 6.0 (Tamura et al., 2013), using the contigs obtained by RNA-seq as a reference.
Annotation
Open reading frames (ORFs) were identified with ORF finder (Wheeler et al., 2005). Alternative start codons were considered functional because they are common in Bivalvia. ORFs were annotated starting from the first available start codon (ATG, ATA, or ATC) downstream of the preceding gene, and ending with the first stop codon in frame (TAA or TAG). tRNA genes and their structure were identified with MITOS (Bernt et al., 2013) and ARWEN (Laslett & Canback, 2008). Secondary structures were predicted using the RNAFold Server, included in the ViennaRNA Web Services (http://rna.tbi.univie.ac.at/; Gruber et al., 2008); the folding temperature was set at 16 °C which is the average annual temperature of the water from which the Ruditapes decussatus specimens used in this work were fished (download RNAFold results from figshare: https://ndownloader.figshare.com/files/8387672). tRNAs and other secondary structures were drawn with the software Varna GUI (Darty, Denise & Ponty, 2009). Ribosomal small subunit (rrnS) and large subunit (rrnL) were identified with BLASTN, and annotated considering the start and the end of the adjacent genes as the boundaries of the rRNA genes. Non-genic regions were annotated as URs. In order to identify the putative D-loop/control region (CR), we analyzed the LUR with the MEME suite (Bailey et al., 2009) to find DNA motifs using the following bivalve species as comparison: Acanthocardia tuberculata, Arctica islandica, Coelomactra antiquata, Fulvia mutica, Hiatella arctica, Loripes lacteus, Lucinella divaricata, Lutraria rhynchaena, Meretrix lamarckii (F-type), Meretrix lamarckii (M-type), Meretrix lusoria, Meretrix lyrata, Meretrix meretrix, Meretrix petechialis, Moerella iridescens, Nuttallia olivacea, Paphia amabilis, Paphia euglypta, Paphia textile, Paphia undulata, Ruditapes philippinarum (F-type), Ruditapes philippinarum (M-type), Semele scabra, Sinonovacula constricta, Solecurtus divaricatus, Solen grandis, Solen strictus, Soletellina diphos and the sea urchin Strongylocentrotus purpuratus (Echinoidea, Strongylocentrotidae). The list of the species used in the phylogenetic analysis and in the comparative analyses of DNA motifs, sequence similarity, and gene order are available in Table S3. The GOMo (Gene Ontology for Motifs; Buske et al., 2010) tool of the MEME suite was used to assign GO terms to the motifs discovered.
The number of repeats in the LUR of the reference sample (F4) was calculated with tandem repeat finder (http://tandem.bu.edu/trf/trf.html), since the complete LUR sequence was available (download tandem repeat finder results from Figshare: https://ndownloader.figshare.com/files/8387666). In the other cases, in which the LUR could not be sequenced without gaps, the number of repeats was inferred from agarose gel electrophoresis.
Other analyses
Comparisons among venerid complete mtDNAs were performed with BLAST Ring Image Generator (BRIG) (Alikhan et al., 2011) and Easyfig (Sullivan, Petty & Beatson, 2011). Descriptive statistics were obtained with MEGA v6.0 (Tamura et al., 2013), except for the codon usage table, which was obtained with the Sequence Manipulation Suite (Stothard, 2000). SP assessment from RNA-Seq reads was performed with the Genome Analysis Toolkit (GATK, McKenna et al., 2010), with the Sanger-sequenced mtDNA as reference. For SP discovery and genotyping we used standard hard filtering parameters or variant quality score recalibration (DePristo et al., 2011). The MitoPhast pipeline (Tan et al., 2015) was used to obtain the Maximum Likelihood (ML) tree, which was visualized with Evolview v2 (He et al., 2016). Briefly, MitoPhast takes as input GenBank files (.gb), extracts the coding sequences, profiles the sequences with Pfam (Finn et al., 2016) and PRINTS (Attwood et al., 2003), performs a multiple sequence alignment with Clustal Omega (Sievers et al., 2011), removes poorly aligned regions with trimAl (Capella-Gutiérrez, Silla-Martinez & Gabaldón, 2009), concatenates the coding sequences, performs data partitioning and model selection, and then carries out a ML analysis using RAxML (Stamatakis, 2014). The species used in the ML analysis, and their GenBank Accession Numbers are listed in Table S3. Amino acid sequences of three different cox3 ORFs inferred from Sanger sequencing and GATK polymorphism data were analyzed with InterProScan (Jones et al., 2014).
Results
De novo assembly and Sanger validation
Despite using HTS on extracts of ripe gonads (i.e., mature gametes), and multiple assembly strategies (see Discussion for details) we could not find evidence for DUI. The de novo assembly process produced 9 contigs, of which 8 included multiple genes, and one included a single gene (see Table 1). The sequences of the contigs in FASTA format are available on figshare (https://ndownloader.figshare.com/files/8906839). In four cases (Contigs 1, 3, 6, and 7) a clear polyadenylation signal was present, in other four cases (Contigs 2, 5, 8, and 9) it was not. Contig 4, the only one including a single gene (cox3), ends with just 8 As, so it is not clear if a polyadenylation signal is present in this case. In Contig 7 (that includes cox2, tRNA-Ile, nd4L, nd2, nd1, tRNA-Leu1, and cox1 genes) there is a polyadenylation signal (56 As) after the cox2 gene.
Table 1. Features of the contigs obtained by de novo assembly of mtDNA.
| Contig | Length | Gene content | Poly-A | Notes |
|---|---|---|---|---|
| 1 | 6,794 | atp6_nd3_nd5_cox1_tRNA-Leu1_nd1_nd2_nd4L | Yes | Chimeric assembly. The contiguity between nd5 and cox1 is an artifact |
| 2 | 1,884 | rrnS_cox3 | No | – |
| 3 | 1,288 | atp6_nd3 | Yes | – |
| 4 | 1,663 | cox3 | ? | The contig ends with just 8 As |
| 5 | 1,934 | atp8_nd4_tRNA-His_tRNA-Glu_tRNA-Ser2_tRNA-Tyr | No | – |
| 6 | 1,831 | atp8_nd4_tRNA-His | Yes | – |
| 7 | 5,478 | cox2_tRNA-Ile_nd4L_nd2_nd1_tRNA-Leu1_cox1 | Yes | There is a polyadenylation signal (56 As) after the cox2 gene |
| 8 | 2,879 | cytb_rrnL | No | – |
| 9 | 952 | nd6_tRNA-Lys_tRNA-Val_tRNA-Phe_tRNA-Trp_tRNA-Arg_tRNA-Leu2 | No | – |
The nine contigs were used as a scaffold for the primer walking procedure used for Sanger validation of the de novo assembly. We first tried to connect the contigs designing primers close to the 5′ and 3′ ends of each contig and pairing them following the gene order of Paphia, because the sequence of genes in the contigs suggested that Ruditapes decussatus gene order might have been similar. During such process, Contig 1 turned out to be a chimeric assembly between two non-contiguous portions of the mtDNA, one including atp6, nd3, and nd5, the other including cox1, tRNA-Leu1, nd1, nd2, and nd4L. Once we amplified and sequenced the portions of mtDNA between the contigs, we proceeded with the Sanger resequencing of the remaining parts.
Annotation and mtDNA features
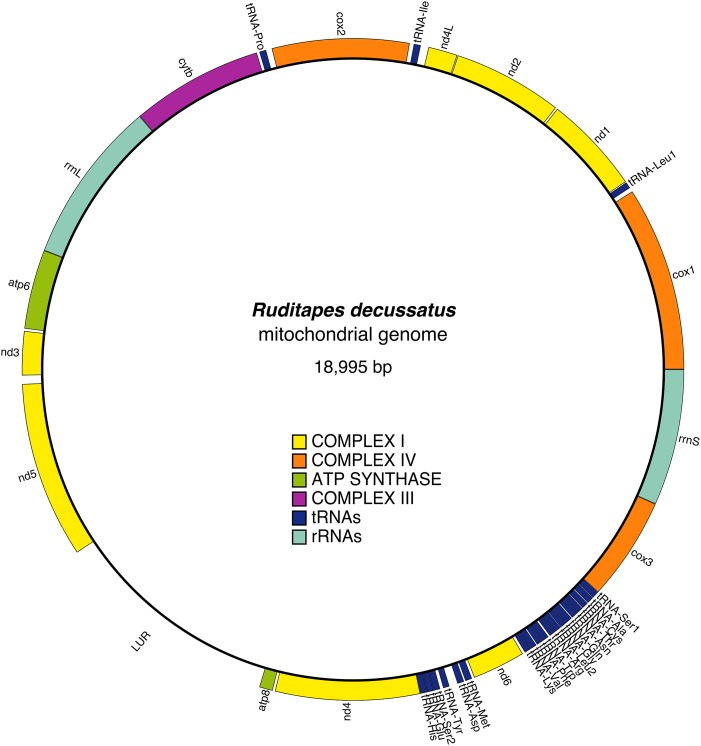
The mitochondrial genome contains 13 protein-coding genes, and in the reference female is 18,995 bp long (Fig. 1); the gene arrangement and other details are shown in Table 2. All genes are located on the heavy strand, and in addition to the classic start codon ATG (Met), the alternative start codons ATA (Met) and ATC (Ile) are present. The most frequently used start codons are: ATA (cox1, nd1, nd4L, cox2, cob, atp8, nd4), and ATG (nd2, atp6, nd3, nd5, nd6, cox3). The stop codons found are TAG (cox1, nd2, nd4L, cox2, cytb, nd4) and TAA (nd1, atp6, nd3, atp8, nd6). The nd4 gene has an incomplete stop codon (TA-). 22 tRNA genes were identified, including two tRNAs for leucine, tRNA-Leu1(TAG) and tRNA-Leu2(TAA), and two for serine, tRNA-Ser1(TCT) and tRNA-Ser2(TGA), both showing degenerate D-arm branches. tRNA structures are shown in Fig. S1. The two rRNAs, rrnS and rrnL, were both identified: the rrnS is located between cox3 and cox1, while rrnL is between cytb and atp6. URs were identified on the basis of unannotated spaces between different genes; we found 24 URs (Table 3).
Figure 1. Ruditapes decussatus mtDNA gene arrangement.
Table 2. MtDNA gene arrangement of Ruditapes decussatus.
| Name | Type | Start | Stop | Length (bp) | Start | Stop | Anticodon |
|---|---|---|---|---|---|---|---|
| cox1 | Coding | 1 | 1,716 | 1,716 | ATA | TAG | |
| tRNA-Leu1 | tRNA | 1,754 | 1,815 | 62 | TAG | ||
| nd1 | Coding | 1,822 | 2,739 | 918 | ATA | TAA | |
| nd2 | Coding | 2,755 | 3,774 | 1,020 | ATG | TAG | |
| nd4l | Coding | 3,780 | 4,052 | 273 | ATA | TAG | |
| tRNA-Ile | tRNA | 4,125 | 4,190 | 66 | GAT | ||
| cox2 | Coding | 4,228 | 5,499 | 1,272 | ATA | TAG | |
| tRNA-Pro | tRNA | 5,553 | 5,616 | 64 | TGG | ||
| cytb | Coding | 5,641 | 6,864 | 1,224 | ATA | TAG | |
| rrnL | rRNA | 6,865 | 8,385 | 1,521 | |||
| atp6 | Coding | 8,386 | 9,123 | 738 | ATG | TAA | |
| nd3 | Coding | 9,145 | 9,552 | 408 | ATG | TAA | |
| nd5 | Coding | 9,631 | 11,268 | 1,638 | ATG | TAG | |
| atp8 | Coding | 13,379 | 13,504 | 126 | ATA | TAA | |
| nd4 | Coding | 13,526 | 14,865 | 1,340 | ATA | TA- | |
| tRNA-His | tRNA | 14,866 | 14,928 | 63 | GTG | ||
| tRNA-Glu | tRNA | 14,929 | 14,990 | 62 | TTC | ||
| tRNA-Ser2 | tRNA | 14,991 | 15,052 | 62 | TGA | ||
| tRNA-Tyr | tRNA | 15,081 | 15,140 | 60 | GTA | ||
| tRNA-Asp | tRNA | 15,218 | 15,280 | 63 | GTC | ||
| tRNA-Met | tRNA | 15,294 | 15,358 | 65 | CAT | ||
| nd6 | Coding | 15,380 | 15,874 | 495 | ATG | TAA | |
| tRNA-Lys | tRNA | 15,897 | 15,959 | 63 | TTT | ||
| tRNA-Val | tRNA | 15,960 | 16,021 | 62 | TAC | ||
| tRNA-Phe | tRNA | 16,030 | 16,092 | 63 | GAA | ||
| tRNA-Trp | tRNA | 16,093 | 16,155 | 63 | TCA | ||
| tRNA-Arg | tRNA | 16,171 | 16,232 | 62 | TCG | ||
| tRNA-Leu2 | tRNA | 16,233 | 16,295 | 63 | TAA | ||
| tRNA-Gly | tRNA | 16,297 | 16,358 | 62 | TCC | ||
| tRNA-Gln | tRNA | 16,359 | 16,427 | 69 | TTG | ||
| tRNA-Asn | tRNA | 16,435 | 16,497 | 63 | GTT | ||
| tRNA-Thr | tRNA | 16,498 | 16,560 | 63 | TGT | ||
| tRNA-Cys | tRNA | 16,565 | 16,626 | 62 | GCA | ||
| tRNA-Ala | tRNA | 16,632 | 16,696 | 65 | TGC | ||
| tRNA-Ser1 | tRNA | 16,698 | 16,764 | 67 | TCT | ||
| cox3 | Coding | 16,765 | 17,730 | 966 | ATG | TAA | |
| rrnS | rRNA | 17,731 | 18,995 | 1,265 |
Note:
The anticodon of tRNAs are reported in the 5′-3′ direction.
Table 3. Unassigned regions (URs).
| UR name | Start | Stop | Length (bp) |
|---|---|---|---|
| UR1 | 1,717 | 1,753 | 37 |
| UR2 | 1,816 | 1,821 | 6 |
| UR3 | 2,740 | 2,754 | 15 |
| UR4 | 3,775 | 3,779 | 5 |
| UR5 | 4,053 | 4,124 | 72 |
| UR6 | 4,191 | 4,227 | 37 |
| UR7 | 5,500 | 5,552 | 53 |
| UR8 | 5,617 | 5,640 | 24 |
| UR9 | 9,124 | 9,144 | 21 |
| UR10 | 9,553 | 9,630 | 78 |
| UR11 (LUR) | 11,269 | 13,378 | 2,110 |
| UR12 | 13,505 | 13,525 | 21 |
| UR13 | 15,053 | 15,080 | 28 |
| UR14 | 15,141 | 15,217 | 77 |
| UR15 | 15,281 | 15,293 | 13 |
| UR16 | 15,359 | 15,379 | 21 |
| UR17 | 15,875 | 15,896 | 22 |
| UR18 | 16,022 | 16,029 | 8 |
| UR19 | 16,156 | 16,170 | 15 |
| UR20 | 16,296 | 16,296 | 1 |
| UR21 | 16,428 | 16,434 | 7 |
| UR22 | 16,561 | 16,564 | 4 |
| UR23 | 16,627 | 16,631 | 5 |
| UR24 | 16,697 | 16,697 | 1 |
The analysis of the nucleotide composition points out that the mitochondrial genome of this bivalve species exhibits high A+T content, totaling 63% vs 37% G+C. The minimum values of A+T are found in cytb (60.1%) and nd4 (61%). The nucleotide composition of every gene is shown in Table 4. According to the analysis above, both A and T occur very frequently at the third position of codons (64.6% on average of A+T), while the less frequent base in third position is C (12%). The most used codons are UUU (Phe), counted 269 times, and UUA (Leu) counted 210 times (6.78% and 5.29% of the total, respectively), while the less used codons are CGC (Arg) counted 6 times (0.15%), ACC (Thr) and CCG (Pro) each counted 16 times (0.4%) (Table 5). Only in four cases over 20 (Lys, Leu, Gln, Val), the most frequently used codon matches the correspondent mitochondrial tRNA anticodon.
Table 4. Nucleotide composition.
| Name | Length (bp) | T (%) | C (%) | A (%) | G (%) | A+T (%) | T3 (%) | C3 (%) | A3 (%) | G3 (%) | A3+T3 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cox1 | 1,716 | 35.8 | 15.5 | 25.8 | 22.9 | 61.6 | 39 | 12.1 | 28.0 | 21.3 | 67.0 |
| nd1 | 918 | 38.7 | 12.5 | 24.0 | 24.8 | 62.7 | 38 | 10.1 | 30.7 | 21.2 | 68.7 |
| nd2 | 1,020 | 38.3 | 11.0 | 24.8 | 25.9 | 63.1 | 35 | 11.5 | 29.4 | 24.4 | 64.4 |
| nd4l | 273 | 39.9 | 12.8 | 25.3 | 22.0 | 65.2 | 34 | 14.3 | 30.8 | 20.9 | 64.8 |
| cox2 | 1,272 | 29.7 | 14.8 | 29.1 | 26.4 | 58.8 | 30 | 15.3 | 27.4 | 27.6 | 57.4 |
| cob | 1,224 | 37.4 | 17.2 | 22.7 | 22.6 | 60.1 | 41 | 14.7 | 21.8 | 22.1 | 62.8 |
| rrnL | 1,749 | 33.2 | 11.5 | 32.6 | 22.6 | 65.8 | 33 | 10.6 | 33.4 | 23.0 | 66.4 |
| atp6 | 510 | 42.0 | 15.7 | 20.8 | 21.6 | 62.8 | 45 | 13.5 | 21.8 | 20.0 | 66.8 |
| nd3 | 408 | 39.5 | 11.0 | 24.8 | 24.8 | 64.3 | 33 | 11.0 | 30.1 | 25.7 | 63.1 |
| nd5 | 1,638 | 37.6 | 11.7 | 27.7 | 23.0 | 65.3 | 35 | 11.0 | 34.2 | 19.8 | 69.2 |
| atp8 | 126 | 44.4 | 11.9 | 19.0 | 24.6 | 63.4 | 45 | 4.8 | 23.8 | 26.2 | 68.8 |
| nd4 | 1,340 | 38.9 | 12.9 | 22.1 | 26.1 | 61.0 | 41 | 10.8 | 24.9 | 23.5 | 65.9 |
| nd6 | 495 | 39.2 | 12.1 | 23.0 | 25.7 | 62.2 | 38 | 13.9 | 27.9 | 20.0 | 65.9 |
| cox3 | 966 | 36.9 | 12.7 | 24.8 | 25.6 | 61.7 | 39 | 9.6 | 28.6 | 23.0 | 67.6 |
| rrnS | 1,265 | 32.7 | 12.3 | 32.9 | 22.1 | 65.6 | 35 | 13.5 | 31.6 | 19.5 | 66.6 |
| All coding | 14,920 | 36.3 | 13.2 | 26.5 | 24.0 | 63.0 | 37 | 12.0 | 28.9 | 22.4 | 65.7 |
| All rRNAs | 3,014 | 32.9 | 23.8 | 32.7 | 22.3 | 65.7 | |||||
| All tRNAs | 1,394 | 35.4 | 12.8 | 30.2 | 21.7 | 65.6 | |||||
| All URs | 2,681 | 28.2 | 14.1 | 34.1 | 23.6 | 62.3 | |||||
| All genic DNA | 16,314 | 36.2 | 13.2 | 26.8 | 23.8 | 63.0 | |||||
| All DNA | 18,995 | 35.1 | 13.3 | 27.9 | 23.7 | 63.0 |
Note:
URs, unassigned regions.
Table 5. Codon usage.
| Amino acid | Codon | # | Frequency | %TOT | Amino acid | Codon | # | Frequency | %TOT |
|---|---|---|---|---|---|---|---|---|---|
| Ala | GCG | 29 | 0.15 | 0.73 | Pro | CCG | 16 | 0.12 | 0.40 |
| GCA | 44 | 0.23 | 1.11 | CCA | 36 | 0.27 | 0.91 | ||
| GCT | 85 | 0.45 | 2.14 | CCT | 58 | 0.43 | 1.46 | ||
| GCC | 30 | 0.16 | 0.76 | CCC | 24 | 0.18 | 0.61 | ||
| Cys | TGT | 94 | 0.76 | 2.37 | Gln | CAG | 25 | 0.44 | 0.63 |
| TGC | 30 | 0.24 | 0.76 | CAA | 32 | 0.56 | 0.81 | ||
| Asp | GAT | 54 | 0.66 | 1.36 | Arg | CGG | 23 | 0.31 | 0.58 |
| GAC | 28 | 0.34 | 0.71 | CGA | 21 | 0.28 | 0.53 | ||
| Glu | GAG | 87 | 0.6 | 2.19 | CGT | 25 | 0.33 | 0.63 | |
| GAA | 58 | 0.4 | 1.46 | CGC | 6 | 0.08 | 0.15 | ||
| Phe | TTT | 269 | 0.78 | 6.78 | Ser | AGG | 69 | 0.19 | 1.74 |
| TTC | 78 | 0.22 | 1.97 | AGA | 69 | 0.19 | 1.74 | ||
| Gly | GGG | 131 | 0.4 | 3.30 | AGT | 55 | 0.15 | 1.39 | |
| GGA | 61 | 0.19 | 1.54 | AGC | 23 | 0.06 | 0.58 | ||
| GGT | 98 | 0.3 | 2.47 | TCG | 18 | 0.05 | 0.45 | ||
| GGC | 36 | 0.11 | 0.91 | TCA | 33 | 0.09 | 0.83 | ||
| His | CAT | 37 | 0.62 | 0.93 | TCT | 76 | 0.21 | 1.92 | |
| CAC | 23 | 0.38 | 0.58 | TCC | 22 | 0.06 | 0.55 | ||
| Ile | ATT | 165 | 0.8 | 4.16 | Thr | ACG | 21 | 0.17 | 0.53 |
| ATC | 40 | 0.2 | 1.01 | ACA | 30 | 0.24 | 0.76 | ||
| Lys | AAG | 61 | 0.41 | 1.54 | ACT | 57 | 0.46 | 1.44 | |
| AAA | 87 | 0.59 | 2.19 | ACC | 16 | 0.13 | 0.40 | ||
| Leu | TTG | 122 | 0.23 | 3.08 | Val | GTG | 113 | 0.3 | 2.85 |
| TTA | 210 | 0.39 | 5.29 | GTA | 121 | 0.32 | 3.05 | ||
| CTG | 43 | 0.08 | 1.08 | GTT | 119 | 0.32 | 3.00 | ||
| CTA | 70 | 0.13 | 1.76 | GTC | 23 | 0.06 | 0.58 | ||
| CTT | 75 | 0.14 | 1.89 | Trp | TGG | 58 | 0.54 | 1.46 | |
| CTC | 20 | 0.04 | 0.50 | TGA | 49 | 0.46 | 1.24 | ||
| Met | ATG | 86 | 0.36 | 2.17 | Tyr | TAT | 103 | 0.69 | 2.60 |
| ATA | 155 | 0.64 | 3.91 | TAC | 47 | 0.31 | 1.18 | ||
| Asn | AAT | 76 | 0.66 | 1.92 | STOP | TAG | 34 | 0.58 | 0.86 |
| AAC | 39 | 0.34 | 0.98 | TAA | 25 | 0.42 | 0.63 |
Note:
The codons corresponding to a tRNA present in the mitochondrial genome are underlined and in bold. The highest frequency among synonymous codons is also underlined and in bold. #, number of codons; Frequency, frequency of each codon among synonymous codons; %TOT, frequency of each codon among all the codons.
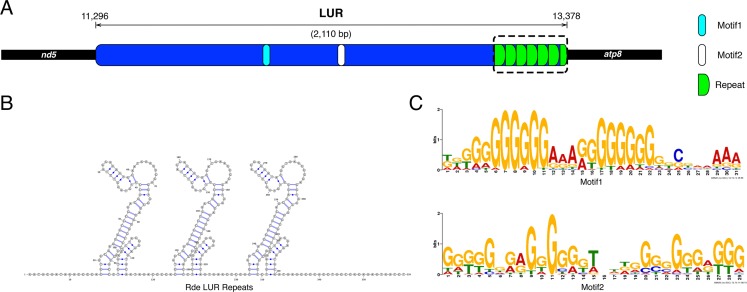
The UR11 is the LUR and is located between atp8 and nd5 (Figs. 1 and 2A). The LUR of the female used for whole mtDNA Sanger sequencing (i.e., the reference female, F4) is 2,110 bp long, and includes 6.5 repeated sequences—each repeat having a length of 54 bp—localized in the 3′ region of the LUR, just upstream the atp8 gene (Fig. 2A). DNA secondary structure analysis predicted three stem-loop structures in such region (Fig. 2B and Supplemental Information files on figshare: https://ndownloader.figshare.com/files/8387672), with a change in Gibbs free energy (ΔG) of −71.38 Kcal/mol. We also amplified and sequenced the LUR of 13 more females. We were not able to completely sequence LURs longer than 2,110 bp, because of the known difficulties in Sanger sequencing of regions including multiple repeats. The sequence alignment of the 13 LURs is available for download from figshare (https://ndownloader.figshare.com/files/8360789). LUR lengths, inferred from gel electrophoresis, are reported in Table 6, and they range from 2,000 to 5,000 bp. Two females (F3 and F17) showed length heteroplasmy of the LUR. The portion of the genome occupied by URs varies between 14.11% and 29.38%, depending on LUR length. The analysis with MEME (output shown in Figs. S2 and S3) unveiled two motifs (Fig. 2C) that show a strong conservation within the Veneridae family, and with S. purpuratus. The sea urchin was included in the analysis because Cao et al. (2004) reported a match between some motifs found in the CR of the marine mussels Mytilus edulis and Mytilus galloprovincialis with regulatory elements of the sea urchin CR. Accordingly, the search with GOMo assigned a series of GO terms related to transcription to the two motifs (Table S4).
Figure 2. Principal features of the Largest Unassigned Region (LUR).
(A): map of the lUR; (B): DNA secondary structure predicted in the repeat region (boxed in A); (C): Logos of the two DNA motifs found in the LUR.
Table 6. LUR length and number of repeats in the 13 female samples analyzed.
| Specimen | Length (bp) | Number of repeats | GenBank Acc. No. |
|---|---|---|---|
| F3 | 2,100–3,500 | 6.5–25 | MF055702 |
| F5 | 5,000 | 45 | MF055703 |
| F7 | 3,500 | 25 | MF055704 |
| F9 | 3,500 | 25 | MF055705 |
| F10 | 3,000 | 20 | MF055706 |
| F11 | 3,000 | 20 | MF055707 |
| F13 | 3,500 | 25 | MF055708 |
| F15 | 3,000 | 20 | MF055709 |
| F16 | 3,500 | 25 | MF055710 |
| F17 | 2,500–3,500 | 8–25 | MF055711 |
| F19 | 3,500 | 25 | MF055712 |
| F20 | 2,500 | 8 | MF055713 |
| F21 | 2,100 | 6.5 | MF055714 |
Note:
F3 and F17 are heteroplasmic with LURs of different length.
Polymorphism
Table 7 (top) shows the statistics associated with the SP analysis performed with GATK on the 12 samples used for RNA-Seq, with the Sanger-sequenced mtDNA as reference. Overall, 257 SPs were called, of which 145 (56.4%) were located in coding sequences (CDS). Interestingly, most of the SPs were called because of private alleles of one single male specimen (mRDI01). More in detail, 151 SPs out of 257 (58.7%) along the whole mtDNA sequence, and 103 SPs out of 145 (71%) in CDS, were private of mRDI01. In CDS, if we exclude the SPs associated with this male, the number of polymorphisms drops to 42 over 14,920 bp of coding mtDNA (GATK output in VCF format and a detailed list of SPs in tabular format is available on figshare: https://ndownloader.figshare.com/files/8902537), of which 18 are represented by indels, 6 of which are located in 4 different coding genes: one each in cox1, cytb, and nd5, plus 3 in cox3 (see Table 8). A file showing the ORF generated by the different variants of cox3, and alignments between them is available on figshare (https://ndownloader.figshare.com/files/8402471). Table 7 (bottom) shows the number of SPs in males, in males except mRDI01, and in females both along the whole mtDNA, and in CDS. The number in brackets represent the number of private SPs for each category.
Table 7. Sequence Polymorphism (SP): SPs and small indels called by GATK.
| Feature | Value | Min | Median | Mean | Max |
|---|---|---|---|---|---|
| Depth (all SPs) | – | 6 | 1,357 | 1,521 | 3,880 |
| Phred score (all SPs) | – | 3.30E+01 | 5.76E+03 | 4.18E+07 | 2.15E+09 |
| Depth (SPs in CDS) | – | 222 | 2,038 | 2,150 | 3,880 |
| Phred score (SPs in CDS) | – | 1.18E+02 | 1.01E+04 | 4.45E+07 | 2.15E+09 |
| Total number of SPs | 257 | – | – | – | – |
| Number of mRDI01 private SPs | 151 (58.7% of the total) | – | – | – | – |
| Number of SPs in CDS | 145 (56.4% of the total) | – | – | – | – |
| Number of mRDI01 private SPs in CDS | 103 (71% of the SP in CDS) | – | – | – | – |
| Number of SPs in CDS (excluding mRDI01) | 42 | – | – | – | – |
| Frequency of SPs in CDS | 0.0097 (∼1 every 103 bp) | – | – | – | – |
| Frequency of SPs in CDS (excluding mRDI01) | 0.0028 (∼1 every 355 bp) | – | – | – | – |
| Total number of indels | 18 | – | – | – | – |
| Number of indels in CDS | 6 | – | – | – | – |
| Number of indels causing frameshift | 4 | – | – | – | – |
| # Of SPs | Whole mtDNA | CDS | |||
| Males | 234 (160) | 136 (107) | |||
| Males (no mRDI01) | 84 (15) | 32 (6) | |||
| Females | 97 (23) | 38 (9) |
Note:
CDS, coding sequences; Whole mtDNA, polymorphism in the whole mitochondrial genome; the number in brackets the bottom of the table represent private SPs (e.g., there are 23 female specific SPs in the whole mtDNA and 9 female specific SPs in CDS); p-value, significance of the Fisher’s exact test on number of SPs between sexes (i.e., all males vs females, males except mRDI01 vs females).
Table 8. Indels located in coding sequences.
| Position | Depth | Qual | Gene | SP | Frameshift | Sample | Allele frequency | Notes |
|---|---|---|---|---|---|---|---|---|
| 1,698 | 3,732 | 1.38E+04 | cox1 | C/CAAA | No | mRDI02, mRDI03 | 0.089, 0.85 | Insertion of 1 Lysine |
| 6,364 | 1,929 | 2.15E+09 | cytb | CT/C | Yes | fRDI04, mRDI05 | 0.80, 0.81 | Yields a shorter Cytb. Possible sequencing error due to the homopolymer CTTTTTTT |
| 10,449 | 1,780 | 2.15E+09 | nd5 | C/CT | Yes | fRDI01, fRDI04, fRDI05 | 0.11, 0.10, 0.11 | Yields a nd5 gene divided in 2 ORFs. Possible sequencing error due to the homopolymer CTTTTTT |
| 17,619 | 2,272 | 5.98E+03 | cox3 | AGCG/A | No | mRDI01 | 0.97 | Deletion of one Alanine |
| 17,621 | 2,188 | 9.99E+04 | cox3 | CG/C | Yes | mRDI01 | 0.99 | Always combined with SP_17624. Together change the last 35 amino acids |
| 17,624 | 2,287 | 5.98E+03 | cox3 | C/CAT | Yes | mRDI01 | 0.99 | Always combined with SP_17621. Together change the last 35 amino acids |
Note:
Depth, sequencing depth; Qual, quality of the called SP expressed in Phred score; Allele frequency, frequency of the alternative allele in each sample indicated in the “Sample” column.
Comparison with other veneridae
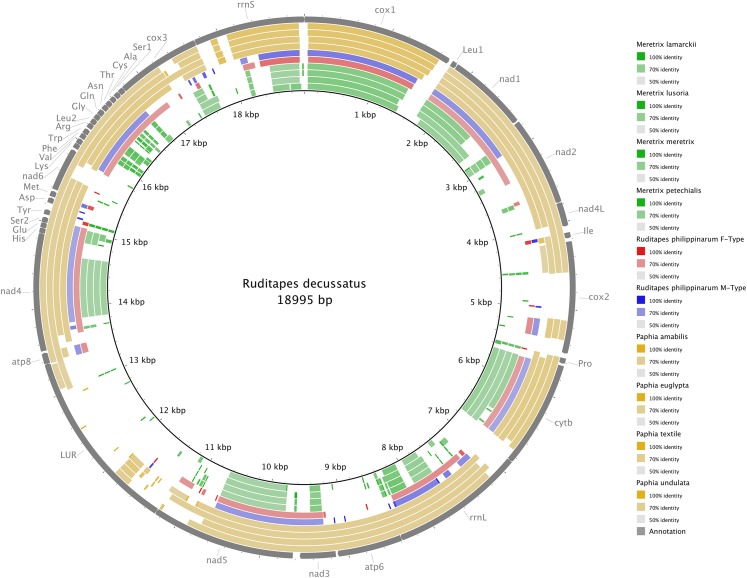
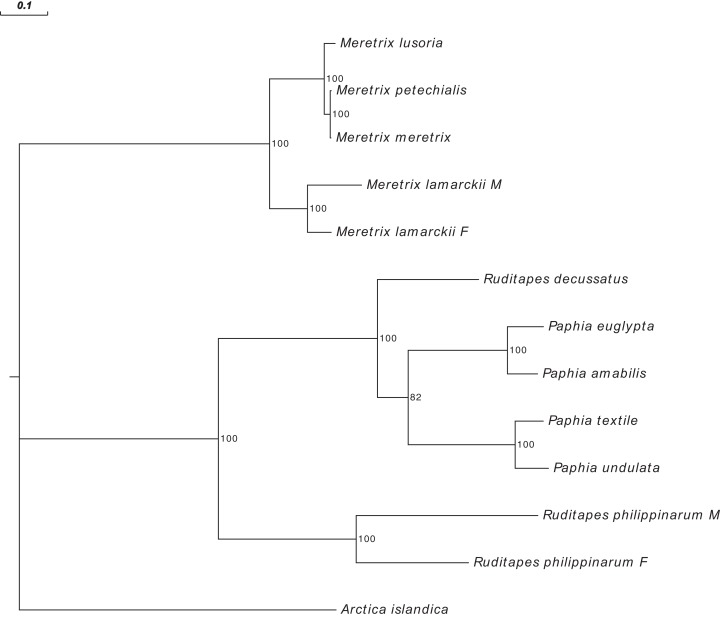
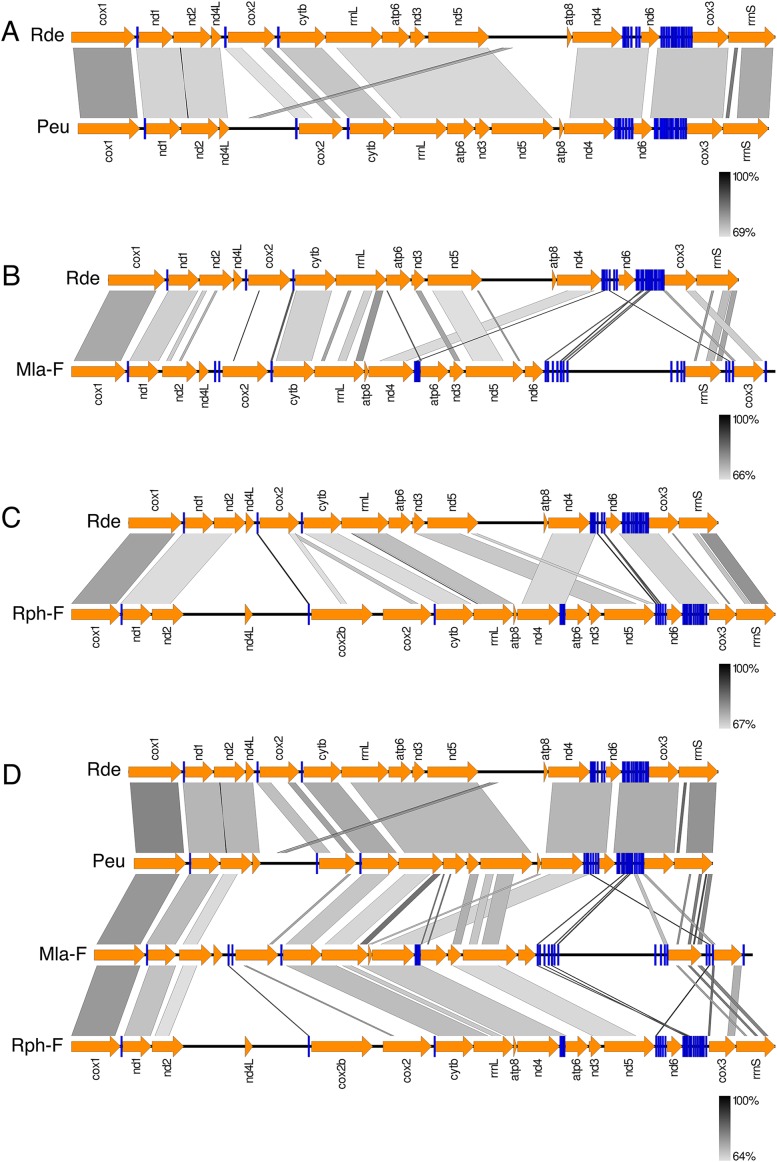
Figure 3 shows the Ruditapes decussatus mtDNA map (external gray circle), and the BLASTN identity (colored inner circles) with complete mtDNAs of other 10 venerid species (see list in Table S3). Figure 4 shows the ML tree obtained with the MitoPhast pipeline; the complete input and output of this analysis is available on figshare (https://ndownloader.figshare.com/files/8360792). Figure 5 shows the variation in gene order between Ruditapes decussatus and P. euglypta (Fig. 5A), M. lamarckii F-type (Fig. 5B), Ruditapes philippinarum F-type (Fig. 5C), and among all the four species (Fig. 5D).
Figure 3. BLASTN comparison of Ruditapes decussatus and other Veneridae.
Ruditapes decussatus mtDNA map (external gray circle), and BLASTN identity (colored inner circles) with complete mtDNAs of other 10 venerid species (see list in Table S3).
Figure 4. Maximum Likelihood (ML) tree of Veneridae obtained with all mitochondrial coding genes.
ML tree obtained with the MitoPhast pipeline; the complete input and output of this analysis is available on figshare (https://doi.org/10.6084/m9.figshare.4970762.v1).
Figure 5. Comparison of gene order in venerid mtDNAs.
Variation in gene order between Ruditapes decussatus and P. euglypta (A) M. lamarckii F-type (B) Ruditapes philippinarum F-type (C) and among all the four species (D).
Discussion
RNA-Seq-guided sequencing of mtDNA
The de novo assembly of the mtDNA from RNA-Seq data turned out to be informative, simplifying the primer walking procedure used for Sanger sequencing. Only one conting (Contig 1) resulted to be a chimeric sequence obtained by the misassembly of two smaller contigs. Most of the contigs (eight out of nine) contained more than one gene, and most of the tRNA genes were included in the de novo assembly. Except for tRNA-Pro, tRNA-Ile, and tRNA-Leu1, all the other tRNA genes are organized in two big clusters: a 13-gene cluster positioned between cox3 and nd6, and a 6-gene cluster between nd6 and nd4. The assembly retrieved 6 out of 13 tRNAs from the first cluster (missing tRNA-Gly, tRNA-Glu, tRNA-Asn, tRNA-Thr, tRNA-Cys, tRNA-Ala, and tRNA-Ser1), and 4 out of 6 tRNAs from the second cluster (missing tRNA-Met and tRNA-Asp). All the tRNA genes not located in these two clusters (tRNA-Pro, tRNA-Ile, and tRNA-Leu1) were included in the contigs. The presence of a clear polyadenylation signal in four of the assembled contigs (see Table 1) seems to indicate the existence of multiple polycistronic transcripts. It is also noteworthy that poly-A sequences seem to be absent in contigs having tRNA of rRNA genes at one end (Contigs 2, 5, 8 and 9). This could be either an evidence supporting the “tRNA punctation model” of RNA processing proposed by Ojala, Montoya & Attardi (1981) for human mitochondria, or a result of difficulties in sequencing/assembly of such regions. More analyses are required to address this point.
General features
The size of the fully Sanger-sequenced mitochondrial genome of Ruditapes decussatus (reference female F4) is of 18,995 bp, and it includes 13 protein-coding genes, 22 tRNAs and 2 rRNAs. Our data support the presence of the atp8 gene in the mtDNA of Ruditapes decussatus; atp8 has been reported as missing in several bivalve species, however, more accurate searches often led to the identification of the gene, so, in most cases, the alleged lack of atp8 is likely ascribable to annotation inaccuracies due to the extreme variability and the small size of the gene (Breton, Stewart & Hoeh, 2010; Breton et al., 2014; Plazzi, Puccio & Passamonti, 2016).
The mitochondrial genome of Ruditapes decussatus shows a high content of A-T (63%), a common feature in bivalve mtDNAs; moreover, T is the most common nucleotide at the third codon base (64.6%). The most common codon is UUU (Phe), which is also the most commonly used in bivalves, as well as in other invertebrates (Passamonti et al., 2011).
Codon usage
As shown in Table 5, in 16 cases out of 20, the most frequently used codon does not correspond to the anticodon of the inferred tRNA. In other words, there is not a correspondence between the most abundant codons and the anticodons of the 22 mitochondrial tRNAs. According to the “wobble hypothesis”—first proposed by Crick (1966)—the conformation of the tRNA anticodon loop enables some flexibility at the first base of the anticodon, so a Watson–Crick type of base pairing in the third position of the codon is not strictly necessary. This allows an amino acid to be correctly incorporated by ribosomes even if the tRNA is not fully complementary to the codon; according to Crick, this explains the degeneracy of the genetic code. This feature is particularly interesting in the light of the debate about natural selection acting at synonymous sites: since the early 1980s, evidence of a correlation between synonymous codon usage and tRNA abundances started accumulating. According to these authors, synonymous codon usage is biased to match skews in tRNA abundance, as a result of selective pressure maximizing protein synthesis rates (reviewed in Chamary, Parmley & Hurst, 2006). Following this rationale, the results here reported and data from other marine bivalves and metazoans (Yu & Li, 2011; Passamonti et al., 2011) would suggest that in some mitochondrial genomes translation efficiency is not maximized, and this observation deserves further investigation.
Length and sequence polymorphism
The mtDNA of Ruditapes decussatus has a high proportion of URs mostly depending on the length of the LUR (Table 6); on average, bivalve mtDNAs have 1.7× the amount of URs in respect to other analyzed Metazoa (Ghiselli et al., 2013), and it is still unclear whether there is an accumulation of non-functional sequences in bivalve mtDNA due to genetic drift, or if such URs are maintained by natural selection because they contain—so far unknown—functional elements (see Milani et al., 2013, 2014b; Breton et al., 2014; Pozzi et al., 2017). The LUR of Ruditapes decussatus most likely includes the mitochondrial CR, as indicated by the presence of two motifs (Fig. 2C; Figs S2 and S3) similar to two regulatory elements identified in the sea urchin CR. These two motifs are the same identified in previous analyses on the clam Ruditapes philippinarum and the mussel Musculista senhousia (Ghiselli et al., 2013; Guerra, Ghiselli & Passamonti, 2014) so they are conserved across distant bivalve taxa, and the GO terms associated with such motifs are related to transcriptional control (Table S4). An interesting feature of Ruditapes decussatus LUR is its variable length (Table 6), most likely due to different repeat content. As a matter of fact, the very same repeat sequence was present in every LUR, and our data strongly suggest that LUR length variation is actually due to repeat CNV (see Supplemental Information files on figshare: https://ndownloader.figshare.com/files/8387666 and https://ndownloader.figshare.com/files/8360789), as observed in other bivalve species (see Ghiselli et al., 2013; Guerra, Ghiselli & Passamonti, 2014). Tandem repeats have been also reported in the mitochondrial genomes of the bivalves Acanthocardia tuberculata (Dreyer & Steiner, 2006), Placopecten magellanicus (La Roche et al., 1990), Moerella iridescens, Sanguinolaria olivacea, Semele scaba, Sinonovacula constricta, Solecurtus divaricatus (Yuan et al., 2012), Ruditapes philippinarum (Ghiselli et al., 2013), and Musculista senhousia (Guerra, Ghiselli & Passamonti, 2014). These repeats are believed to arise from duplications caused by replication slippage (Buroker et al., 1990; Hayasaka, Ishida & Horai, 1991; Broughton & Dowling, 1994). The tandem repeats found at the 3′ end of Ruditapes decussatus LUR are predicted to form a secondary structure (see Fig. 2B and Supplemental Information files on figshare) composed by multiple stem-loops, which obviously increase in number with the increment of the number of tandem repeats. The effect, if any, of tandem repeats in mtDNA is unknown: since the repeats are almost always localized in proximity of the CR, they might interact with regulatory elements—or even contain some—influencing replication and/or transcription initiation, and such interactions might also be altered by the formation of secondary structures (Passamonti et al., 2011; Ghiselli et al., 2013; Guerra, Ghiselli & Passamonti, 2014).
We assessed the genetic variability of Ruditapes decussatus mtDNA using two different approaches: by SP calling in CDS (RNA-Seq data on 12 individuals), and by analysis of the LUR (Sanger sequencing of 14 individuals). The CR and its flanking regions are known to be hypervariable, so they are commonly used to assess polymorphism at low taxonomic levels. Our data strongly support a very low genetic variability: the number of SPs in CDS is 145, of which 103 are private of a single individual (mRDI01)—thus reducing the number to 42—while the number of variable sites in the analyzed LURs is 98 over 3,095 aligned positions. Considering the known variability of mtDNA in bivalves (Gissi, Iannelli & Pesole, 2008; Ghiselli et al., 2013; Breton et al., 2014; Plazzi, Puccio & Passamonti, 2016), this is a surprising result. Even more if we compare the results of the present work to a methodologically identical analysis performed on 12 Ruditapes philippinarum samples from the Pacific coast of USA, performed by Ghiselli et al. (2013): in that work, GATK yielded 194 SPs in the M-type mtDNA and 293 in the F-type. Strikingly, the 12 Ruditapes philippinarum samples analyzed were actually two families (6 siblings + 6 siblings). This means that randomly sampled individuals of Ruditapes decussatus used in this work showed a much lower mtDNA variability than Ruditapes philippinarum siblings. A previous analysis on the cox1 gene of Ruditapes decussatus reported a nucleotide diversity (π) of 0.15 for a population from the Northern Adriatic Sea (Cordero, Peña & Saavedra, 2014). Another analysis on the same gene of Ruditapes philippinarum from the same range resulted in a π = 0.25 (Cordero et al., 2017), so Ruditapes decussatus has a lower nucleotide diversity at the cox1 locus. The difference between the variability in mtDNA of Ruditapes decussatus that we are reporting here and that of Ruditapes philippinarum reported in Ghiselli et al. (2013) appears to be more marked. It is known that the genetic variability of Ruditapes philippinarum in the Adriatic Sea is lower than in populations from its native range in Asia (Cordero et al., 2017), probably because of the bottlenecks that this species had to go through during the multiple colonization events. The introduction in North America from Asia happened first (in the 1930s), and from there the Manila clam was introduced in Atlantic Europe (in the 1970s and 1980s), and lastly into the Adriatic Sea (1983 and 1984), and it is plausible that the genetic diversity decreased at each introduction event. Accordingly, Cordero et al. (2017) observed that Ruditapes philippinarum genetic variability in Europe is lower compared to that of the Pacific coast of the USA, so the samples analyzed in Ghiselli et al. (2013) could have been more polymorphic than those analyzed in Cordero, Peña & Saavedra (2014), thus explaining the more pronounced differences in genetic variability between the Manila clam and the European clam discussed above. In any case, all the available data point to a lower genetic diversity of Ruditapes decussatus mtDNA, and it would be interesting to know whether it is a cause or an effect of the ongoing replacement of Ruditapes decussatus with the invasive Ruditapes philippinarum. It will also be important to investigate genetic variability of the nuclear genes, especially after Cordero, Peña & Saavedra (2014) reported contrasting levels of differentiation between mitochondrial and nuclear markers.
With respect to SP effects, we found six indels in CDS, 2 of which do not cause frameshift, but a simple insertion/deletion of one amino acid (SP_1698, and SP_17619, see Table 8). Of the remaining four, SP_6364 and SP_10449 consist of a deletion and an insertion of a single T in two homopolymeric sequences (CTTTTTTT and CTTTTTT, respectively), raising the possibility of a sequencing error. In any case, the two SPs yield a shorter CDS (cytb and nd5, respectively), and are present at relatively low frequencies in the specimens carrying them, except for SP_6364 which has a frequency of 80% in fRDI04. The cox3 gene shows three SPs: the first one, SP_17619, does not cause a frameshift, and results in the deletion of one alanine residue, and its frequency in mRDI01 is 97%. The second one, SP_17621, consists of a deletion of a G with respect to the reference sequence, which is the Sanger-sequenced mtDNA of sample F4; all the individuals analyzed with RNA-Seq carry this deletion except for mRDI01 which, at that position, has the same sequence of the reference mtDNA (reference-like allele frequency in mRDI01 = 99%). The third indel, SP_17624, consists of an insertion of two nucleotides, and its frequency in mRDI01 is 99%. So, basically, for cox3 we have three types of sequences: (i) the Sanger-sequenced reference, which yields a 966 bp (321 aa) ORF; (ii) a sequence found in 11/12 of samples analyzed with RNA-Seq (except mRDI01) that carries a single-nucleotide deletion (SP_17621), and yields a 963 bp (320 aa) ORF; (iii) a sequence, private of mRDI01, which is obtained by combining SP_17624 and SP_17621 (both 99% of frequency, so most likely co-occurring), which produces a 963 bp (320 aa) ORF. Interestingly, the ORFs obtained from the sequences described in (ii) and (iii), are almost identical, namely the sequence obtained by RNA-seq in 11/12 samples and the sequence obtained by RNA-Seq in mRDI01 are basically the same, and differ from the Sanger-sequenced reference, yielding an amino acid sequence that differs in the last 35 residues (all data available in Supplemental Information files on figshare: https://doi.org/10.6084/m9.figshare.4970762.v3). Given this consistent difference between the sequence obtained by Sanger-sequencing of DNA, and those obtained by RNA-Seq, it is tempting to speculate that this difference might be caused by RNA editing, a mechanism observed in mtDNA of some animals (Lavrov & Pett, 2016), and recently reported to be common in cephalopods (Liscovitch-Brauer et al., 2017). Actually, Liscovitch-Brauer et al. (2017) reported only A-to-I editing, which is not the kind of change we are observing here, but other types of editing are known across eukaryotes (see Gott & Emeson, 2000 for a review), and some others, still unknown, might exist as well. Post-transcriptional modifications (thus including RNA-editing) are still poorly understood mechanisms, but they appear to be responsible for most of the mitochondrial gene expression regulation (Scheibye-Alsing et al., 2007; Scheffler, 2008; Milani et al., 2014a). What we propose here is a pure conjecture, but we think in the future it might be worthy to investigate mitochondrial transcriptomes looking for such kind of “unexpected” biological features.
Interestingly, in contrast with a low nucleotide variability along the entire mitochondrial genome, we observed a pretty high polymorphism in LUR length due to CNV of tandem repeats, and even a LUR length heteroplasmy: two females yielded two electrophoretic bands each (∼2,100 and ∼3,500 bp in F3; ∼2,500 and ∼3,500 bp in F17; see Table 6). A possible explanation is that the diversity (CNV) detected in the LURs could be recent: the accumulation of nucleotide variation at different sites along the mitochondrial genome needs time, while the kind structural variability we observed can be achieved in few generations (or even one) considering that replication slippage is common in repeat-rich regions.
Phylogenetic relationship with Ruditapes philippinarum
Despite Ruditapes decussatus and Ruditapes philippinarum being morphologically similar and being ascribed to the same genus, the results here reported clearly show that they are quite different both for mtDNA sequence (Figs. 3 and 4) and mtDNA gene arrangement (Fig. 5). This is an unusual finding, even among bivalves, which are known to be fast-evolving for these characters. This may point to the fact that these two species are less related than previously thought. Actually, this is not the first clue that Ruditapes decussatus and Ruditapes philippinarum are quite different genetically, as allozyme electrophoresis (Passamonti, Mantovani & Scali, 1997, 1999) and satellite DNA content (Passamonti, Mantovani & Scali, 1998) pointed out. More in-depth analyses are therefore needed to correctly trace the phylogenetic relationships of these two Ruditapes species, which may eventually end up in two different Genera. As shown in Figs. 3–5, the Genus Paphia is the most similar to Ruditapes decussatus.
Presence/absence of DUI
We could not find evidence for sex-specific mtDNAs, typical of DUI. As stated in the Introduction, the search for DUI is not a straightforward process. HTS can help thanks to a much deeper sequencing coverage (in respect to the cloning-and-Sanger-sequencing approach), and because it overcomes the problem of primer specificity, a limitation of the classical approach. One possible concern about using HTS approaches based on short reads in presence of DUI is about the ability of softwares to detect divergent reads and assembly them correctly. More specifically, one could ask what is the divergence threshold under which the assemblers are not able to partition the contigs into two sex-linked groups. We do not know such a threshold, but we used different assembly strategies trying to retrieve sex-specific mtDNA sequences from our data. Other than the approach reported in Materials and Methods (which is the one that produced the data reported here), we tried other techniques. After identifying reads that blasted to bivalve mitochondrial sequences present in GenBank and discarding all the other reads, we generated A5+CAP3 assemblies: (i) for each of the samples (obtaining 12 separate assemblies), and (ii) pooling the six males together and the six females together, and assembling the two sex-specific pools. Both these approaches did not show evidence of sex-specific mtDNAs. Then we took the assembly obtained from the females and removed the reads from each of the samples that mapped (<8 mismatches) to these sequences. We then used the remaining reads as A5 input. The program could not assemble anything. Lastly, we tried the software MetaVelvet (Namiki et al., 2012)—that assembles metagenomes—on all the reads matching bivalve mtDNAs, and only one genome was produced. After all these alternative approaches failed to find two sex-linked mtDNAs, we decided to proceed with the assembly as indicated in Materials and Methods, because it was the technique that yielded the best quality contigs, most likely because using the reads from all 12 the individuals granted a higher coverage of the mtDNA. Given these results, we can propose three different explanations.
Ruditapes decussatus is characterized by SMI of mitochondria, so a male-transmitted mtDNA is not present in this species.
- The divergence between the two sex-specific mtDNAs is too low to be detected. This could be the outcome of two different situations.
- DUI is very young in this species, so the two sex-linked mtDNAs did not have the time to diverge.
- A role-reversal event occurred recently. Role reversal (a.k.a. “route reversal” or “masculinization”) is a process—observed so far only in species of the Mytilus complex—by which F-type genomes invades the male germ line becoming sperm-transmitted, thus turning into M-type mtDNAs (Hoeh et al., 1997). This event actually resets to zero the divergence between F- and M-type, although substantial differences in the control regions were reported between the original F-type and the “masculinized” one (see Zouros, 2013 for a thorough review). The hypothesis that role reversal could have occurred multiple times in the evolutionary history of bivalves and could have led to the complete replacement of M or F mtDNAs in several species was proposed by Hoeh et al. (1997) to explain the scattered phylogenetic distribution of DUI across Bivalvia. Indeed, according to the hypothesis of a single origin, DUI arose >400 Mya, approximately at the origin of Autolamellibranchia, but, as said, such hypothesis requires the assumption of multiple role-reversal and/or DUI loss events in several branches of the bivalve tree (see Zouros, 2013 for a detailed discussion). Recently, a multiple origin of DUI was proposed (Milani et al., 2013, 2014b; Milani, Ghiselli & Passamonti, 2016; Mitchell et al., 2016), and in such case there would be no need of multiple role-reversal events to explain its phylogeny. In our opinion, until further evidence will be provided, role-reversal should not be considered a rule, but rather an exception. Of course, we cannot rule out that a masculinization event might have occurred in Ruditapes decussatus, so this hypothesis must be taken into consideration.
In our data, even if there is no clear evidence of a male-specific mtDNA, a male sample (mRDI01) clearly stood out from the others, both males and females (see Table 7). Overall, the divergence between mRDI01 and the other 11 samples calculated considering its private SPs is of 151 sites over 18,995 bp (considering the whole mtDNA), and of 103 sites over 14,920 bp (considering only CDS). In both cases the divergence is very low (0.8% and 0.7%, respectively), which explains why the mtDNA of mRDI01, although different, was not assembled as a separate genome. We have no sufficient data to evaluate if such divergence is normal within Ruditapes decussatus populations, but considered the variability usually observed in bivalves, we find the difference unsurprising. On the contrary, the lack of variability among the other 11 samples is remarkable. For these reasons, we are inclined to believe that mRDI01 divergence is compatible with hypotheses (1) and (2). That said, there still could be a third, quite conjectural, hypothesis by which these data might indicate an incipient DUI, not yet fixed in the population.
All in all, we have a preference for the first explanation, but the present data are not sufficient to exclude the others, and a more thorough investigation is necessary to assess this point.
Up to now DUI was identified in only three Veneridae species: Cyclina sinensis, Ruditapes philippinarum, and Meretrix lamarckii (Gusman et al., 2016). The status of Paphia is still unknown, and in future works it would be interesting to investigate more Heterodonta species to understand better the distribution of DUI in this derived group of bivalves.
Supplemental Information
BP, Biological Process; CC, Cellular Component; MF, Molecular Function.
Acknowledgments
We would like to thank Edoardo Turolla (Istituto Delta Ecologia Applicata, Ferrara, Italy) for providing the specimens, and Massimo Milan for bibliographic suggestions. We also gratefully thank the Editor Tim Collins, and the reviewers Carlos Saavedra, Shallee Page, and one anonymous colleague for their comments and suggestions.
Funding Statement
This study was supported by the Italian Ministry of Education, University and Research (MIUR) FIR Programme no. RBFR13T97A funded to FG, MIUR SIR Programme no. RBSI14G0P5 funded to LM, Zumberge Foundation to SVN, and by the Canziani bequest funded to MP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Fabrizio Ghiselli conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Liliana Milani conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Mariangela Iannello performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Emanuele Procopio performed the experiments, analyzed the data.
Peter L. Chang analyzed the data.
Sergey V. Nuzhdin conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Marco Passamonti conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
GenBank accession numbers MF055702 to MF055714, and KP089983.
GenBank BioProject PRJNA170478.
Data Availability
The following information was supplied regarding data availability:
Ghiselli, Fabrizio; Milani, Liliana; Iannello, Mariangela; Procopio, Emanuele; L. Chang, Peter; Nuzhdin, Sergey; Passamonti, Marco (2017): The Complete Mitochondrial Genome of the Grooved Carpet Shell, Ruditapes decussatus (Bivalvia, Veneridae). figshare.
References
- Alikhan et al. (2011).Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12(1):402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Pérez et al. (2016).Arias-Pérez A, Cordero D, Borrell Y, Sánchez JA, Blanco G, Freire R, Insua A, Saavedra C. Assessing the geographic scale of genetic population management with microsatellites and introns in the clam Ruditapes decussatus. Ecology and Evolution. 2016;6(10):3380–3404. doi: 10.1002/ece3.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood et al. (2003).Attwood TK, Bradley P, Flower DR, Gaulton A, Maudling N, Mitchell AL, Moulton G, Nordle A, Paine K, Taylor P, Uddin A, Zygouri C. PRINTS and its automatic supplement, prePRINTS. Nucleic Acids Research. 2003;31(1):400–402. doi: 10.1093/nar/gkg030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey et al. (2009).Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt et al. (2013).Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution. 2013;69(2):313–319. doi: 10.1016/j.ympev.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Breton et al. (2014).Breton S, Milani L, Ghiselli F, Guerra D, Stewart DT, Passamonti M. A resourceful genome: updating the functional repertoire and evolutionary role of animal mitochondrial DNAs. Trends in Genetics. 2014;30(12):555–564. doi: 10.1016/j.tig.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Breton, Stewart & Hoeh (2010).Breton S, Stewart DT, Hoeh WR. Characterization of a mitochondrial ORF from the gender-associated mtDNAs of Mytilus spp. (Bivalvia: Mytilidae): identification of the “missing” ATPase 8 gene. Marine Genomics. 2010;3(1):11–18. doi: 10.1016/j.margen.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Breton et al. (2011).Breton S, Stewart DT, Shepardson S, Trdan RJ, Bogan AE, Chapman EG, Ruminas AJ, Piontkivska H, Hoeh WR. Novel protein genes in animal mtDNA: a new sex determination system in freshwater mussels (Bivalvia: Unionoida)? Molecular Biology and Evolution. 2011;28(5):1645–1659. doi: 10.1093/molbev/msq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton & Dowling (1994).Broughton RE, Dowling TE. Length variation in mitochondrial DNA of the minnow Cyprinella spiloptera. Genetics. 1994;138:179–190. doi: 10.1093/genetics/138.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buroker et al. (1990).Buroker NE, Brown JR, Gilbert TA, O’Hara PJ, Beckenbach AT, Thomas WK, Smith MJ. Length heteroplasmy of sturgeon mitochondrial DNA: an illegitimate elongation model. Genetics. 1990;124:157–163. doi: 10.1093/genetics/124.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske et al. (2010).Buske FA, Bodén M, Bauer DC, Bailey TL. Assigning roles to DNA regulatory motifs using comparative genomics. Bioinformatics. 2010;26(7):860–866. doi: 10.1093/bioinformatics/btq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2004).Cao L, Kenchington E, Zouros E, Rodakis GC. Evidence that the large noncoding sequence is the main control region of maternally and paternally transmitted mitochondrial genomes of the marine mussel (Mytilus spp.) Genetics. 2004;167(2):835–850. doi: 10.1534/genetics.103.026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez, Silla-Martinez & Gabaldón (2009).Capella-Gutiérrez S, Silla-Martinez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamary, Parmley & Hurst (2006).Chamary JV, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nature Reviews Genetics. 2006;7(2):98–108. doi: 10.1038/nrg1770. [DOI] [PubMed] [Google Scholar]
- Cordero et al. (2017).Cordero D, Delgado M, Liu B, Ruesink J, Saavedra C. Population genetics of the Manila clam (Ruditapes philippinarum) introduced in North America and Europe. Scientific Reports. 2017;7:39745. doi: 10.1038/srep39745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero, Peña & Saavedra (2014).Cordero D, Peña JB, Saavedra C. Phylogeographic analysis of introns and mitochondrial DNA in the clam Ruditapes decussatus uncovers the effects of Pleistocene glaciations and endogenous barriers to gene flow. Molecular Phylogenetics and Evolution. 2014;71:274–287. doi: 10.1016/j.ympev.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Crick (1966).Crick FHC. Codon—anticodon pairing: the wobble hypothesis. Journal of Molecular Biology. 1966;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Darty, Denise & Ponty (2009).Darty K, Denise A, Ponty Y. VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009;25(15):1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo et al. (2011).DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa et al. (2014).de Sousa JT, Milan M, Bargelloni L, Pauletto M, Matias D, Joaquim S, Matias AM, Quillien V, Leitão A, Huvet A. A microarray-based analysis of gametogenesis in two Portuguese populations of the European clam Ruditapes decussatus. PLOS ONE. 2014;9:e92202. doi: 10.1371/journal.pone.0092202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz, Dudley & Skibinski (2012).Diz AP, Dudley E, Skibinski DOF. Identification and characterisation of highly expressed proteins in sperm cells of the marine mussel Mytilus edulis. Proteomics. 2012;12(12):1949–1956. doi: 10.1002/pmic.201100500. [DOI] [PubMed] [Google Scholar]
- Dreyer & Steiner (2006).Dreyer H, Steiner G. The complete sequences and gene organisation of the mitochondrial genomes of the heterodont bivalves Acanthocardia tuberculata and Hiatella arctica—and the first record for a putative Atpase subunit 8 gene in marine bivalves. Frontiers in Zoology. 2006;3:13. doi: 10.1186/1742-9994-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn et al. (2016).Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research. 2016;44(D1):D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli et al. (2012).Ghiselli F, Milani L, Chang PL, Hedgecock D, Davis JP, Nuzhdin SV, Passamonti M. De Novo assembly of the Manila clam Ruditapes philippinarum transcriptome provides new insights into expression bias, mitochondrial doubly uniparental inheritance and sex determination. Molecular Biology and Evolution. 2012;29(2):771–786. doi: 10.1093/molbev/msr248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli et al. (2013).Ghiselli F, Milani L, Guerra D, Chang PL, Breton S, Nuzhdin SV, Passamonti M. Structure, transcription, and variability of metazoan mitochondrial genome: perspectives from an unusual mitochondrial inheritance system. Genome Biology and Evolution. 2013;5(8):1535–1554. doi: 10.1093/gbe/evt112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli, Milani & Passamonti (2011).Ghiselli F, Milani L, Passamonti M. Strict sex-specific mtDNA segregation in the germ line of the DUI species Venerupis philippinarum (Bivalvia: Veneridae) Molecular Biology and Evolution. 2011;28(2):949–961. doi: 10.1093/molbev/msq271. [DOI] [PubMed] [Google Scholar]
- Gissi, Iannelli & Pesole (2008).Gissi C, Iannelli F, Pesole G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity. 2008;101(4):301–320. doi: 10.1038/hdy.2008.62. [DOI] [PubMed] [Google Scholar]
- Gosling (2003).Gosling EM. Bivalve Molluscs: Biology, Ecology and Culture. Hoboken: Wiley Online Library; 2003. [Google Scholar]
- Gott & Emeson (2000).Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annual Review of Genetics. 2000;34(1):499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- Gruber et al. (2008).Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. The Vienna RNA websuite. Nucleic Acids Research. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, Ghiselli & Passamonti (2014).Guerra D, Ghiselli F, Passamonti M. The Largest Unassigned Regions of the male- and female-transmitted mitochondrial DNAs in Musculista senhousia (Bivalvia Mytilidae) Gene. 2014;536(2):316–325. doi: 10.1016/j.gene.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Gusman et al. (2016).Gusman A, Lecomte S, Stewart DT, Passamonti M, Breton S. Pursuing the quest for better understanding the taxonomic distribution of the system of doubly uniparental inheritance of mtDNA. PeerJ. 2016;4:e2760. doi: 10.7717/peerj.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka, Ishida & Horai (1991).Hayasaka K, Ishida T, Horai S. Heteroplasmy and polymorphism in the major noncoding region of mitochondrial DNA in Japanese monkeys: association with tandemly repeated sequences. Molecular Biology and Evolution. 1991;8:399–415. doi: 10.1093/oxfordjournals.molbev.a040660. [DOI] [PubMed] [Google Scholar]
- He et al. (2016).He Z, Zhang H, Gao S, Lercher MJ, Chen W-H, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Research. 2016;44(W1):W236–W241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeh et al. (1997).Hoeh WR, Stewart DT, Saavedra C, Sutherland BW, Zouros E. Phylogenetic evidence for role-reversals of gender-associated mitochondrial DNA in Mytilus (Bivalvia: Mytilidae) Molecular Biology and Evolution. 1997;14(9):959–967. doi: 10.1093/oxfordjournals.molbev.a025839. [DOI] [PubMed] [Google Scholar]
- Huang & Madan (1999).Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Research. 1999;9(9):868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al. (2014).Jones P, Binns D, Chang H-YY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong S-Y, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al. (2011).Ju YS, Kim J-I, Kim S, Hong D, Park H, Shin J-Y, Lee S, Lee W-C, Kim S, Yu S-B, Park SS, Seo S-H, Yun J-Y, Kim H-J, Lee D-S, Yavartanoo M, Kang HP, Gokcumen O, Govindaraju DR, Jung JH, Chong H, Yang K-S, Kim H, Lee C, Seo J-S. Extensive genomic and transcriptional diversity identified through massively parallel DNA and RNA sequencing of eighteen Korean individuals. Nature Genetics. 2011;43(8):745–752. doi: 10.1038/ng.872. [DOI] [PubMed] [Google Scholar]
- King et al. (2014).King JL, LaRue BL, Novroski NM, Stoljarova M, Seo SB, Zeng X, Warshauer DH, Davis CP, Parson W, Sajantila A, Budowle B. High-quality and high-throughput massively parallel sequencing of the human mitochondrial genome using the Illumina MiSeq. Forensic Science International: Genetics. 2014;12:128–135. doi: 10.1016/j.fsigen.2014.06.001. [DOI] [PubMed] [Google Scholar]
- La Roche et al. (1990).La Roche J, Snyder M, Cook DI, Fuller K, Zouros E. Molecular characterization of a repeat element causing large-scale size variation in the mitochondrial DNA of the sea scallop Placopecten magellanicus. Molecular Biology and Evolution. 1990;7(1):45–64. doi: 10.1093/oxfordjournals.molbev.a040586. [DOI] [PubMed] [Google Scholar]
- Laslett & Canback (2008).Laslett D, Canback B. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 2008;24(2):172–175. doi: 10.1093/bioinformatics/btm573. [DOI] [PubMed] [Google Scholar]
- Lavrov & Pett (2016).Lavrov DV, Pett W. Animal Mitochondrial DNA as We Do Not Know It: mt-Genome Organization and Evolution in Nonbilaterian Lineages. Genome Biology and Evolution. 2016;8(9):2896–2913. doi: 10.1093/gbe/evw195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite et al. (2013).Leite RB, Milan M, Coppe A, Bortoluzzi S, dos Anjos A, Reinhardt R, Saavedra C, Patarnello T, Cancela ML, Bargelloni L. mRNA-Seq and microarray development for the Grooved Carpet shell clam, Ruditapes decussatus: a functional approach to unravel host-parasite interaction. BMC Genomics. 2013;14(1):741. doi: 10.1186/1471-2164-14-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch-Brauer et al. (2017).Liscovitch-Brauer N, Alon S, Porath HT, Elstein B, Unger R, Ziv T, Admon A, Levanon EY, Rosenthal JJC, Eisenberg E. Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell. 2017;169(2):191–202.e11. doi: 10.1016/j.cell.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubośny et al. (2017/2).Lubośny M, Przyłucka A, Sańko TJ, Śmietanka B, Rosenfeld S, Burzyński A. Next generation sequencing of gonadal transcriptome suggests standard maternal inheritance of mitochondrial DNA in Eurhomalea rufa (Veneridae) Marine Genomics. 2017/2;31:21–23. doi: 10.1016/j.margen.2016.11.002. [DOI] [Google Scholar]
- McKenna et al. (2010).McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani & Ghiselli (2015).Milani L, Ghiselli F. Mitochondrial activity in gametes and transmission of viable mtDNA. Biology Direct. 2015;10:22. doi: 10.1186/s13062-015-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani et al. (2013).Milani L, Ghiselli F, Guerra D, Breton S, Passamonti M. A comparative analysis of mitochondrial ORFans: new clues on their origin and role in species with doubly uniparental inheritance of mitochondria. Genome Biology and Evolution. 2013;5(7):1408–1434. doi: 10.1093/gbe/evt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani et al. (2014a).Milani L, Ghiselli F, Iannello M, Passamonti M. Evidence for somatic transcription of male-transmitted mitochondrial genome in the DUI species Ruditapes philippinarum (Bivalvia: Veneridae) Current Genetics. 2014a;60(3):163–173. doi: 10.1007/s00294-014-0420-7. [DOI] [PubMed] [Google Scholar]
- Milani et al. (2014b).Milani L, Ghiselli F, Maurizii MG, Nuzhdin SV, Passamonti M. Paternally transmitted mitochondria express a new gene of potential viral origin. Genome Biology and Evolution. 2014b;6(2):391–405. doi: 10.1093/gbe/evu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani, Ghiselli & Passamonti (2016).Milani L, Ghiselli F, Passamonti M. Mitochondrial selfish elements and the evolution of biological novelties. Current Zoology. 2016;62(6):687–697. doi: 10.1093/cz/zow044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbury et al. (2010).Milbury CA, Lee JC, Cannone JJ, Gaffney PM, Gutell RR. Fragmentation of the large subunit ribosomal RNA gene in oyster mitochondrial genomes. BMC Genomics. 2010;11(1):485. doi: 10.1186/1471-2164-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell et al. (2016).Mitchell A, Guerra D, Stewart D, Breton S. In silico analyses of mitochondrial ORFans in freshwater mussels (Bivalvia: Unionoida) provide a framework for future studies of their origin and function. BMC Genomics. 2016;17(1):597. doi: 10.1186/s12864-016-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi et al. (2008).Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Namiki et al. (2012).Namiki T, Hachiya T, Tanaka H, Sakakibara Y. MetaVelvet: an extension of Velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Research. 2012;40(20):e155. doi: 10.1093/nar/gks678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala, Montoya & Attardi (1981).Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Passamonti & Ghiselli (2009).Passamonti M, Ghiselli F. Doubly uniparental inheritance: two mitochondrial genomes, one precious model for organelle DNA inheritance and evolution. DNA and Cell Biology. 2009;28(2):79–89. doi: 10.1089/dna.2008.0807. [DOI] [PubMed] [Google Scholar]
- Passamonti, Mantovani & Scali (1997).Passamonti M, Mantovani B, Scali V. Allozymic characterization and genetic relationships among four species of Tapetinae (Bivalvia, Veneridae) Italian Journal of Zoology. 1997;64:117–124. doi: 10.1080/11250009709356183. [DOI] [Google Scholar]
- Passamonti, Mantovani & Scali (1998).Passamonti M, Mantovani B, Scali V. Characterization of a highly repeated DNA family in tapetinae species (mollusca bivalvia: veneridae) Zoological Science. 1998;15(4):599–605. doi: 10.2108/zsj.15.599. [DOI] [PubMed] [Google Scholar]
- Passamonti, Mantovani & Scali (1999).Passamonti M, Mantovani B, Scali V. Allozymic analysis of some Mediterranean Veneridae (Mollusca: Bivalvia): preliminary notes on taxonomy and systematics of the family. Journal of the Marine Biological Association of the UK. 1999;79(5):899–906. doi: 10.1017/s0025315498001064. [DOI] [Google Scholar]
- Passamonti et al. (2011).Passamonti M, Ricci A, Milani L, Ghiselli F. Mitochondrial genomes and Doubly Uniparental Inheritance: new insights from Musculista senhousia sex-linked mitochondrial DNAs (Bivalvia Mytilidae) BMC Genomics. 2011;12(1):442. doi: 10.1186/1471-2164-12-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesole et al. (2012).Pesole G, Allen JF, Lane N, Martin W, Rand DM, Schatz G, Saccone C. The neglected genome. EMBO Reports. 2012;13:473–474. doi: 10.1038/embor.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzi, Puccio & Passamonti (2016).Plazzi F, Puccio G, Passamonti M. Comparative large-scale mitogenomics evidences clade-specific evolutionary trends in mitochondrial DNAs of Bivalvia. Genome Biology and Evolution. 2016;8(8):2544–2564. doi: 10.1093/gbe/evw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi et al. (2017).Pozzi A, Plazzi F, Milani L, Ghiselli F, Passamonti M. SmithRNAs: could mitochondria “bend” nuclear regulation? Molecular Biology and Evolution. 2017;34(8):1960–1973. doi: 10.1093/molbev/msx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen & Skaletsky (2000).Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Scheffler (2008).Scheffler IE. Mitochondria. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- Scheibye-Alsing et al. (2007).Scheibye-Alsing K, Cirera S, Gilchrist MJ, Fredholm M, Gorodkin J. EST analysis on pig mitochondria reveal novel expression differences between developmental and adult tissues. BMC Genomics. 2007;8(1):367. doi: 10.1186/1471-2164-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serb & Lydeard (2003).Serb JM, Lydeard C. Complete mtDNA sequence of the North American freshwater mussel, Lampsilis ornata (Unionidae): an examination of the evolution and phylogenetic utility of mitochondrial genome organization in Bivalvia (Mollusca) Molecular Biology and Evolution. 2003;20(11):1854–1866. doi: 10.1093/molbev/msg218. [DOI] [PubMed] [Google Scholar]
- Sievers et al. (2011).Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7(1):539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski, Gallagher & Beynon (1994a).Skibinski DO, Gallagher C, Beynon CM. Mitochondrial DNA inheritance. Nature. 1994a;368:817–818. doi: 10.1038/368817b0. [DOI] [PubMed] [Google Scholar]
- Skibinski, Gallagher & Beynon (1994b).Skibinski DO, Gallagher C, Beynon CM. Sex-limited mitochondrial DNA transmission in the marine mussel Mytilus edulis. Genetics. 1994b;138:801–809. doi: 10.1093/genetics/138.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith (2013).Smith DR. RNA-Seq data: a goldmine for organelle research. Briefings in Functional Genomics. 2013;12(5):454–456. doi: 10.1093/bfgp/els066. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard (2000).Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- Sullivan, Petty & Beatson (2011).Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan et al. (2015).Tan MH, Gan HM, Schultz MB, Austin CM. MitoPhAST, a new automated mitogenomic phylogeny tool in the post-genomic era with a case study of 89 decapod mitogenomes including eight new freshwater crayfish mitogenomes. Molecular Phylogenetics and Evolution. 2015;85:180–188. doi: 10.1016/j.ympev.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Theologidis et al. (2008).Theologidis I, Fodelianakis S, Gaspar MB, Zouros E. Doubly uniparental inheritance (DUI) of mitochondrial DNA in Donax trunculus (Bivalvia: Donacidae) and the problem of its sporadic detection in Bivalvia. Evolution; International Journal of Organic Evolution. 2008;62(4):959–970. doi: 10.1111/j.1558-5646.2008.00329.x. [DOI] [PubMed] [Google Scholar]
- Tritt et al. (2012).Tritt A, Eisen JA, Facciotti MT, Darling AE. An integrated pipeline for de novo assembly of microbial genomes. PLOS ONE. 2012;7(9):e42304. doi: 10.1371/journal.pone.0042304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler et al. (2005).Wheeler DL, Church DM, Federhen S, Lash AE, Madden TL, Pontius JU, Schuler GD, Schriml LM, Sequeira E, Tatusova TA, Wagner L. Database resources of the National Center for Biotechnology. Nucleic Acid Research. 2005;33:D39–D45. doi: 10.1093/nar/gki062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu & Li (2011).Yu H, Li Q. Mutation and selection on the wobble nucleotide in tRNA anticodons in marine bivalve mitochondrial genomes. PLOS ONE. 2011;6(1):e16147. doi: 10.1371/journal.pone.0016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2012).Yuan Y, Li Q, Yu H, Kong L. The complete mitochondrial genomes of six heterodont bivalves (Tellinoidea and Solenoidea): variable gene arrangements and phylogenetic implications. PLOS ONE. 2012;7(2):e32353. doi: 10.1371/journal.pone.0032353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2016).Yuan S, Xia Y, Zheng Y, Zeng X. Next-generation sequencing of mixed genomic DNA allows efficient assembly of rearranged mitochondrial genomes in Amolops chunganensis and Quasipaa boulengeri. PeerJ. 2016;4:e2786. doi: 10.7717/peerj.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouros (2013).Zouros E. Biparental inheritance through uniparental transmission: the doubly uniparental inheritance (DUI) of mitochondrial DNA. Evolutionary Biology. 2013;40(1):1–31. doi: 10.1007/s11692-012-9195-2. [DOI] [Google Scholar]
- Zouros et al. (1994a).Zouros E, Ball AO, Saavedra C, Freeman KR. Mitochondrial DNA inheritance. Nature. 1994a;368:818. doi: 10.1038/368818a0. [DOI] [PubMed] [Google Scholar]
- Zouros et al. (1994b).Zouros E, Oberhauser Ball A, Saavedra C, Freeman KR. An unusual type of mitochondrial DNA inheritance in the blue mussel Mytilus. Proceedings of the National Academy of Sciences of USA. 1994b;91(16):7463–7467. doi: 10.1073/pnas.91.16.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BP, Biological Process; CC, Cellular Component; MF, Molecular Function.
Data Availability Statement
The following information was supplied regarding data availability:
Ghiselli, Fabrizio; Milani, Liliana; Iannello, Mariangela; Procopio, Emanuele; L. Chang, Peter; Nuzhdin, Sergey; Passamonti, Marco (2017): The Complete Mitochondrial Genome of the Grooved Carpet Shell, Ruditapes decussatus (Bivalvia, Veneridae). figshare.