Abstract
Microparticles (MPs), membrane fragments of 0.1–1.0 μm, are derived from many cell types in response to systemic inflammation. Acute liver failure (ALF) is a prototypical syndrome of systemic inflammatory response syndrome (SIRS) associated with a procoagulant state. We hypothesized that patients with ALF develop increased procoagulant MPs in proportion to the severity of systemic complications and adverse outcome. Fifty patients with acute liver injury (ALI), 78% of whom also had hepatic encephalopathy (HE; ALF), were followed until day 21 after admission. MPs were characterized by Invitrox Sizing, Antigen Detection and Enumeration, a light-scattering technology that can enumerate MPs as small as 0.15 μm, and by flow cytometry. Procoagulant activity was assessed by a functional MP-tissue factor (MP-TF) assay. Sixteen patients (32%) died and 27 (54%) recovered without liver transplantation (LT). Total MPs (0.15–1.0 μm) were present in nearly 19-fold higher concentrations in ALI/ALF patients, compared to healthy controls (P < 0.0001). MP-TF assays revealed high procoagulant activity (9.05 ± 8.82 versus 0.24 ± 0.14 pg/mL in controls; P = 0.0008). MP concentrations (0.28–0.64 μm) were higher in patients with the SIRS and high-grade HE, and MPs in the 0.36–0.64-μm size range increased in direct proportion to SIRS severity (P < 0.001) and grade of HE (P < 0.002). Day 1 MPs (0.28–0.64 μm) correlated with laboratory predictors of death/LT (higher phosphate and creatinine; lower bicarbonate), and day 1 and 3 MPs were higher in patients who died or underwent LT, compared to spontaneous survivors (P ≤ 0.01). By flow cytometry, 87% of patients had circulating CD41+ MPs, indicating platelet origin. Conclusion: Highly procoagulant MPs of specific size ranges are associated with the SIRS, systemic complications, and adverse outcome of ALI/ALF. MPs may contribute to the multiorgan system failure and high mortality of ALF.
Acute liver failure (ALF), the clinical syndrome subsequent to acute liver injury (ALI), is characterized by coagulopathy, hepatic encephalopathy (HE), and, frequently, death without liver transplantation (LT).1 An intense systemic inflammatory response syndrome (SIRS),2 often in the absence of infection, predicts multiorgan system failure (MOSF) and death.3 Although proinflammatory cytokines originating from the necrotic liver may trigger the systemic complications of ALF, mediators of the syndrome are incompletely defined, and others with effects on vascular endothelium and hemostasis likely coexist.4
Although abnormalities in hemostasis are an invariable feature of ALF syndrome, patients rarely develop bleeding complications despite dramatically elevated international normalized ratio of prothrombin time (INR).5 Indeed, patients with ALF appear more prone to thrombotic, rather than bleeding, complications,6 and intrahepatic thrombosis may exacerbate the initial injury.7 Recently, we6,8 and others9 have suggested that patients with ALF generally maintain normal or hypercoagulable global hemostasis, as determined by thromboelastography (TEG) and thrombin generation assays. Moreover, maximal clot strength by TEG increases in proportion to the number of SIRS components, possibly resulting from increased release of factor VIII and von Willebrand factor from activated/injured endothelial cells (ECs),10 providing a plausible explanation for the absence of bleeding, even in the most critically ill subjects with the highest INR.
In the presence of a relatively prothrombotic state, patients with ALF also frequently develop thrombocytopenia. 11 In other acute illnesses characterized by a prominent SIRS, such as sepsis, thrombocytopenia portends an ominous prognosis,12–14 particularly in patients with declining platelet counts after admission. 15 Although platelet fragmentation is well recognized in sepsis as part of disseminated intravascular coagulation (DIC), platelet fragmentation has not been studied in patients with ALF, who often have a DIClike phenotype, except for factor VIII levels, which tend to be low in DIC, but very high in ALF.10,16
Microparticles (MPs) are membrane fragments (ranging in size from 0.1–1.0 μm) derived from many cell types.17 Activation of cells or platelets by systemic inflammation initiates an enzymatically catalyzed reaction whereby chards of plasma membrane bleb inside out into the circulation, exposing procoagulant phosphatidylserine and cellular epitopes conferring functionality. MPs are particularly prothrombotic when they display tissue factor (TF), a transmembrane protein.18,19 Increasing experimental evidence suggests that MPs play a functional role in regulating vascular tone in patients with cirrhosis20 and sepsis,21 conditions that bear many similarities to ALF syndrome.22 Recent advances in light-scattering technology have permitted the enumeration and sizing of very small MPs of 0.15–0.5 μm, below the limit of detectability by standard flow cytometry, allowing an exploration of the role of MPs in disease pathogenesis.23
We hypothesized that patients with ALI/ALF may develop increased procoagulant MPs in plasma as a function of the severity of the SIRS. Furthermore, we sought to explore a potential pathogenic role of MPs in the systemic complications and outcome of patients with ALI/ALF.
Patients and Methods
Patients
This work was approved by the Ancillary Studies Committee of the Acute Liver Failure Study Group (ALFSG), and patients or their nearest of kin provided informed consent under the ALFSG Registry modified for the collection of platelet-poor plasma (PPP) by the institutional review board of Virginia Commonwealth University (VCU; Richmond, VA). Fifty consecutive patients with ALI/ALF were recruited prospectively from admissions at VCU Medical Center. ALI was defined as liver injury in a patient with no known previous liver disease, an admission INR of ≥1.5, and a duration of illness of ≤26 weeks. ALF was defined as ALI in the presence of HE. Some patients in the current study population also participated in two previous studies exploring hemostasis in ALI/ALF.6,8 For the present study, 13 healthy volunteer controls were also recruited for the collection of 5 mL of whole blood for plasma. Controls were of similar age (39 years) and gender distribution (54% female) as the study population (P = 0.6 and 0.2, respectively).
SIRS components were determined at time of admission to the study by standard criteria, and the presence of the SIRS was defined as two to four positive SIRS components.24
Complications of ALI/ALF, including bleeding, thrombosis, and infection, were defined previously6 and occurred late after admission (on or after day 3). Bleeding sites included gastric mucosal erosions (N = 6) and cutaneous (N = 3), none of which lead to the need for blood transfusion. Thrombotic events included occlusion of renal replacement therapy (RRT) catheters (N = 6), portal venous thrombosis (N = 2), and limb vessel thrombosis (N = 1). Sites of infection included lung (N = 5), urine (N = 4), blood (N = 3), and ascites (N = 1) and were identified relatively late after admission (>3 days after admission). As per ALFSG protocol, outcomes (death, LT, or transplantfree survival [TFS]) were determined at day 21 after admission.
Laboratory Methods
Standard laboratories were collected on admission to the hospital (day 1) and daily for 7 days. For the analyses herein, laboratories drawn on days 1 and 3 after admission were analyzed. Whole blood from days 1 and 3 was also collected for PPP in 5-mL citrated Vacutainer tubes. Because enrolled patients were purposely chosen to represent a wide range of liver injury severity, blood was drawn by in-dwelling venous catheters, radial artery catheters, and butterfly needle catheters, depending upon whether patients were in a floor bed or intensive care unit, and the availability of vascular access. Blood was centrifuged at 1,500×g for 20 minutes at room temperature, aliquotted, and PPP was frozen at −80°C within 2 hours of drawing.
MP Sizing and Enumeration
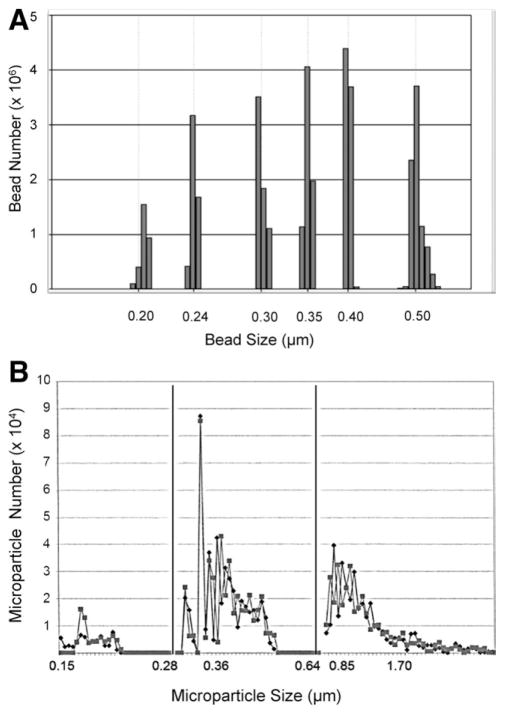
MPs were analyzed by Invitrox Sizing, Antigen Detection, and Enumeration (ISADE; Invitrox, Inc., Research Triangle Park, NC).23 Batches of 10–20 PPP samples, randomly selected, were injected into the detection chamber using a fixed volume of 200 μL/sample. Testing time for sizing and enumeration was 6 minutes/sample. To eliminate any contribution from buffer/diluent, background counts were subtracted from each sample result. The concentration of MPs was recorded as log10 MP/mL for the following size distributions: 0.15–0.27, 0.28–0.35, 0.36–0.64, and >0.64 μm. The ability of ISADE to resolve a mixture of standard control polystyrene beads with known sizes (0.2, 0.24, 0.3, 0.35, 0.4, and 0.5 μm) is shown in Fig. 1A. Both the size and number of beads were accurately reported with a small scatter of size around each peak, which resulted from a small variation in bead size, confirmed by scanning electron microscopy (SEM).
Fig. 1.
MP charcterization by ISADE. (A) Demonstration of the ability of ISADE to resolve a mixture of polystyrene beads with sizes of 0.2, 0.24, 0.3, 0.35, 0.4, and 0.5 μm. Both the size and number of beads were accurately reported. The low counts noted on either side of each bead size results from a small variation in bead size, confirmed by SEM. (B) Enumeration and sizing of MPs in PPP from a patient with ALF from disulfuram. Tracings from two different freeze-thawed aliquots are shown, demonstrating the small intersample variability of the results. Three distinct size ranges were identified, separated by solid black lines. Mid-sized MPs of 0.28–0.64 μm were most closely associated with ALF complications, laboratories, and outcome.
MP tissue factor (MP-TF) activity assay. MPs were isolated from 250 uL of PPP by centrifugation (20,000×g for 30 minutes at 4°C). The MP pellet was resuspended by sonication in 250 uL of HEPES-buffered saline containing 0.5% bovine serum albumin (BSA) (20 mM of HEPES, 120 mM of NaCl, and 1 mg/mL of BSA). A previously described25 two-stage chromogenic assay was employed with the following modifications. First, MPs were incubated for 2 hours with 2.5 mM of CaCl2, 1 nM of factor VIIa, and 150 nM of factor X (FX) in the presence and absence of a TF blocking antibody (Ab). Next, absorbance measurements (to measure generated FXa) were made for every 30 seconds for 30 minutes after the addition of ethylenediaminetetraacetic acid and FXa chromogenic substrate (Pefachrome 8595; Centerchem, Inc., Norwalk, CT). TF activity was calculated in relation to an Innovin TF standard.
Flow cytometry was performed on a Becton Dickinson BD LSRII (Becton Dickinson, Franklin Lakes, NJ) as per International Society on Thrombosis and Hemostasis standardization.26 Briefly, PPP (10 μL) at 37°C was stained with Ab for 15 minutes. Secondary Ab was added for an additional 10 minutes. Samples were then diluted with 0.9 mL of Annexin V binding buffer (BD) with or without calcium. An equal volume of Beckman Coulter Flow-Count beads (Beckman Coulter, Inc., Brea, CA) were added to the samples. Ten thousand sample events were collected within the MP gate, and results were compared to isotope controls.
Statistical Analyses
MP concentrations in each size distribution were log10-transformed for analysis. Continuous variables were analyzed for normality of distribution and expressed as mean ± standard deviation (SD) or median (range) and analyzed by analysis of variance or Wilcoxon’s/Kruskal-Wallis’ rank-sums test, as appropriate. Categorical variables were analyzed by chi-square test and correlation of continuous data by Pearson’s correlation (r value). Both uni- and multivariate logistic regression was used to model TFS using demographic and MP data. For stepwise logistic regression modeling, a P = 0.25 significance level was required for entry into the model, whereas a P = 0.05 significance was required for a covariate to remain in the model. Data were analyzed using JMP 8.0, and multivariate analyses were performed with SAS (SAS Institute Inc., Cary, NC). Significance was defined as a P value ≤0.05.
Results
Relationship of Baseline Patient Characteristics to Outcome
Demographic, clinical, and laboratory parameters of the study population are depicted in Table 1 according to outcome, either spontaneous recovery (TFS) or LT/death. Mean age of the entire population was 43 years, approximately two thirds were women, and half had acetaminophen (APAP) hepatotoxicity. Etiologies of liver injury in the non-APAP group included hepatitis B (in 7), idiosyncratic drug reactions (in 6), autoimmune hepatitis (in 5), indeterminate (in 3), and ischemia/herpes simplex virus/heat shock/Amanita mushroom poisoning (in 1 each). Hepatic encephalopathy (ALF) was present in 39 patients (78%) on admission, 24 of whom (62%) developed high-grade (grade 3/4) encephalopathy within the first 7 days of admission. The SIRS was present on admission in 28 patients (56%). In univariate analysis, predictors of death/LT included older age (P = 0.017), non-APAP etiology (P = 0.010), development of high-grade HE (P = 0.005), presence of SIRS on admission (P = 0.019), higher admission lactate (P < 0.0001), phosphate (P = 0.037), total bilirubin (P = 0.016), activated partial thromboplastin time (aPTT; P = 0.010), and factor VIII (P = 0.013), and lower alanine aminotransferase (ALT; P = 0.0003), bicarbonate (P = 0.019), and fibrinogen (P = 0.007).
Table 1.
Demographic and Clinical Characteristics of Study Population on Admission to the Hospital for ALI/ALF
| Clinical Feature | Total Study Population | Transplant-Free Survivors | Death/LT | P* |
|---|---|---|---|---|
|
|
|
|
||
| (N = 50) | (N = 27) | (N = 23) | ||
| Demographic and clinical characteristics | ||||
| Age, years | 43.1 ± 13.5 | 38.9 ± 13.2 | 48.0 ± 12.5 | 0.017 |
| Female gender, % | 64 | 67 | 61 | 0.670 |
| BMI, kg/m2 | 28.2 ± 6.8 | 27.4 ± 5.0 | 29.1 ± 8.4 | 0.381 |
| Etiology of ALF, % | 0.010 | |||
| APAP | 50 | 67 | 33 | |
| Non-APAP | 50 | 33 | 70 | |
| HE grade, % grade 3–4 | 48 | 30 | 70 | 0.005 |
| SIRS N, % 2–4 | 56 | 41 | 74 | 0.019 |
| Laboratories | ||||
| Ammonia, μM (venous) | 80.3 ± 45.2 | 72.5 ± 39.3 | 88.8 ± 50.5 | 0.227 |
| Lactate, mg/dL | 5.9 ± 5.6 | 3.1 ± 3.4 | 9.8 ± 5.7 | <0.0001 |
| Phosphate, mg/dL | 3.3 ± 2.2 | 2.7 ± 1.3 | 4.0 ± 2.8 | 0.037 |
| pH (arterial) | 7.35 ± 0.13 | 7.38 ± 0.13 | 7.33 ± 0.14 | 0.230 |
| Bicarbonate, mg/dL | 19.7 ± 7.7 | 22.0 ± 5.8 | 17.0 ± 8.8 | 0.019 |
| Creatinine, mg/dL | 1.0 (0.4–7.5) | 0.9 (0.4–7.5) | 1.3 (0.4–5.2) | 0.340 |
| Total bilirubin, mg/dL | 5.0 (0.3–44.2) | 4.4 (0.9–29.4) | 13.3 (0.3–44.2) | 0.016 |
| INR | 3.4 ± 1.8 | 3.1 ± 1.3 | 3.8 ± 2.1 | 0.175 |
| aPTT, seconds | 47.4 ± 14.7 | 42.4 ± 9.8 | 53.0 ± 17.3 | 0.010 |
| ALT, IU/L | 3,579 ± 2,765 | 4,813 ± 2,840 | 2,129 ± 1,852 | 0.0003 |
| Fibrinogen, mg/dL | 183 ± 71 | 211 ± 63 | 142 ± 63 | 0.007 |
| Factor VIII, % normal | 435 ± 206 | 364 ± 143 | 537 ± 243 | 0.013 |
P refers to comparison of transplant-free survivors and those who died or underwent LT.
Abbreviation: BMI, body mass index.
Enumeration, Sizing, and Procoagulant Activity of Microparticles in Plasma of Patients With ALI/ALF, Compared to Healthy Controls
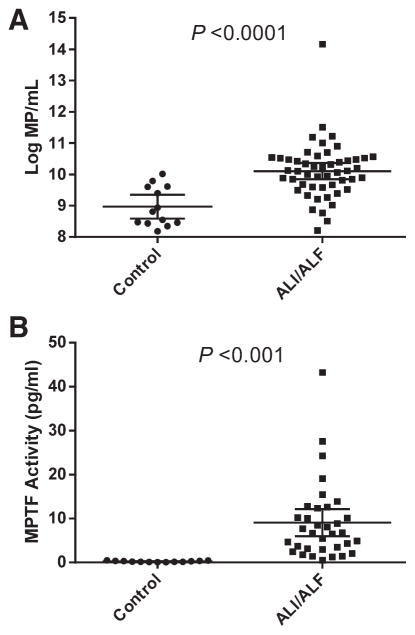
Three dominant MP size ranges were detected in plasma from ALF patients and healthy controls (0.15–0.27, 0.28–0.64, and >0.64 μm; Fig. 1B). Of total MPs in the range of 0.15–1.0 μm, a mean of 99.5% were <0.5 μm, the size limit of detection of standard flow cytometry (data not shown). Mean total MPs (0.15–1.0 μm) in patients with ALI/ALF were present in nearly 19-fold greater number than healthy controls of similar mean age and gender distribution (Fig. 2A; P < 0.0001). MPs of all size ranges were present in significantly greater concentrations in patients with ALI/ALF than in healthy controls (data not shown). TF-dependent procoagulant activity of MPs was determined using an in-house MPTF assay. Mean MP-TF activity was 38-fold higher in PPP from 34 ALI/ALF patients, compared to 13 healthy control patients (9.05 ± 8.82 versus 0.24 ± 0.14 pg/mL, respectively; Fig. 2B; P = 0.0008).
Fig. 2.
MP concentration and procoagulant activity in patients with ALI/ALF and normal healthy controls. (A) Total log10MP/mL of size range 0.15–1.0 μm in 50 ALI/ALF patients on admission to the hospital and 13 healthy controls of similar age and gender distribution (P < 0.0001). (B). MP-TF activity in 34 ALI/ALF patients on admission to the hospital and 13 healthy controls (P = 0.0008). Error bars indicate mean ± SD.
Relationship of MP Number and Size to Admission Laboratories and Late Complications of ALF
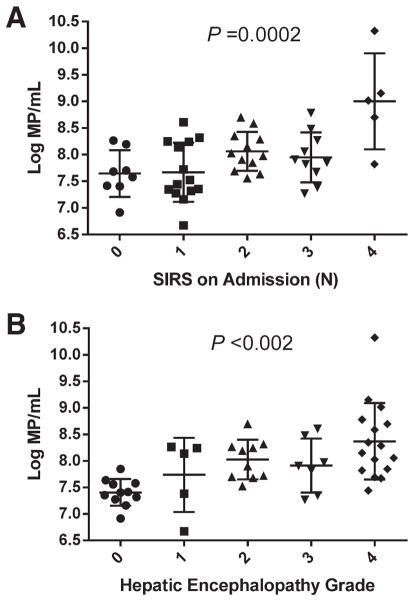
Table 2 depicts the relationship of log10 MP number/mL according to size with complications and laboratories on admission for ALI/ALF. Concentrations of large MPs (>0.64 μm) were present in significantly greater number in plasma from patients with non-APAP, compared to those with APAP hepatotoxicity, but were otherwise similar in patients with and without the SIRS on admission and those who developed specific complications of ALF. Significant differences were also not observed in concentrations of the smallest MPs (0.15–0.27 μm) according to etiology of liver injury, the presence of the SIRS, or specific complications of ALF. In contrast, concentrations of MPs of intermediate size (0.28–0.64 μm) were higher in patients with the presence of the SIRS on admission (9.19 ± 0.91 with 2–4 SIRS versus 8.71 ± 0.51/mL with 0–1 SIRS; P = 0.033), and those in the 0.36–0.64-μm size range were particularly closely related to the number of SIRS on admission (Fig. 3A; P = 0.0002). Similarly, MPs of intermediate size (0.28–0.64 μm) were present in higher concentration in patients with high-grade HE (grade 3–4) than those with grade 0–2 encephalopathy (9.24 ± 0.95μm versus 8.74 ± 0.52/mL, respectively; P = 0.026), and those in the 0.36–0.64-μm size range were again particularly closely related to the individual grade of encephalopathy (Fig. 3B; P < 0.002). MPs of intermediate size range were also present in higher concentration in patients who developed late (after day 3) complications of minor bleeding (none requiring blood transfusion) and renal failure requiring RRT. MP concentrations of any size range were not significantly different in patients who developed infectious or thrombotic complications, compared to those who did not (Table 2A).
Table 2.
Relationship Between Admission Microparticle Concentration, Clinical Characteristics (A), and Laboratory Variables (B) on Admission for ALI/ALF
| A. | |||||
|---|---|---|---|---|---|
|
| |||||
| Clinical Variable (N) | Microparticle Size (μm) | ||||
|
| |||||
| 0.15–0.27 | 0.28–0.35 | 0.36–0.64 | >0.64 | ||
|
| |||||
| Log10MP/mL | |||||
| Etiology | APAP (25) | 9.98 ± 1.22 | 8.83 ± 1.04 | 7.84 ± 0.81 | 7.65 ± 0.62 |
| Non-APAP (25) | 9.98 ± 1.00 | 8.98 ± 0.48 | 8.07 ± 0.38 | 8.00 ± 0.46* | |
| SIRS N | 0–1 (22) | 9.68 ± 1.01 | 8.65 ± 0.53 | 7.66 ± 0.50 | 7.71 ± 0.55 |
| 2–4 (28) | 10.21 ± 1.13 | 9.11 ± 0.93* | 8.19 ± 0.64† | 7.92 ± 0.57 | |
| HE grade | 0–2 (26) | 9.72 ± 0.98 | 8.68 ± 0.56 | 7.71 ± 0.48 | 7.72 ± 0.50 |
| 3–4 (24) | 10.25 ± 1.18 | 9.15 ± 0.96* | 8.23 ± 0.69† | 7.95 ± 0.62 | |
| Bleeding | No (41) | 9.84 ± 0.93 | 8.77 ± 0.52 | 7.86 ± 0.53 | 7.78 ± 0.50 |
| Yes (9) | 10.60 ± 1.60 | 9.50 ± 1.45† | 8.39 ± 0.91* | 8.06 ± 0.82 | |
| Thrombosis | No (41) | 10.01 ± 1.15 | 8.90 ± 0.86 | 7.93 ± 0.66 | 7.83 ± 0.58 |
| Yes (9) | 9.84 ± 0.87 | 8.93 ± 0.49 | 8.09 ± 0.52 | 7.83 ± 0.53* | |
| Infection | No (37) | 9.96 ± 0.93 | 8.84 ± 0.57 | 7.88 ± 0.58 | 7.81 ± 0.50 |
| Yes (13) | 10.02 ± 1.53 | 9.08 ± 1.27 | 8.17 ± 0.76 | 7.89 ± 0.74 | |
| RRT | No (32) | 9.83 ± 0.94 | 8.72 ± 0.57 | 7.77 ± 0.55 | 7.69 ± 0.48 |
| Yes (18) | 10.24 ± 1.32 | 9.23 ± 1.05* | 8.30 ± 0.65† | 8.07 ± 0.64* | |
| B. | ||||
|---|---|---|---|---|
|
| ||||
| Laboratory Variables | Microparticle Size (μm) | |||
|
| ||||
| 0.15–0.27 | 0.28–0.35 | 0.36–0.64 | >0.64 | |
| Phosphate | 0.35† | 0.52§ | 0.52§ | 0.51‡ |
| Bicarbonate | −0.06 | −0.23 | −0.44‡ | −0.14 |
| Creatinine | 0.22 | 0.10 | 0.31* | 0.18 |
| ALT | −0.19 | −0.32* | −0.37† | −0.46‡ |
| Factor VIII | 0.07 | 0.36* | 0.34* | 0.43† |
| MP-TF activity | 0.33* | 0.42† | 0.28 | 0.22 |
Numbers in (A) represent log10MP/mL of the indicated size ± SD. Numbers in (B) represent Pearson’s r values, with negative numbers denoting inverse correlation.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
P ≤ 0.0001.
Fig. 3.
Relationship of MP concentration to number of positive SIRS components on admission for ALI/ALF and grade of HE. (A) Log10MP/mL (0.36–0.64 μm) versus number of positive SIRS components on admission (P = 0.0002). (B) Log10 MP/mL (0.36–0.64 μm) versus maximal grade of HE during the first 7 days of admission (P = 0.0015). Error bars indicate mean ± SD.
Concentrations of MPs of intermediate size range (0.28–0.64 μm), and particularly those in the 0.36–0.64-μm range, were also the most strongly related to laboratories associated with the SIRS and poor outcome after ALF (Table 2B). Specifically, higher MP concentrations were associated with higher phosphate (r = 0.52; P < 0.0001), creatinine (r = 0.31; P = 0.030), and factor VIII (r = 0.38; P = 0.029) as well as lower bicarbonate (r = −0.44; P = 0.002) and ALT (r = −0.37; P = 0.009). MP concentrations in the 0.28–0.64-μm size range also directly correlated with MP-TF activity in the 34 patients in whom these assays were performed (r = 0.43; P = 0.012).
Relationship of MP Concentration and Size to Outcome of ALI/ALF
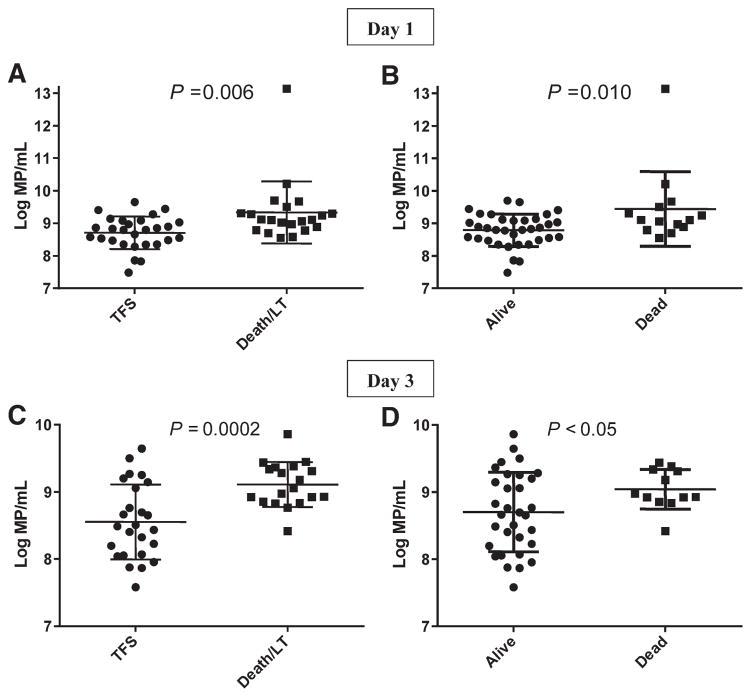
MPs of intermediate size (0.28–0.64 μm) were significantly related to the outcome of ALI/ALF at day 21 (Fig. 4), whereas MPs of smallest (0.15–0.27 μm) and largest (>0.64 μm) size ranges were not (data not shown). MP log10 concentrations of MPs of 0.28–0.64 μm on day 1 were greater in patients who died or were transplanted by day 21 than in transplantfree survivors (9.31 ± 0.94 versus 8.71 ± 0.51/mL; P = 0.006; Fig. 4A). Similarly, MP concentrations in plasma from day 1 were higher in patients who died, compared to those who survived overall (Fig. 4B; P = 0.010). MP concentrations in plasma from day 1 correlated modestly with concentrations in samples from day 3 (r = 0.39; P = 0.012), which were available in 43 patients; 3 patients died between days 1 and 3. MP concentrations in the intermediate size range increased from days 1 to 3 in 20 patients and decreased in 23 patients, but the changes between days 1 and 3 were not significantly related to outcome (data not shown). However, MP concentrations in plasma from day 3 were also higher in those who died or underwent LT by day 21 than in transplant-free survivors (Fig. 4C; P = 0.0002) and in patients who died, compared to those who survived overall (Fig. 4D; P < 0.05). Concentrations of MPs greater than ~log108.5 in day 3 plasma identified all but 1 patient who died or underwent LT (Figs. 4C,D).
Fig. 4.
Relationship of MP concentration (0.28–0.64 μm) to outcome of ALI/ALF. (A) Log10MP/mL on day 1 of admission according to outcome by day 21, TFS, or death/LT (P = 0.006). (B) Log10MP/mL on day 1 according to overall survival by day 21 (P = 0.010). (C) Log10MP/mL on day 3 of admission according to TFS versus death/LT by day 21 (P = 0.0002). (D) Log10MP/mL on day 3 according to overall survival by day 21 (P < 0.05). The range of MP concentration on day 3 was lower than the range on day 1 samples as a result of early mortality of 3 patients with high day 1 MP concentrations. Error bars indicate mean ± SD.
Because certain static patient characteristics were found in univariate analyses to affect outcome and MP concentrations (Tables 1 and 2), we performed stepwise multivariate logistic regression analysis using predictors with P < 0.25 (age, gender, and etiology) to determine whether MP concentrations were independently associated with death/LT. Only MP concentration (0.28–0.35, 0.36–0.64, or 0.28–0.64 μm) was found to be independently associated with outcome in the final multivariate models across the three size ranges (Table 3). In the first model, each 10-fold increase in the number of MPs of 0.28–0.35 μm size increased the likelihood of death/LT by 4.9-fold (P = 0.042), whereas APAP etiology decreased the likelihood of death/LT by approximately 75% (P = 0.038). In the second model, each 10-fold increase in MP of 0.36–0.64 μm size increased the likelihood of death/LT by 11-fold (P = 0.003), whereas APAP was not an independent predictor of outcome. In the third model, each 10-fold increase in MP of 0.28–0.64 μm size increased the likelihood of death/LT by 6.8-fold (P = 0.027), whereas APAP etiology was also not an independent predictor of outcome.
Table 3.
Step-Wise Multivariate Logistical Regression Analyses of Death/LT Using Demographic and Clinical Variables and Microparticle Number According to Size
| Model | Variables | P | OR | 95% CI |
|---|---|---|---|---|
| 1 | Etiology APAP | 0.038 | 0.253 | 0.069–0.928 |
| MP 0.28–0.35 μm day 1 | 0.042 | 4.932 | 1.060–22.943 | |
| 2 | Etiology APAP | 0.078 | 0.272 | 0.064–1.159 |
| MP 0.36–0.64 μm day 1 | 0.003 | 11.093 | 2.270–54.223 | |
| 3 | Etiology APAP | 0.056 | 0.280 | 0.070–1.031 |
| MP 0.28–0.64 μm day 1 | 0.027 | 6.776 | 1.245–36.889 |
Step-wise logistic regression was performed on variables with a P = 0.25 entry criterion in univariate analysis and P = 0.05 criterion to remain in the model. Odds ratios for continuous variables are per unit change.
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
Phenotyping of MPs
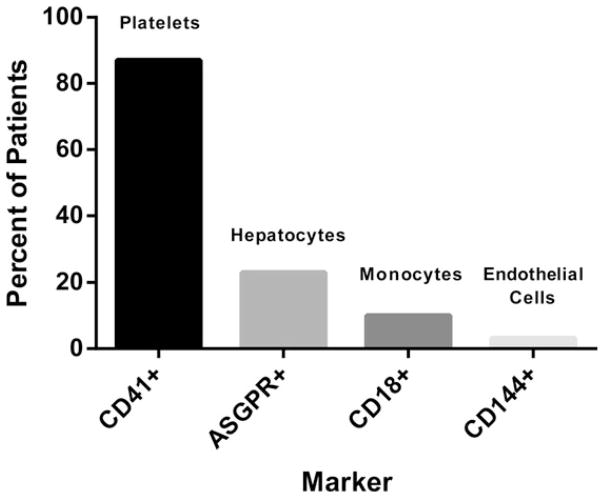
Using Abs against specific cell membrane markers, we performed flow cytometry on PPP from a subset of 31 patients with ALI/ALF. Markers were chosen according to sites of injury in ALF and known sources of MPs in circulation in patients with prominent SIRS (platelets, hepatocytes, monocytes, and ECs). CD41, a marker of platelet membranes, was detected in PPP from 27 of 31 (87%) patients (Fig. 5). Asialoglycoprotein receptor (ASGPR), a specific marker of hepatocyte plasma membranes, was present in the MP fraction of 7 (23%) patients. In contrast, CD18+ MPs derived from monocytes and CD144+ MPs derived from ECs were detected in a small minority of plasma samples (3 and 1 patients, respectively). Although there were no significant associations between phenotypes and severity of ALI/ALF, the numbers of patients in these subgroups was too small to analyze. Thus, flow cytometry determined that platelets are the predominant source of circulating MPs in patients with ALI/ALF.
Fig. 5.
Prevalence of MP phenotypes in plasma of patients with ALI/ALF by flow cytometry.
Discussion
The data presented suggest that plasma MP concentrations of a specific size range are associated with the systemic complications and adverse outcome of patients with ALI/ALF, and that MPs thereby represent an important link between systemic inflammation and activation of hemostasis in this syndrome. Specifically, higher concentrations of MPs (0.28–0.64 μm) were observed in patients with the SIRS, high-grade HE, and in those who developed renal failure and/or minor bleeding complications, and correlated with laboratory predictors of poor outcome (phosphate, bicarbonate, and creatinine). Furthermore, plasma MP concentrations were significantly higher in patients who died or underwent LT than in spontaneous survivors and higher in patients who died, compared to those who survived; multivariate logistic regression analysis identified MPs in the 0.28–0.64-μm range as independently associated with death/LT, particularly in the 0.36–0.64-μm range. Although MPs of multiple phenotypes were present, the majority of patients had detectable MPs of platelet origin, and MP-TF assays demonstrated that circulating MPs in these patients were markedly procoagulant.
In most previous publications, the detection and characterization of MPs has been impaired by limitations in technology that relied on flow cytometry.23 Specifically, flow cytometry cannot reliably size and enumerate MPs <0.5 μm, an important point of emphasis considering our finding that >99% of circulating MPs in patients with ALF were <0.5 μm. ISADE, a novel light-scattering technology, determines particle size directly from the intensity of light scattered at a defined angle, assessing single particles one at a time, and resolving MPs accurately to a size of 0.15 μm. The current work demonstrates the power of this technology over standard flow cytometry because it allowed the accurate enumeration of MPs in the 0.28–0.64-μm range, where the most important differences were observed in our study population. A recent investigation of hemostasis in 20 patients with ALF found a 4-fold increase in TF-independent procoagulant activity in the MP fraction of PPP, compared to healthy controls,9 supporting our findings using ISADE and flow cytometry. However, such functional assays do not provide information about MP size distribution or cell of origin.17
The ability of ISADE to enumerate MPs by size may represent a distinct advantage of this technology, because size profoundly affects MP physical properties and functionality and therefore likely determines specificity. For example, MPs of specific size differ in surface area and angles of curvature, which, in turn, influences the surface chemistry and stability of the MP. Smaller MPs carry smaller numbers of epitopes and are more adherent to cell surfaces because the entropy term for the interaction is smaller. They also display greater distortion of epitopes bound to their surface because of their greater angle of curvature. In contrast, larger MPs require higher amounts of energy to stabilize interaction between a target cell and the MP. Particle size also affects its distribution within the microcirculation. Therefore, the findings in the present work that MPs of 0.28–0.64 μm correlate with many aspects of ALF syndrome, and that the 0.36–0.64-μm size range correlates particularly strongly, may be highly relevant.
Increasing experimental evidence suggests that MPs are effectors of inflammation and coregulators of hemostasis and/or thrombosis in acute and chronic diseases.27–30 In patients with sepsis, MPs play an important role as messengers from inflammatory cells to ECs, myocardial cells, and smooth muscle cells, leading to microcirculatory thrombosis, peripheral tissue ischemia, and circulatory collapse.21 These features of septic shock also characterize patients with ALF with MOSF.2 Platelet MPs, in particular, are candidate effectors of sepsis and ALF syndromes, because patients with both conditions may develop microvascular thrombosis leading to peripheral tissue hypoxia. 31,32 The data reported herein support a pathogenic role for MPs in the 0.28–0.64-μm size range in mediating ALF syndrome. The direct correlation between MP number and factor VIII levels also suggests that MPs may play a role in vascular endothelial cell activation/injury of ALF, the severity of which directly correlates with mortality. 10,33 Whether MPs serve as mediators of the systemic complications of ALF or are simply biomarkers of inflammation cannot be determined conclusively from our data; however, it appears likely that they represent both the cause and the effect of systemic inflammation.
Recent studies have also incriminated MPs in the pathogenesis of chronic liver diseases (CLDs).30 Patients with cirrhosis have increased circulating MPs derived from leukocytes, ECs, and hepatocytes, compared to healthy controls, and concentrations of MPs increase with increasing severity of cirrhosis.20 MPs isolated from PPP of subjects with cirrhosis were shown in vitro and in experimental animals to impair vasoconstrictor response and may thereby cause the vasoplegia of end-stage liver disease. Similarly, T-lymphocyte-derived CD4+ and CD8+ MP numbers were higher in patients with nonalcoholic fatty liver disease and chronic hepatitis C than healthy controls and correlated with disease activity.34,35 In contrast to the present work, the number of CD41+ (platelet-derived) MPs in these populations with CLD were not significantly higher than healthy controls nor were they proportional to the severity of disease. However, both of these studies were performed using flow cytometry and may have thereby missed a possible effect of platelet-derived MPs, most of which (as shown herein) are below the limit of detection by flow cytometry. These studies and the present work suggest that increased production of platelet MPs may be restricted to acute conditions characterized by a prominent SIRS.
In addition to systemic effects of MPs implied by the association of MP concentrations and systemic complications of ALF, procoagulant MPs may also serve to exacerbate the primary liver injury. In a mouse model of APAP hepatotoxicity, activation of coagulation within the necrotic liver increases the primary APAP-induced injury and is greatly ameliorated by heparin administration.7 Furthermore, the prothrombotic effect of APAP is also greatly ameliorated in mice expressing low levels of TF, providing indirect evidence that liver-derived TF may mediate the activation of coagulation.7 Other experimental models also support a role for secondary activation of coagulation within the acutely injured liver in the pathogenesis of liver failure.36,37 Because thrombin generation requires exposure of anionic phospholipids on cellular and/or MP surfaces, intrahepatic MPs would be reasonable candidate platforms on which coagulation occurs.
MP-TF assays have also shown that the population of circulating MPs is highly procoagulant in a TF-dependent manner. Although this study did not determine the cellular origin of the TF-expressing MPs, the measured levels are among the highest we have ever observed in a variety of prothrombotic conditions, including cancer,38 sickle cell disease,39 and human immunodeficiency virus (Baker and Key, unpublished data). Furthermore, these levels exceed, by 3- to 4-fold, the transient peak level of monocyte-derived MPTF activity in plasma that we have measured in healthy volunteers receiving endotoxin.40 Although these intriguing observations might be explained by the release of TF from the necrotic liver into the circulation, proof of this hypothesis awaits confirmation.
There are important limitations to the current study. First, we recognize that the use of flow cytometry to phenotype MPs could not determine the cellular origin of most of the MPs in the 0.28–0.64-μm size range because of the above-noted poor sensitivity of this technology to detect MPs <0.5 μm. Unfortunately, the current state of technology for phenotyping MPs is limited to flow cytometry, which indicated that platelets are the major species of larger MPs in the circulation. We assume that the smaller MPs of 0.28–0.50 μm are part of a size continuum, but proof requires the development of new methods. Second, the manner in which blood was drawn for PPP could not be standardized, because the study population represented a wide range of acuity of illness. Therefore, less acutely ill patients were more likely to have had blood sampled from a butterfly catheter during a brief use of a venous tourniquet, and those more acutely ill were more likely to have had blood samples from indwelling central venous or radial artery catheters without the use of a tourniquet. We speculate that MP number would be increased by the former mode of blood collection. However, MP number was higher in the latter population, which would argue that the manner of collection did not bias our results.
In conclusion, the data presented suggest that MPs of 0.28–0.64 μm are independent predictors of systemic complications and poor outcome in patients with ALI/ALF and support a pathogenic role of MPs in ALF syndrome, rather than simply representing markers of disease acuity. The marked elevation of MP-TF activity provides an additional mechanism by which patients with ALI/ALF maintain normal or hypercoagulable global hemostasis and rarely experience significant bleeding complications.
Acknowledgments
This work was partially supported by UL1TR00058 from the National Center for Advancing Translational Sciences.
This work was an ancillary study of the Acute Liver Failure Study Group (NIH NIDDK U01 DK58369, William M. Lee, M.D., Principal Investigator).
Abbreviations
- Ab
antibody
- ALF
acute liver failure
- ALFSG
the Ancillary Studies Committee of the Acute Liver Failure Study Group
- ALI
acute liver injury
- ALT
alanine aminotransferase
- APAP
acetaminophen (paracetamol)
- aPTT
activated partial thromboplastin time
- ASGPR
asialoglycoprotein receptor
- BSA
bovine serum albumin
- CLD
chronic liver disease
- DIC
disseminated intravascular coagulation
- ECs
endothelial cells
- FX
factor X
- FXa
FX assay
- HE
hepatic encephalopathy
- INR
international normalized ratio of prothrombin time
- ISADE
Invitrox Sizing, Antigen Detection and Enumeration
- LT
liver transplantation
- MOSF
multiorgan system failure
- MPs
microparticles
- MP-TF
microparticle tissue factor assay/activity
- PPP
platelet-poor plasma
- RRT
renal replacement therapy
- SD
standard deviation
- SEM
scanning electron microscopy
- SIRS
systemic inflammatory response syndrome
- TEG
thromboelastogram/thromboelastography
- TF
tissue factor
- TFS
transplant-free survival
- VCU
Virginia Commonwealth University
Footnotes
Potential conflict of interest: D.A.G. acknowledges his role as Chief Medical Officer of Invitrox, Inc. The focus of Invitrox is to develop new technology to detect and phenotype microparticles and to explore potential clinical applications of this technology.
References
- 1.Bernal W, Auzinger G, Sizer E, Wendon J. Intensive care management of acute liver failure. Semin Liver Dis. 2008;28:188–200. doi: 10.1055/s-2008-1073118. [DOI] [PubMed] [Google Scholar]
- 2.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 4.Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Munoz SJ, Stravitz RT, Gabriel DA. Coagulopathy of acute liver failure. Clin Liver Dis. 2009;13:95–107. doi: 10.1016/j.cld.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129–136. doi: 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, et al. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46:1177–1186. doi: 10.1002/hep.21779. [DOI] [PubMed] [Google Scholar]
- 8.Lisman T, Bakhtiari K, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10:1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal B, Wright G, Gatt A, Riddell A, Vemela V, Mallett S, et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol. 2012;57:780–786. doi: 10.1016/j.jhep.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Pereira LM, Langley PG, Hayllar KM, Tredger JM, Williams R. Coagulation factor V and VIII/V ratio as predictors of outcome in paracetamol induced fulminant hepatic failure: relation to other prognostic indicators. Gut. 1992;33:98–102. doi: 10.1136/gut.33.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiodt FV, Balko J, Schilsky M, Harrison ME, Thornton A, Lee WM. Thrombopoietin in acute liver failure. Hepatology. 2003;37:558–561. doi: 10.1053/jhep.2003.50113. [DOI] [PubMed] [Google Scholar]
- 12.Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115:1363–1370. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 13.Olmez I, Zafar M, Shahid M, Amarillo S, Mansfield R. Analysis of significant decrease in platelet count and thrombocytopenia, graded according to NCI-CTC, as prognostic risk markers for mortality and morbidity. J Pediatr Hematol Oncol. 2011;33:585–588. doi: 10.1097/MPH.0b013e318234622f. [DOI] [PubMed] [Google Scholar]
- 14.Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993;104:1243–1247. doi: 10.1378/chest.104.4.1243. [DOI] [PubMed] [Google Scholar]
- 15.Moreau D, Timsit JF, Vesin A, Garrouste-Org, de Lassence A, Zahar JR, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 16.Pereira SP, Langley PG, Williams R. The management of abnormalities of hemostasis in acute liver failure. Semin Liver Dis. 1996;16:403–414. doi: 10.1055/s-2007-1007253. [DOI] [PubMed] [Google Scholar]
- 17.Owens AP, III, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Key NS. Analysis of tissue factor positive microparticles. Thromb Res. 2010;125(Suppl 1):S42–S45. doi: 10.1016/j.thromres.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 20.Rautou PE, Bresson J, Sainte-Marie Y, Vion AC, Paradis V, Renard JM, et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology. 2012;143:166–176. doi: 10.1053/j.gastro.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: circulating microparticles—a new player in sepsis? Crit Care. 2010;14:236. doi: 10.1186/cc9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer DJ, Canabal JM, Arasi LC. Application of intensive care medicine principles in the management of the acute liver failure patient. Liver Transpl. 2008;14(Suppl 2):S85–S89. doi: 10.1002/lt.21649. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel DA, Giordano K. Microparticle sizing and counting using light scattering methods. Semin Thromb Hemost. 2010;36:824–832. doi: 10.1055/s-0030-1267036. [DOI] [PubMed] [Google Scholar]
- 24.American College of Chest Physicians/Society of Critical Care Medicine. Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 25.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 27.Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010;36:907–916. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- 28.Chironi GN, Boulanger CM, Simon A, Gnat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 29.Chironi GN, Simon A, Boulanger CM, Gnat-George F, Hugel B, Megnien JL, et al. Circulating microparticles may influence early carotid artery remodeling. J Hypertens. 2010;28:789–796. doi: 10.1097/HJH.0b013e328335d0a8. [DOI] [PubMed] [Google Scholar]
- 30.Kornek M, Schuppan D. Microparticles: modulators and biomarkers of liver disease. J Hepatol. 2012;57:1144–1146. doi: 10.1016/j.jhep.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 32.Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology. 1996;23:1067–1072. doi: 10.1053/jhep.1996.v23.pm0008621135. [DOI] [PubMed] [Google Scholar]
- 33.Williams AM, Langley PG, Osei-Hwediah J, Wendon JA, Hughes RD. Hyaluronic acid and endothelial damage due to paracetamol-induced hepatotoxicity. Liver Int. 2003;23:110–115. doi: 10.1034/j.1600-0676.2003.00808.x. [DOI] [PubMed] [Google Scholar]
- 34.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirata K, Ogata I, Ohta Y, Fujiwara K. Hepatic sinusoidal cell destruction in the development of intravascular coagulation in acute liver failure of rats. J Pathol. 1989;158:157–165. doi: 10.1002/path.1711580211. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, et al. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103–1108. doi: 10.1136/gut.29.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, et al. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 40.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]