Abstract
Purpose
Diindolylmethane (DIM), a bioactive compound found in cruciferous vegetables, has proposed breast cancer chemoprevention activity. There is limited evidence of clinically relevant activity or long-term safety. A randomized, double-blind, placebo-controlled trial was conducted to determine the activity and safety of combined use of DIM (BR-DIM) with tamoxifen.
Methods
Women prescribed tamoxifen (n=130) were assigned to receive BioResponse-DIM® (BR-DIM), providing 150 mgs DIM twice daily, or placebo, for 12 months. The primary study endpoint was change in urinary 2/16 -hydroxyestrone (2/16 -OHE1) ratio. Change in 4-hydroxyestrone (4-OHE1), serum estrogens and sex hormone-binding globulin (SHBG), breast density, and tamoxifen metabolites also were assessed.
Results
Ninety-eight women (51 placebo, 47 DIM) completed intervention; compliance with treatment was >91%. DIM increased the 2/16 -OHE1 ratio (+3.2[0.8, 8.4]) compared to placebo (−0.7 [−1.7, 0.8], p <0.001). Serum SHBG also increased with DIM as compared to placebo (+ 25±22 and +1.1 ±19 nmol/L, respectively). No change in breast density measured by mammography or by MRI was observed. Plasma tamoxifen metabolites (endoxifen, 4-OH tamoxifen, and N-desmethyl-tamoxifen) were reduced in women receiving DIM versus placebo (P <0.001). Minimal adverse events were reported and overall did not differ by treatment arm.
Conclusion
In patients taking tamoxifen for breast cancer, daily DIM promoted favorable changes in estrogen metabolism and circulating levels of SHBG. Further research is warranted to determine whether decreases in tamoxifen metabolites, including endoxifen, with DIM would attenuate the clinical benefit of tamoxifen.
Introduction
Tamoxifen is a selective estrogen receptor modulator (SERM) used in the treatment and prevention of estrogen receptor positive (ER+) breast cancers. The primary action of tamoxifen is competition with estradiol for binding ER in breast tissue. The efficacy of tamoxifen is well established. A notable subgroup of patients exhibits poor response to therapy and combining tamoxifen with other compounds that complement anti-tumor activity [1,2] or reduce side-effects could increase tamoxifen'schemopreventive benefit [3,4].
Breast cancer survivors frequently self-prescribe bioactive dietary supplements with the intention to obtain survival benefit [5–8]. Of these, 3,3′-diindolylmethane (DIM), a stable in vivo metabolite of indole-3-carbinol (I3C) found in cruciferous vegetables [9], is among the most well-studied [10]. We previously reported, from a secondary analysis, a 52% reduction in breast cancer recurrence among women consuming more cruciferous vegetables [11], hypothesized to be attributed to higher exposure to indolyl-3-carbinol and the related dimer, DIM [12,13].
Accumulating evidence favors several anti-tumor actions of DIM, including favorable changes in estrogen metabolism toward 2-hydroxylation of estrogen metabolism [14]; increasing the ratio of 2-hydroxyestrone (2-OHE1) (anti-tumorigenic) to 16α-hydroxyestrone(16α-OHE1) (pro-tumorigenic) estrogens [15,16], a shift associated with lower breast cancer risk [17]. Support of anti-estrogenic effect of DIM are evident in thyroid disease [18], cell culture studies demonstrating diminished ERα levels [19] and activation of ERβ target genes [20].
Despite interest in the anti-cancer activity of DIM and availability for dietary supplementation, limited data support benefits or risks of DIM in patients receiving tamoxifen. Here we report on findings from a double-blind, placebo-controlled, randomized trial of absorption-enhanced DIM in patients on tamoxifen. We hypothesized that DIM supplementation would be associated with (1) an increase in urinary 2/16α-OHE1 ratio, (2) a reduction in circulating estrogens and an increase in sex hormone-binding globulin (SHBG), and (3) a reduction in breast density. We also described the effects of regular DIM use on tamoxifen metabolites in order to understand any possible interactions between the two agents.
Methods
Trial Design and Eligibility
A randomized, double-blind, placebo-controlled trial was conducted in women prescribed tamoxifen. Study staff enrolled cancer-free patients residing in south and central Arizona who had mammograms within 6 months and BIRADs score ≥ 2 who were taking tamoxifen for ≥ 3 months for primary prevention or adjuvant therapy for early stage breast cancer. Women >18 y not expected to remain on tamoxifen for >2 y, those with hyponatremia or abnormal liver or renal function, pregnant or lactating women, and those not completing the study run-in activities were ineligible. Written, informed consent was obtained from all participants; the study was approved by The University of Arizona Institutional Review Board in accord with an assurance filed with and approved by the DHHS. All data are anonymized to protect the identities of the research subjects. The study underwent regular safety and data quality review at The University of Arizona Cancer Center Data Safety and Monitoring Board.
Intervention and Dose Escalation
Women were randomized, using computer-assisted software by Biometry Shared Resource, 1:1 to receive microencapsulated DIM (BioResponse-DIM®, a patented, absorption-enhancing formulation of diindolylmethane, Indolplex® or BR-DIM®) or placebo twice daily for up to 18 months. Participants, study coordinator and study faculty were blinded to treatment allocation. At study initiation, data available for dose selection for the stated endpoints were limited. To establish a dose with minimum side-effects, a nested, dose-escalation sub-study was conducted over a 12-week period in the first 10 randomized participants (5 per arm) to evaluate safety. Dosing was initiated at 75 mg DIM from BR-DIM twice daily. Review by the Data Safety & Monitoring Committee resulted in permission to increase to 150 mg twice daily for initial recruits as well as all subsequent enrollees. Active and placebo capsules were supplied by BioResponse, LLC, Boulder, CO.
Study Endpoints
The study was statistically powered for primary and secondary outcomes. The primary outcome was originally breast density; however, with DSMB advisement, high rates of bilateral mastectomy which challenged recruitment resulted in a change in primary endpoint to urinary 2/16αOHE ratio. Other urinary estrogen metabolites and serum estradiol (E2), estrone (E1), and SHBG also were assessed. To test the effect of DIM on tissue, breast density was assessed from mammogram and separately from fat-water ratio magnetic resonance imaging (FWR-MRI). Adverse event reporting was completed using NIH Common Terminology Criteria for Adverse Events (CTCAE) v3.
Biomarker Measures
Urinary estrogens
Urinary estrogen metabolites were assessed using modified high performance liquid cromograpthy methods at baseline, 6 months, and 12 months [21]. Briefly, 0.5 mL urine was incubated with β-glucuronidate/sulfatase at 37 C for 4 hours. Samples were extracted bymethylene chloride, followed by dansyl chloride derivatization and extraction with hexane, evaporated to dryness. Dry residues were reconstituted with 50% methanol and injected on the HPLC-MS. Chromatographic separation was achieved on a PhenomenexSynergi Hydro-RP column with a gradient of methanol and 0.1% formic acid in water. Mass spectrometric analysis was performed using electrospray ionization, operated in positive ion mode. The analytes were detected by multiple reaction monitoring. The detection limit was 1 pg/mL; coefficients of variations (CV) for blinded samples were,<10%.
Serum estrogens
Serum hormones (E1 and E2) were measured by HPLC-MS at baseline, 6 and 12 months [22] with minor modifications. Briefly, 0.5 mL serum was extracted with methylene chloride followed by dansyl chloride derivatization. Samples were further extracted with hexane, evaporated to dryness. Dry residues were reconstituted with 50% acetonitrile and injected on the HPLC-MS. Chromatographic separation was achieved on a Phenomenex Synergi Max-RP column with a gradient of acetonitrile and 0.1% formic acid in water. Mass spectrometric analysis was performed using atmospheric pressure chemical ionization, operated in the positive ion mode. Analytes were detected by multiple reaction monitoring. The detection limit was 0.25 pg/mL; CVs were <12%. Serum SHBG was measured using an ELISA based immunoassay (GenWay Biotech, Inc).
Mammographic density assessment
Craniocaudal views of de-identified mammograms performed as part of routine care were obtained for density analysis using published methodology [23,24]. Computer-assisted density assessment was performed using the Cumulus package; all images for one participant were assessed during the same session with the reader blinded to treatment status and time sequence. Reader-selected threshold values that best distinguished the breast and the mammographically dense areas for percent density (PD) calculation as the ratio of the dense to the total breast area in pixels. As quality control, 27 images were assessed in duplicate; correlations were 0.99 for total breast area, 0.96 for dense breast area, and 0.92 for PD.
Fat/water MRI
MRI scans were performed in <5 minutes on a 1.5T GE Signa NV-CV/i scanner on the axial plane using radial gradient-and spin-echo (GRASE) pulse sequence [25] with 8 performed on a 3T Siemens Skyra using a Cartesian echo method [26], automated breast segmentation was applied [27,28]. For patients with breast implants, implanted breasts were subtracted prior to segmentation. For participants with prior breast cancer, only the contralateral, unaffected breast was analyzed.
Fra80 represents the ratio of breast voxels with < 80% apparent fat fraction. Fra80 is correlated with mammograhic breast density (Spearman ρ=0.86, p<0.001) [29] and has excellent reliability (r2=0.985) [30]. Here, breast Fra80 was calculated for all GE scanner data; for the Siemens scanner, a threshold was matched to the GE Fra80 using data from the same patient acquired on both scanners on the same day.
Tamoxifen metabolites
An aliquot of human plasma was added into four-volume of 0.5 mM ammonium formate buffer, pH 3.0 spiked with internal standard. The sample mixture was then extracted by solid-phase extraction using MCX cartridge from Waters. Tamoxifen and its metabolites were separated by high-performance liquid chromatography and mass spectrometry using mobile phase A: 5 mM ammonium formate buffer, pH4.5 + 2% methanol and mobile phase B: 70:20:10acetonitrile:methanol:50mM ammonium formate buffer, pH 4.5 and eluted by 5% NH 4 OH in methanol [31].
Statistical Analysis
Target accrual for 95% statistical power was 77 per group for urinary estrogen metabolites, based on a pilot clinical trial [14] using 2-sided statistical significance; with 50 women per group the power was 88%. Baseline participant characteristics were calculated by treatment arm. DIM adherence was investigated as presence versus absence of urinary DIM at each time point in a 50% random sub-sample of women. Intent-to-treat analysis was used for percent mammographic density and FWR-MRI based density. Assays for urinary estrogen metabolites, serum hormones, and tamoxifen metabolites were performed for women who completed baseline, 6 month and 12 month measures. Skewed urinary estrogen metabolite levels over time were compared between arms using Wilcoxon rank-sum tests. E2 analyses were limited to premenopausal women, since 66% of postmenopausal women had values below detection limits. For levels below detection (< 1% and 23%, E1 and E2, respectively in premenopausal women), half the lower limit was used. Changes in serum hormone levels, percent mammographic density, FWR-MRI based density, and tamoxifen metabolites from baseline to 12 months were evaluated using paired t-tests. The incidence of adverse events was compared between arms using Fisher's exact tests. The sample was restricted to women with > 80% adherence (based on pill-counts) and > 11 months on study in a series of sensitivity analyses (74% of total). Findings were confirmed using all available data in linear mixed effects models, testing interactions between time and arm (data not shown). All analyses were conducted using Stata 14.1 (StataCorp, College Station, TX), and all tests were two-sided with alpha set to 0.05.
Results
Study Population and Adherence
Between March 2011 and October 2015, 156 women consented, 65 to each arm (Figure 1). A total of 98 women (51 placebo, 47 DIM) completed at least 12 months of study-assigned agent and a final study visit, of which 86 (41 placebo, 45 DIM) provided a final mammogram to confirm cancer-free status and breast density. There were no differences in characteristics by study arm (Table 1). The majority were educated, non-Hispanic white women, mean age of 53.0 y and BMI of 26.5 kg/m2. Mean time on tamoxifen was 1.7 y. Adherence to intervention was by pill count data (91.9%) and urinary DIM measures (97%).
Figure 1.
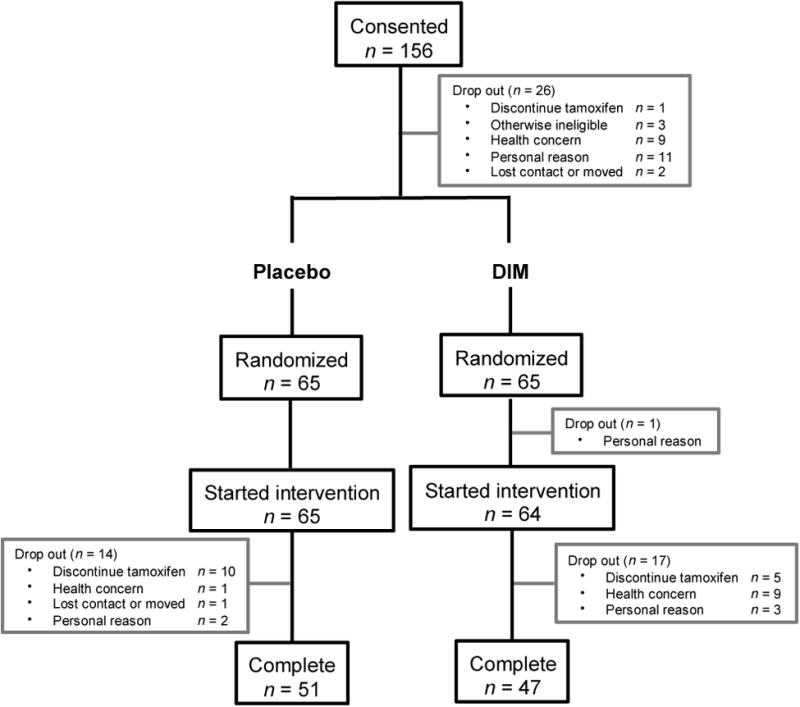
CONSORT diagram for the Di-indolylmethane versus placebo randomized, placebo-controlled trial in women taking tamoxifen.
Table 1. Baseline characteristics of randomized participants (n = 130).
| Characteristic | mean± SDor n (%) | |
|---|---|---|
|
| ||
| Placebo n = 65 |
DIM n = 65 |
|
| Age at baseline (y) | 52.7 ± 9.0 | 53.3 ± 9.7 |
| Time since diagnosis (y) | 2.2 ± 1.6 | 3.4 ± 5.1 |
| Time on tamoxifen (y) | 1.5 ± 1.6 | 1.9 ± 3.5 |
| BMI (kg/m2) | 26.4 ± 5.5 | 26.5 ± 5.4 |
| Ethnicity | ||
| Hispanic | 7 (10.8) | 10 (15.4) |
| Non-Hispanic | 58 (89.2) | 55 (84.6) |
| Race | ||
| White | 61 (93.9) | 61 (93.9) |
| Asian | 2 (3.08) | 1 (1.54) |
| Black or African American | 1 (1.54) | 2 (3.08) |
| Native Hawaiian or other Pacific Islander | 1 (1.54) | 0 (0.00) |
| American Indian or Alaska Native | 0 (0.00) | 1 (1.59) |
| Education | ||
| ≤ High school | 6 (9.23) | 7 (10.8) |
| Some college / college graduate | 35 (53.9) | 33 (50.8) |
| Some post-graduate / post-graduate degree | 24 (36.9) | 25 (38.5) |
| Smoking status | ||
| Never smoker | 44 (67.7) | 44 (67.7) |
| Former smoker | 18 (27.7) | 19 (29.2) |
| Current smoker | 3 (4.62) | 2 (3.08) |
| Stage at diagnosis | ||
| No diagnosis (primary prevention) | 3 (4.62) | 2 (3.08) |
| 0 (DCIS) | 8 (12.3) | 10 (15.4) |
| I | 29 (44.6) | 29 (44.6) |
| II | 18 (27.7) | 20 (30.8) |
| IIIa | 7 (10.8) | 3 (4.62) |
| Don't know | 0 (0.00) | 1 (1.54) |
| Radiation therapy | 42 (64.6) | 48 (73.9) |
| Chemotherapy | 23 (35.4) | 17 (26.2) |
Effect of DIM on Urinary and Serum Hormone Biomarkers
Participants randomized to DIM (Table 2) experienced an increase in 2OHE1 (1.3 pmol/mg Cr) compared to placebo (−0.8pmol/mg Cr). Both arms had lower 16αOHE1 values at the study end with greater reduction in DIM arm (−0.7 vs.−0.2 pmol/mg Cr, p=0.003). The 2OHE1/16α-OHE1 ratio increased with DIM (3.2) and decreased with placebo (−0.7; P <0.001). No differences were observed for serum E1 nor E2 by treatment status (Table 3). For SHBG, a marked increase was shown at 6 months and 12 months with DIM, (25±22 and 1.1±19 nmol/L, DIM and placebo, respectively).
Table 2. Urinary estrogen metabolite levels (pmol/mg Cr) at each time point, by arm.
| Metabolite | Time point | Placebo n = 51 median (IQR) |
DIM n = 47 median (IQR) |
Wilcoxon rank-sum P |
|---|---|---|---|---|
| 2-OHE1 | ||||
| Baseline | 2.9 (1.0–9.1) | 4.2 (1.1–12.5) | ||
| 6 months | 2.0 (0.5–3.8) | 4.4 (1.6–13.1) | ||
| 12 months | 2.4 (0.3–5.6) | 5.9 (1.7–24.8) | ||
| B to 12 moa | −0.8 (−4.8, 2.2) | 1.3 (−0.3, 10.9) | 0.003 | |
| 16-OHE1 | ||||
| Baseline | 2.1 (1.0–7.9) | 2.4 (0.8–7.2) | ||
| 6 months | 1.3 (0.8–3.4) | 0.9 (0.4–3.2) | ||
| 12 months | 1.4 (0.9–3.9) | 1.1 (0.4–3.5) | ||
| B to 12 moa | −0.2 (–1.4, 0.5) | −0.7 (−2.7, −0.1) | 0.049 | |
| 4-OHE1 | ||||
| Baseline | 2.8 (0.4–14.3) | 3.5 (0.9–29.9) | ||
| 6 months | 3.2 (0.1–8.9) | 3.3 (0.2–13.5) | ||
| 12 months | 4.7 (0.6–21.4) | 6.7 (0.6–22.4) | ||
| B to 12 moa | 0.2 (−5.4, 12.6) | 0.0 (−9.5, 6.9) | 0.534 | |
| 2/16 -OHE1 ratio | ||||
| Baseline | 1.4 (0.4–3.6) | 1.6 (0.3–2.5) | ||
| 6 months | 1.0 (0.5–2.3) | 4.2 (0.7–12.9) | ||
| 12 months | 0.9 (0.2–2.4) | 5.0 (2.0–12.7) | ||
| B to 12 moa | −0.7 (−1.7, 0.8) | 3.2 (0.8, 8.4) | < 0.001 | |
Δ B to 12 mo= 12 month minus the baseline value
Table 3. Serum hormone levels at each time point, by treatment arm.
| Hormone | Time point | Placebo | DIM | t -test P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | mean ± SD | n | mean ± SD | |||
| SHBG (nmol/L) | ||||||
| Baseline | 51 | 103 ± 37 | 46 | 97 ± 35 | ||
| 6 months | 51 | 104 ± 38 | 47 | 122 ± 39 | ||
| 12 months | 51 | 104 ± 35 | 47 | 121 ± 35 | ||
| B to 12 moa | 51 | 1.1 ± 19 | 46 | 25 ± 22 | < 0.001 | |
| Estradiol (pg/mL) | ||||||
| Baseline | 16 | 139 ± 182 | 17 | 97 ± 125 | ||
| 6 months | 16 | 72 ± 97 | 17 | 90 ± 110 | ||
| 12 months | 16 | 88 ± 130 | 17 | 111 ± 132 | ||
| B to 12 moa | 16 | −51 ± 210 | 17 | 15 ± 139 | 0.297 | |
| Estrone (pg/mL) | ||||||
| Baseline | 51 | 37 ± 70 | 46 | 42 ± 73 | ||
| 6 months | 51 | 31 ± 43 | 47 | 24 ± 30 | ||
| 12 months | 51 | 33 ± 64 | 47 | 25 ± 32 | ||
| B to 12 moa | 51 | −4.8 ± 11 | 46 | −16 ± 10 | 0.312 | |
Δ B to 12 mo= 12 month minus the baseline value
Breast density as assessed by mammography (n = 102 and 86 at baseline and 12 months, respectively) and separately by FWR-MRI (n = 73 and 60 at baseline and 12 months, respectively) did not change by treatment arm (Table 4a, b).
Table 4. Percent breast density (BD) from clinical mammography (4a.) and FWR-MRI-based breast density (range 0-1, higher number indicates higher density) (4b.) baseline and after 12 months on study, by arm.
| 4a. BD by mammography | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time point | Placebo | DIME | ||||
|
|
|
|||||
| n | Mean ± SD | Median | n | Mean ± SD | Median | |
| First mammogram | 49 | 26.3 ± 14.2 | 23.9 | 53 | 23.9 ± 15.5 | 18.8 |
| Follow-up mammogram | 41 | 25.4 ± 13.9 | 24.4 | 45 | 22.4 ± 15.2 | 19.2 |
| Change (P = 0.744) a | 41 | −1.9 ± 7.4 | 45 | −1.3 ± 7.6 | ||
|
| ||||||
| 4b. BD by FWR MRI | ||||||
|
| ||||||
| Time point | Placebo | DIM | ||||
|
|
|
|||||
| n | Mean ± SD | Median | n | Mean ± SD | Median | |
|
| ||||||
| Baseline | 37 | 0.32 ± 0.20 | 0.24 | 34 | 0.31 ± 0.26 | 0.20 |
| 12 months | 33 | 0.31 ± 0.18 | 0.26 | 27 | 0.28 ± 0.24 | 0.20 |
| B to 12 mo (P=0.772)b | 30 | −0.008 ± 0.1 | 24 | −0.001 ± 0.1 | ||
t-test for differences in change between placebo and DIM groups
Δ B to 12 months = 12 month minus the baseline value
Effect of DIM on Tamoxifen Metabolites
DIM treatment was associated with a non-statistically significant reduction in plasma tamoxifen levels (P = 0.06), and with a significant reduction in plasma levels of endoxifen (P =<0.001), 4-OH tamoxifen (P =<0.001), and N-desmethyltamoxifen (P = 0.001) (Figure 2). Effects were evident at 6 weeks, stabilizing over time. A sensitivity analysis restricted to women with high adherence demonstrated similar (Supplemental Table 1). Furthermore, treatment with DIM resulted in a greater number of women who were below the proposed therapeutic threshold of 5.6 ng/mL for endoxifen[32] (Supplemental Figure 1).
Figure 2.
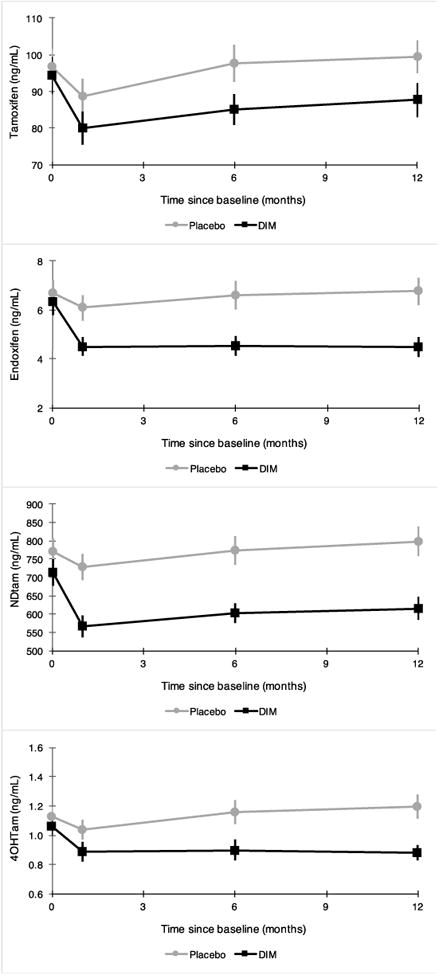
Change in plasma tamoxifen metabolites over time (baseline to 6 weeks, 6 and 12 months) by treatment arm.
Adverse Events
There was no difference in adverse events by treatment arm except discolored urine reported by 40% of DIM participants (P < 0.001; Supplemental Table 2). Report of tamoxifen-associated symptoms did not vary by treatment, including hot flashes. There were 14 reports of vaginal bleeding, 6 in DIM arm and 8 in placebo arm; none resulted in a diagnosis of cancer or hyperplasia; one patient on placebo discontinued tamoxifen and subsequently was removed from the study due to endometrial thickening.
Discussion
This randomized, controlled trial of DIM supplementation is among the first and largest to demonstrate in support of our hypothesis that oral DIM provided as BioResponse-DIM® increases the urinary ratio of 2OHE1/16α-OHE1 along with circulating SHBG in patients taking tamoxifen. No changes in breast density or circulating estrogens were observed. Importantly, this study is the first to demonstrate a decrease in serum levels of the more bioactive forms of tamoxifen, including endoxifen, findings that suggest an effect of DIM on the pharmacokinetics of tamoxifen.
Our finding that DIM modulates estrogen metabolism through increased 2-hydroxylation of estrogen metabolism and, ultimately, increasing the ratio of this “favorable” metabolite to the “punitive” 16α-hydroxylated metabolite confirms results in a pilot clinical trial using one third the DIM dose in breast cancer survivors not on tamoxifen[14]. The present results support prior observations in 3 patients taking tamoxifen where the 2-OHE1/16α-OHE1 ratio increased 126–229% with a daily dose of 100 mg DIM from BR-DIM for 30 days [33]. Mechanistically, 2OHE1 has been shown to bind ERα with high affinity, but lacks the ability to induce transcriptional activity resulting in the reported anti-estrogenic/anti-growth effects [34,35,16]. Higher urinary 2-OHE1/16α-OHE1 ratio has been associated with a lower risk of breast cancer in most, [36,17,37,38], but not all studies [39]. While a higher 2OHE1/16α-OHE1 has been hypothesized as being anti-tumorigenic, direct evidence that manipulating 2OHE1/16α-OHE1 prevents breast cancer or improves outcomes are lacking.
In our study, DIM did not affect serum estrogen levels, but increased SHBG, exposures which have been extensively studied in relation to breast cancer risk. In an analysis of worldwide data, the Endogenous Hormones and Breast Cancer Collaborative Group [40] confirmed that breast cancer risk increased with higher total E2, free E2, non-SHBG-bound E2,E1, E1 sulfate, and testosterone, despite some contrary findings [41]. As in our study, low levels and the high variations in measures may limit interpretation. SHBG has been inversely associated with breast cancer risk [40,42] with recent evidence suggesting a strong inverse association with breast cancer specific mortality [41]. As noted by Duggan et al, SHBG has been shown to induce apoptosis and inhibit growth of breast cancer cells via a receptor-mediated binding [43,44], suggesting that the effects of DIM on SHBG may be beneficial to breast cancer outcomes independent of serum hormone or tamoxifen metabolite levels.
The lack of change in breast density as assessed in mammographic images or by a more quantitative FWR-MRI method is consistent with evidence that breast density may be only weakly associated with urinary estrogens and SHBG [45,46]. Further, our ability to detect effects of DIM on breast density is limited by tamoxifen treatment, as administration is related to a time-dependent reduction in breast density [47]. Breast cancer recurrence and mortality are lower in women who show a ≥10% decrease in breast density in response to tamoxifen[48]. A reduction in breast density, particularly for women with greater breast density, over 12 months was also reported in a Kaiser-based study [49]. Our study participants had been prescribed tamoxifen for an average of 1.7 years, thus, tamoxifen effects on breast density had likely occurred prior to enrollment, limiting our ability to detect changes.
DIM has several mechanisms of bioactivity, beyond estrogen metabolism and modulation of breast density, that were not evaluated in this trial. These mechanisms of action have been described in a recent review [10]. Important areas of bioactivity relevant to breast cancer chemoprevention include antioxidant effects, inhibition on COX-2 and other inflammatory response pathways [50]. Recent evidence of DIM derivatives as chemopreventive agents against triple negative breast cancer hold promise from early demonstration studies of in vitro models[51]. Tumor-specific responses have also been described including aryl hydrocarbon receptor (AhR) agonistic activity [52] and sensitizing activity against gamma radiation [53]. These alternate pathways for chemopreventive activity are under active study and could be further explored using stored biosamples from the current trial.
In this study, DIM lowered plasma levels of all three major Phase I tamoxifen metabolites. We compared the tamoxifen metabolite-to-parent drug ratio and observed a decrease in the endoxifen-to-tamoxifen ratio, but no change in the ratios of the other two metabolites (data not shown). Rodent studies showed that DIM had no effect on the Phase I metabolites of tamoxifen [54] and/or activities of multiple cytochrome (CYP) P450 isozymes[55]. However, clinical studies suggest that DIM modulates human CYPs that mediate 2-hydroxylation and 16-hydroxylation of estrogen metabolism, which could alter in Phase I metabolism of tamoxifen. Additionally, a rodent study showed that intravenous DIM induced Phase II enzymes including UDP glucuronosyltransferases (UGTs) [56]. Induction of UGTs could lead to lower levels of tamoxifen and Phase I metabolites. No human evidence for Phase II enzymes induction by DIM exist.
The clinical significance of lowered levels of tamoxifen metabolites remains unclear. Endoxifen and 4-OHtamoxifen, which exhibit higher activity for ER at the tissue level, have been postulated as the active agents in tamoxifen therapy. While early work in the field failed to demonstrate a direct relationship between tamoxifen dose and tissue Ki67 levels as a biomarker of effects on proliferation, subsequent work suggested that low circulating endoxifen levels may limit the efficacy of tamoxifen. For example, in breast cancer survivors on tamoxifen circulating endoxifen levels > 5.6 ng/ml (upper four quintiles) were associated with a marginally significant lower risk of recurrence (26%) [32]. Despite study limitations, this evidence has promoted inclusion of the 5.6 ng/ml endoxifen cut-point as a putative therapeutic threshold of tamoxifen[32]. Lower doses of tamoxifen, including 1 and 5 mg daily, have demonstrated similar antitumor effects to therapeutic dosing and challenge the validity of the endoxifen cut-point for determining efficacy [57,58]. Further, DIM did not result in unfavorable change in breast density or SHBG, exposures which have been associated with tamoxifen efficacy [59,60].
Strengths of this study are the randomized, placebo-controlled design, biologically assessed and high adherence to DIM, comprehensive assessment of breast density and inclusion of several biologically relevant biomarkers. A limitation of this study is the inability to assess the DIM effect on estrogen receptor target genes directly in breast tissue. The trial is limited in generalizability to women taking tamoxifen and not directly applicable for those prescribed aromatase inhibitors.
Conclusion
In this first randomized, placebo-controlled study of DIM effects on biomarkers associated with breast cancer risk in women receiving tamoxifen, we found evidence of a favorable effect on estrogen metabolism, with an increase in urinary 2/16α-OHE1 ratio with DIM supplementation. Our observation of increased SHBG with DIM suggests that DIM may act independently on SHBG or by interaction with tamoxifen to promote a favorable hormone environment for cancer prevention. DIM had no influence on breast density beyond the effects of tamoxifen therapy alone. Finally, the reduction in endoxifen and other metabolites of tamoxifen are concerning given the presumptive relationship between these metabolites and tamoxifen efficacy. Additional studies are needed prior to recommending DIM supplementation for breast cancer patients receiving tamoxifen.
Supplementary Material
Supplemental Figure 1. Changes in endoxifen applying a proposed clinical threshold of <5.6 ng/mL by treatment arm, over time
Supplemental Table 1. Change in tamoxifen metabolite levels (ng/mL) from baseline to 12 months, by treatment arm restricted to women with >80% adherence to tamoxifen therapy (n=92)
Supplemental Table 2. Most frequent reported adverse events (number of participants reporting each event) throughout the 12-month study period, by treatment arm
Acknowledgments
The investigators wish to acknowledge the contributions of Julie West, study coordinator, Amelia Lobos, study agent manager, Catherine Cordova for performing the estrogen assays and Jean-Phillippe Galons and Jie Ding for assisting with FWR-MRI image processing. The investigators also would like to thank Drs. Steven Stratton, Robert Livingston, and Chiu-Hsieh (Paul) Hsu for serving as members of the ad hoc Data and Safety Monitoring Committee.
Acknowledgements of research support: This research supported by National Institutes of Health, National Cancer Institute grant numbers CAT R01 CA149417 and CCSG-P30CA023074 as well as a research grant from the Academy of Nutrition and Dietetics, Oncology Nutrition Practice Group
Funding: This work was support by The National Cancer Institute (NCI) at the National Institutes of Health (NIH) (CAT R01 CA149417), as well as NCI funding provided to The University of Arizona Comprehensive Cancer Center Support Grant (CCSG-P30CA023074) including support of the Behavioral Measurements and Interventions, Analytical Chemistry, Cancer Imaging, and Biostatistics Shared Resources as well as the Clinical Trials Office and the Data Safety and Monitoring Board.
Footnotes
Portions of this work were presented in preliminary data set of breast density results at the 2014 San Antonio Breast Cancer Symposium
No disclaimers
Trial Registration: ClinicalTrials.gov NCT01391689
Conflicts Of Interest: The authors have no conflicts of interest to disclose
References
- 1.Shin SC, Choi JS, Li X. Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int J Pharm. 2006;313(1-2):144–149. doi: 10.1016/j.ijpharm.2006.01.028. doi:S0378-5173(06)00077-9. [pii]10.1016/j.ijpharm.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Sommer AK, Hermawan A, Mickler FM, Ljepoja B, Knyazev P, Brauchle C, Ullrich A, Wagner E, Roidl A. Salinomycin co-treatment enhances tamoxifen cytotoxicity in luminalA breast tumor cells by facilitating lysosomal degradation of receptor tyrosine kinases. Oncotarget. 2016 doi: 10.18632/oncotarget.10459. 10459 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manni A, El-Bayoumy K, Skibinski CG, Thompson HJ, Santucci-Pereira J, Bidinotto LT, Russo J. Combination of Antiestrogens and Omega-3 Fatty Acids for Breast Cancer Prevention. Biomed Res Int. 2015;2015:638645. doi: 10.1155/2015/638645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason JK, Thompson LU. Flaxseed and its lignan and oil components: can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl Physiol Nutr Metab. 2014;39(6):663–678. doi: 10.1139/apnm-2013-0420. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee H, Kwan ML, Ergas IJ, Strizich G, Roh JM, Wilson AT, Lee M, Sherman KJ, Ambrosone CB, Hershman DL, Neugut AI, Kushi LH. Changes in vitamin and mineralsupplement use after breast cancer diagnosis in the Pathways Study: a prospective cohort study. BMC Cancer. 2014;14:382. doi: 10.1186/1471-2407-14-382. 1471-2407-14-382[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunther S, Patterson RE, Kristal AR, Stratton KL, White E. Demographic and health-related correlates of herbal and specialtysupplement use. J Am Diet Assoc. 2004;104(1):27–34. doi: 10.1016/j.jada.2003.10.009. S0002822303014342 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Miller MF, Bellizzi KM, Sufian M, Ambs AH, Goldstein MS, Ballard-Barbash R. Dietarysupplement use in individuals living with cancer and other chronic conditions: a population-based study. J Am Diet Assoc. 2008;108(3):483–494. doi: 10.1016/j.jada.2007.12.005. S0002-8223(07)02206-7 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Henderson JW, Donatelle RJ. Complementary and alternative medicine use by women after completion of allopathic treatment for breast cancer. Altern Ther Health Med. 2004;10(1):52–57. [PubMed] [Google Scholar]
- 9.Ciska E, Verkerk R, Honke J. Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3′-diindolylmethane in fermented cabbage. J Agric Food Chem. 2009;57(6):2334–2338. doi: 10.1021/jf803477w. [DOI] [PubMed] [Google Scholar]
- 10.Thomson CA, Ho E, Strom MB. Chemopreventive properties of 3,3′-diindolylmethanein breast cancer: evidence from experimental and human studies. Nutr Rev. 2016;74:432–443. doi: 10.1093/nutrit/nuw010. nuw010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, Pierce JP. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women's Healthy Eating and Living Study. Breast Cancer Res Treat. 2011;125(2):519–527. doi: 10.1007/s10549-010-1014-9. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka N, Ainslie-Waldman CE, Upadhyaya P, Carmella SG, Fritz VA, Rohwer C, Fan Y, Rauch D, Le C, Hatsukami DK, Hecht SS. Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev. 2014;23(2):282–287. doi: 10.1158/1055-9965.EPI-13-0645. 1055-9965.EPI-13-0645 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables andhuman cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. doi: S1043-6618(07)00032-1 [pii].10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 15.Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7(2):112–129. [PubMed] [Google Scholar]
- 16.Martucci C, Fishman J. Direction of estradiol metabolism as a control of its hormonal action–uterotrophic activity of estradiol metabolites. Endocrinology. 1977;101(6):1709–1715. doi: 10.1210/endo-101-6-1709. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids. 2015;99(Pt A):67–75. doi: 10.1016/j.steroids.2015.02.015. S0039-128X(15)00071-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajoria S, Suriano R, Parmar PS, Wilson YL, Megwalu U, Moscatello A, Bradlow HL, Sepkovic DW, Geliebter J, Schantz SP, Tiwari RK. 3,3′-diindolylmethane modulatesestrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21(3):299–304. doi: 10.1089/thy.2010.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okino ST, Pookot D, Basak S, Dahiya R. Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Phila) 2009;2(3):251–256. doi: 10.1158/1940-6207.CAPR-08-0146. 1940-6207.CAPR-08-0146 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Vivar OI, Saunier EF, Leitman DC, Firestone GL, Bjeldanes LF. Selective activation of estrogen receptor-beta target genes by 3,3′-diindolylmethane. Endocrinology. 2010;151:1662–1667. doi: 10.1210/en.2009-1028. en.2009-1028 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77(20):6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 22.Nelson RE, Grebe SK, OK DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384. doi: 10.1373/clinchem.2003.025478. clinchem.2003.025478 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Byng JW, Yaffe MJ, Jong RA, Shumak RS, Lockwood GA, Tritchler DL, Boyd NF. Analysis of mammographic density and breast cancer risk from digitized mammograms. Radiographics. 1998;18(6):1587–1598. doi: 10.1148/radiographics.18.6.9821201. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78(9):1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C. AM Multi-Mask Multi-Seed Free Growing Field Map Estimation Algorithm for Iterative Multi-Point Water-Fat Decomposition. ISMRM 17th Annual Scientific Meeting & Exhibition; Honolulu, Hawaii, USA. 2009. [Google Scholar]
- 26.Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. MagnReson Med. 2014;72(5):1353–1365. doi: 10.1002/mrm.25054. [DOI] [PubMed] [Google Scholar]
- 27.Arthur D. Proceedings of the eighteenth annual ACM-SIAM symposium on Discrete algorithms. Society for Industrial and Applied Mathematics; 2007. Vassilvitskii S k-means++: The advantages of careful seeding; pp. 1027–1035. [Google Scholar]
- 28.Rosado-Toro JA, Barr T, Galons JP, Marron MT, Stopeck A, Thomson C, Thompson P, Carroll D, Wolf E, Altbach MI, Rodriguez JJ. Automated breast segmentation of fat and water MR images using dynamic programming. Acad Radio. 2015;22(2):139–148. doi: 10.1016/j.acra.2014.09.015. S1076-6332(14)00379-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson CA, Thompson PA, Wertheim BC, Roe D, Marron MT, Galons JP, Kupinski MA, Altbach MI, Maskarinec G, Stopeck A. Abstract P6-01-18: 2-Hydroxyestrone isassociated with breast density measured by mammography and fat: water ratio magneticresonance imaging in women taking tamoxifen. Cancer Research. 2015;75(9 Supplement) P6-01-18-P06-01-18. [Google Scholar]
- 30.Ding Jie, PA T, Marron Marilyn T, Altbach Maria, Roe Denise, Galons Jean-Philippe, Thomson Cynthia A, Wang Fang, Stopeck Alison, Huang Chuan. The test-retest reliability of fat-water ratio MRI derived breast density measurements and automated breast segmentation. ISMRM 24th Annual Scientific Meeting & Exhibition; Singapore. 2016. [Google Scholar]
- 31.Teunissen SF, Rosing H, Schinkel AH, Schellens JH, Beijnen JH. Bioanalyticalmethods for determination of tamoxifen and its phase I metabolites: a review. Anal Chim Acta. 2010;683(1):21–37. doi: 10.1016/j.aca.2010.10.009. S0003-2670(10)01269-9 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–725. doi: 10.1038/clpt.2011.32. clpt201132 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradlow HL. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo. 2008;22(4):441–445. [PubMed] [Google Scholar]
- 34.Schneider J, Huh MM, Bradlow HL, Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J BiolChem. 1984;259(8):4840–4845. [PubMed] [Google Scholar]
- 35.Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrino. 1989;61(2):239–246. doi: 10.1016/0303-7207(89)90135-4. doi:0303-7207(89)90135-4 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schunemann HJ, Stanulla M, Yang J, Sepkovic DW, Trevisan M, Berrino F. Estrogen metabolism and risk of breastcancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11(6):635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: anested case-control study. Breast Cancer Res. 2013;15(2):R34. doi: 10.1186/bcr3416. bcr3416 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, Buys SS, Isaacs C, Keefer LK, Veenstra TD, Berg CD, Hoover RN, Ziegler RG. Estrogen metabolism and risk ofbreast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104(4):326–339. doi: 10.1093/jnci/djr531. djr531 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, Pike MC. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(12):1067–1072. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 40.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 41.Duggan C, Stanczyk F, Campbell K, Neuhouser ML, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard R, McTiernan A. Associations of sex steroid hormones with mortality in women with breast cancer. Breast Cancer Res Treat. 2016;155:559–567. doi: 10.1007/s10549-016-3704-4. 10.1007/s10549-016-3704-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He XY, Liao YD, Yu S, Zhang Y, Wang R. Sex hormone binding globulin and risk of breast cancer in postmenopausal women: a meta-analysis of prospective studies. HormMetab Res. 2015;47(7):485–490. doi: 10.1055/s-0034-1395606. [DOI] [PubMed] [Google Scholar]
- 43.Catalano MG, Frairia R, Boccuzzi G, Fortunati N. Sex hormone-binding globulin antagonizes the anti-apoptotic effect of estradiol in breast cancer cells. Mol Cell Endocrinol. 2005;230(1-2):31–37. doi: 10.1016/j.mce.2004.11.005. doi: S0303-7207(04)00421-6 [pii] 10.1016/j.mce.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Kahn SM, Li YH, Hryb DJ, Nakhla AM, Romas NA, Cheong J, Rosner W. Sex hormone-binding globulin influences gene expression of LNCaP and MCF-7 cells in response to androgen and estrogen treatment. AdvExp Med Biol. 2008;617:557–564. doi: 10.1007/978-0-387-69080-3_57. [DOI] [PubMed] [Google Scholar]
- 45.Gierach GL, Patel DA, Falk RT, Pfeiffer RM, Geller BM, Vacek PM, Weaver DL, Chicoine RE, Shepherd JA, Mahmoudzadeh AP, Wang J, Fan B, Herschorn SD, Xu X, Veenstra T, Fuhrman B, Sherman ME, Brinton LA. Relationship of serum estrogens and metabolites with area and volume mammographic densities. Horm Cancer. 2015;6(2-3):107–119. doi: 10.1007/s12672-015-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riza E, dos Santos Silva I, De Stavola B, Bradlow HL, Sepkovic DW, Linos D, Linos A. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10(6):627–634. [PubMed] [Google Scholar]
- 47.Chow CK, Venzon D, Jones EC, Premkumar A, O'Shaughnessy J, Zujewski J. Effect of tamoxifen on mammographic density. Cancer Epidemiol Biomarkers Prev. 2000;9(9):917–921. [PubMed] [Google Scholar]
- 48.Mullooly M, Pfeiffer RM, Nyante SJ, Heckman-Stoddard BM, Perloff M, Jatoi I, Brinton LA, Aiello Bowles EJ, Hoover RN, Glass A, Berrington de Gonzalez A, Sherman ME, Gierach GL. Mammographic Density as a Biosensor of Tamoxifen Effectiveness in Adjuvant Endocrine Treatment of Breast Cancer: Opportunities and Implications. J ClinOncol. 2016;34:2093–2097. doi: 10.1200/JCO.2015.64.4492. JCO.2015.64.4492 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Nes JG, Beex LV, Seynaeve C, Putter H, Sramek A, Lardenoije S, Duijm-de Carpentier M, Van Rongen I, Nortier JW, Zonderland HM, van de Velde CJ. Minimal impact ofadjuvantexemestane or tamoxifen treatment on mammographic breast density inpostmenopausal breast cancer patients: a Dutch TEAM trial analysis. Acta Onco. 2015;54(3):349–360. doi: 10.3109/0284186X.2014.964809. [DOI] [PubMed] [Google Scholar]
- 50.Fuentes F, Paredes-Gonzalez X, Kong AT. Dietary GlucosinolatesSulforaphane, PhenethylIsothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr Pharmacol Rep. 2015;1(3):179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godugu C, Doddapaneni R, Safe SH, Singh M. Novel diindolylmethane derivatives based NLC formulations to improve the oral bioavailability and anticancer effects in triple negative breast cancer. Eur J Pharm Biopharm. 2016;108:168–179. doi: 10.1016/j.ejpb.2016.08.006. doi:S0939-6411(16)30433-7. 10.1016/j.ejpb.2016.08.006 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safe S, Cheng Y, Jin UH. The Aryl Hydrocarbon Receptor (AhR) as a Drug Target for Cancer Chemotherapy. Curr Opin Toxicol. 2017;2:24–29. doi: 10.1016/j.cotox.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Lv M, Huangfu C, Wang F, Zhang J. 3,3′-Diindolylmethane: A Promising Sensitizer of gamma-Irradiation. Biomed Res Int. 2015;2015:465105. doi: 10.1155/2015/465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parkin DR, Malejka-Giganti D. Differences in the hepatic P450-dependent metabolism of estrogen and tamoxifen in response to treatment of rats with 3,3′-diindolylmethane and itsparent compound indole-3-carbinol. Cancer Detect Prev. 2004;28(1):72–79. doi: 10.1016/j.cdp.2003.11.006. S0361090X03002058 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Crowell JA, Page JG, Levine BS, Tomlinson MJ, Hebert CD. Indole-3-carbinol, butnot its major digestive product 3,3′-diindolylmethane, induces reversible hepatocyte hypertrophyand cytochromes P450. Toxicol Appl Pharmacol. 2006;211(2):115–123. doi: 10.1016/j.taap.2005.06.011. doi:S0041-008X(05)00376-5. 10.1016/j.taap.2005.06.011 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Wu TY, Huang Y, Zhang C, Su ZY, Boyanapalli S, Khor TO, Wang H, Lin H, Gounder M, Kagan L, Androulakis IP, Kong AN. Pharmacokinetics and pharmacodynamics of 3,3′-diindolylmethane (DIM) in regulating gene expression of phase II drug metabolizing enzymes. J Pharmacokinet Pharmacodyn. 2015;42(4):401–408. doi: 10.1007/s10928-015-9421-5. [DOI] [PubMed] [Google Scholar]
- 57.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, Veronesi P, Torrisi R, Cazzaniga M, Mora S, Sandri MT, Pelosi G, Luini A, Goldhirsch A, Lien EA, Veronesi U. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95(11):779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 58.Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S, Gulisano M, Johansson H, Galimberti V, Cassano E, Moroni SM, Formelli F, Lien EA, Pelosi G, Johnson KA, Bonanni B. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27(23):3749–3756. doi: 10.1200/JCO.2008.19.3797. JCO.2008.19.3797 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–752. doi: 10.1093/jnci/djr079. djr079 [pii] [DOI] [PubMed] [Google Scholar]
- 60.Johansson H, Bonanni B, Gandini S, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, MacisD, Puccio A, Sandri MT, Gulisano M, Formelli F, Decensi A. Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose tamoxifen and fenretinide. Breast Cancer Res Treat. 2013;142(3):569–578. doi: 10.1007/s10549-013-2768-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Changes in endoxifen applying a proposed clinical threshold of <5.6 ng/mL by treatment arm, over time
Supplemental Table 1. Change in tamoxifen metabolite levels (ng/mL) from baseline to 12 months, by treatment arm restricted to women with >80% adherence to tamoxifen therapy (n=92)
Supplemental Table 2. Most frequent reported adverse events (number of participants reporting each event) throughout the 12-month study period, by treatment arm


