Abstract
Background
In Japan, there are limited options for switching opioid analgesics. Hydromorphone is an opioid analgesic that is routinely used instead of morphine for cancer pain; however, it is not yet available in Japan. The aim of this study was to assess the efficacy and safety of hydromorphone (DS-7113b) extended-release tablets in opioid-naïve patients with cancer pain not relieved by non-opioid analgesics.
Subjects and methods
This was a multicenter, randomized, double-blind, parallel-group trial. A double-dummy method was used for blinding. Each randomized subject received either hydromorphone extended-release tablets plus placebo oxycodone hydrochloride extended-release tablets 4 mg/day (n=88) or placebo hydromorphone extended-release tablets plus oxycodone hydrochloride extended-release tablets 10 mg/day (n=93) orally for 7 days (once-daily dosing for hydromorphone and twice-daily dosing for oxycodone). The doses were adjusted as necessary. Efficacy was evaluated by change in visual analog scale (VAS) score from baseline to completion of treatment.
Results
The between-group difference in least squares mean changes in VAS score from baseline to completion or discontinuation of treatment was −0.4 mm (95% CI −5.9 to 5 mm) by analysis of covariance where the baseline VAS score was used as a covariate. The upper limit of the 95% CI was below 10 mm, which was predefined as the noninferiority limit. This verified the noninferiority of hydromorphone tablets relative to oxycodone tablets. The incidence of adverse events was 80.7% (71 of 88) in the hydromorphone group and 83.7% (77 of 93) in the oxycodone group. The most common adverse events were nausea, vomiting, somnolence, diarrhea, and constipation, most of which are commonly observed with opioid analgesics.
Conclusion
The efficacy and safety of hydromorphone extended-release tablets were equivalent to those of the oxycodone extended-release formulation.
Keywords: hydromorphone, oxycodone, cancer pain, palliative medicine, double-blind study
Introduction
Pharmacotherapy for cancer pain is based on the World Health Organization (WHO) guidelines for cancer-pain relief released in 1986.1 The WHO’s three-step ladder for cancer-pain relief recommends the use of potent opioid analgesics for moderate–severe pain; indeed, these agents have been found to be the most effective treatment for cancer pain.2 At present, morphine, oxycodone, and fentanyl are mainly used in Japan as step 3 opioid analgesics.
The selective μ-opioid receptor-agonist analgesic hydromorphone is currently used clinically in 45 countries and regions in the world.3 It is the standard alternative to morphine,4–7 but has not been developed for use in Japan. The metabolites of hydromorphone have been found to be inactive,8 making hydromorphone a potential treatment option for patients with reduced renal function as an alternative to morphine.9,10 Therefore, hydromorphone is expected to expand the treatment options for pain relief.
The efficacy and safety of hydromorphone has been assessed in clinical studies.11 During the development of hydromorphone in Japan, Daiichi Sankyo made a once-daily extended-release formulation.12,13 We conducted a Phase III randomized double-blind study to verify the noninferiority of hydromorphone extended-release tablets (DS-7113b; Daiichi Sankyo, Tokyo, Japan) to oxycodone hydrochloride extended-release tablets (Oxycontin; Shionogi, Osaka, Japan), with the objective of investigating the efficacy of the hydromorphone formulation in Japanese patients.
Subjects and methods
This study was conducted from 2014 to 2015 as a multicenter, active-controlled, randomized, double-blind, parallel-group comparison study, enrolling 184 patients at 49 institutions. The institutes participating in the study are listed in Box S1. The study was approved by the institutional review board of each study site and carried out in compliance with ethical principles based on the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all subjects prior to study participation. The registered clinical trial number: JapicCTI-142666.
Participants
The study participants were cancer patients aged 20 years and older receiving nonopioid analgesics for cancer pain who had not used opioid analgesics within 2 weeks prior to enrollment. At enrollment, visual analog scale (VAS) score (average pain within the last 24 hours) was required to be ≥35 mm (moderate–severe pain that interferes with functioning),14–16 with an Eastern Cooperative Oncology Group performance status ≤3. All patients were judged by the investigator to require treatment with potent opioid analgesics. Patients presenting with symptoms for which oxycodone or morphine are contraindicated or relatively contraindicated, those receiving a monoamine oxidase inhibitor within 14 days prior to enrollment, those participating in another clinical trial within 28 days prior to enrollment, and those with serious hepatic, renal, or respiratory disorder of Common Terminology Criteria for Adverse Events grade 3 were excluded.
Study design
Subjects were randomized at a ratio of 1:1 to either the hydromorphone group or the oxycodone group. A double-dummy method was used for blinding, and each randomized subject received either hydromorphone extended-release tablets plus placebo oxycodone hydrochloride extended-release tablets or placebo hydromorphone extended-release tablets plus oxycodone hydrochloride extended-release tablets orally for 7 days (once-daily dosing for hydromorphone and twice-daily dosing for oxycodone). The initial dose was 4 mg/day of hydromorphone extended-release tablets and 10 mg/day of oxycodone hydrochloride extended-release tablets. Investigators were allowed to increase the doses of the study drugs every 24 hours during the treatment period if necessary due to insufficient analgesic efficacy. Doses could be increased in five stages up to a maximum of 24 mg/day of hydromorphone and 80 mg/day of oxycodone (Table 1). Treatment was switched to appropriate analgesics after completion of study treatment, and subjects were followed up. The initial dose of hydromorphone extended-release tablets was determined as 4 mg/day, which was assumed to be equivalent to 20 mg/day of morphine on the basis of the fivefold-higher efficacy ratio of hydromorphone compared with morphine (Exalgo; Mallinckrodt Brand Pharmaceuticals, Hazelwood, MO, USA). The initial dose of oxycodone hydrochloride extended-release tablets was determined as 10 mg/day, which is specified as the dose for opioid-naïve patients in the Japanese package insert.
Table 1.
Doses of investigational product and rescue medication
| Hydromorphone group | Oxycodone group | Morphine hydrochloride for rescue medication |
|---|---|---|
| 4 mg/day | 10 mg/day | 5 mg |
| 6 mg/day | 20 mg/day | 5 mg |
| 8 mg/day | 30 mg/day | 5 mg |
| 12 mg/day | 40 mg/day | 10 mg |
| 18 mg/day | 60 mg/day | 15 mg |
| 24 mg/day | 80 mg/day | 20 mg |
From the start of study treatment to the completion (or discontinuation) of treatment, each subject evaluated their average pain severity once daily for the last 24 hours using the VAS and recorded the score in the patient diary. Oral morphine hydrochloride solution was used for rescue analgesia to avoid using the investigational agents for rescue (Table 1). The immediate-release preparations of hydromorphone and oxycodone faced challenges of being unapproved and potential confounding of safety assessments, respectively.
Concomitant use of monoamine oxidase inhibitors, opioid analgesics, and narcotic antagonists was prohibited. In addition, starting new treatment with/changing the dosing regimen of systemic nonopioid analgesics, adjuvant analgesics for pain relief, bisphosphonates, or anti-RANKL antibody preparations was prohibited. Furthermore, it was prohibited for subjects to undergo radiotherapy, nerve block, percutaneous vertebroplasty, or surgery, or receive any new cancer chemotherapy or immunotherapy for the first time. Magnesium oxide at 2 g/day and prochlorperazine maleate at 15 mg/day were administered to all subjects to ensure balanced evaluation of constipation and nausea/vomiting.
Outcomes
The primary efficacy end point was the change in VAS score from baseline to completion or discontinuation of treatment. Secondary end points evaluated were change in VAS score and sleep quality from baseline to each evaluation day. Sleep quality was assessed using a 4-point rating scale. The safety population included all subjects who were randomized to receive at least one dose of either treatment option. Adverse events (AEs) were recorded throughout the study period. AEs could be determined by investigators during examination or could be reported voluntarily by patients. Incidence, intensity, and relationship to the study drug were reported for AEs. Serious AEs, including death, were reported for both treatment arms, and attribution to treatment was determined. Clinical and laboratory assessments were performed at periodic intervals (day 1, day 8, and discontinuation) throughout the study period. Investigators coded the AEs by system organ class and preferred terms based on the MedDRA (Medical Dictionary for Regulatory Activities, version 18.1; http://www.meddra.org). Severity of AEs was rated on a three-grade scale (mild, moderate, and severe).
Statistical analysis
The full-analysis set (FAS) consisted of all patients with at least one measurement of the primary-efficacy parameter, at least one dose of study medication, and no serious Good Clinical Practice violation, and was the primary analysis population for efficacy. The level of significance used for the hypothesis test was 5% (two-sided), and the CI was 95% (two-sided). SAS 9.2 (SAS Inc, Cary, NC, USA) was used for statistical analysis. Summary statistics were calculated for VAS scores at baseline, at the completion or discontinuation of treatment, and for changes in VAS score. For primary efficacy evaluation, analysis of covariance was performed using baseline VAS score as a covariate to calculate the two-sided 95% CI for the difference in the hydromorphone and oxycodone groups (hydromorphone group – oxycodone group) in least squares mean changes in VAS score, in order to ensure that the upper limit did not exceed 10 mm, which was defined as the noninferiority limit. P-values and least squares means for paired comparisons for each group were calculated.
To assess changes in VAS scores over time, summary statistics were calculated for VAS scores on each evaluation day and changes in VAS scores from baseline to each evaluation day. A between-group t-test was performed to calculate two-sided 95% CIs for differences in means. A paired t-test was also performed for VAS scores at baseline and on each evaluation day to calculate two-sided 95% CIs for differences in means. For sleep-quality assessments, a cross-frequency table was created for the data at completion or discontinuation of treatment. Between-group comparisons by Wilcoxon rank-sum test and comparisons with baseline values by Wilcoxon signed-rank test were also performed.
Results
Patients and treatment exposure
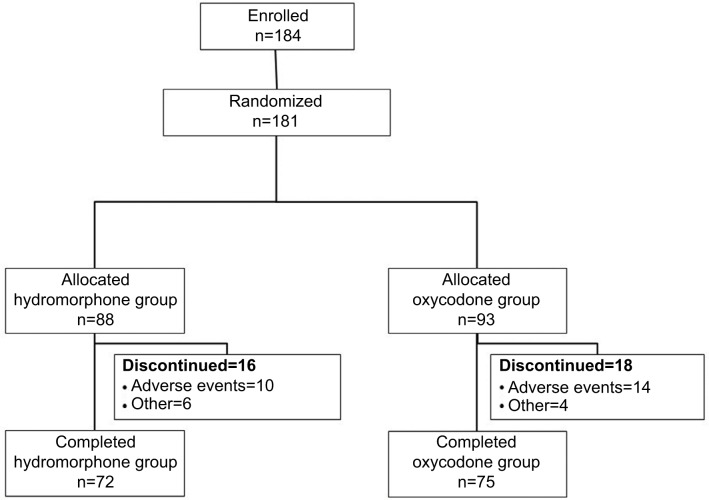
Patient disposition is shown in Figure 1. Of the 184 patients enrolled, 181 were randomized: 88 to the hydromorphone group, and 93 to the oxycodone group. All of these patients received the study drugs. Of these, 72 patients in the hydromorphone group and 75 in the oxycodone group completed the study. Sixteen patients in the hydromorphone group and 18 in the oxycodone group discontinued the study. The commonest reason for study discontinuation was AEs (ten and 14 patients in the hydromorphone and oxycodone groups, respectively).
Figure 1.
Patient disposition.
A total of 178 patients were included in the FAS. Two patients in the hydromorphone group were excluded from the FAS because they had no evaluable efficacy data after completion of study treatment, and one patient in the oxycodone group was excluded because of a major deviation. Eight and 12 patients in the hydromorphone and oxycodone groups, respectively, were excluded from the per protocol set due to protocol deviations.
Patient demographics in the FAS are shown in Table 2. The mean age was 70.1 years in the hydromorphone group and 68.4 years in the oxycodone group. The proportion of patients aged 65 years or older was 77.9% (67 of 86) in the hydromorphone group and 67.4% (62 of 92) in the oxycodone group, being slightly higher in the hydromorphone group. The proportion of females in the hydromorphone group was 45.3% (39 of 86), which was slightly higher than that (33.7% [31 of 92]) in the oxycodone group. There was no notable difference between the two groups for other factors.
Table 2.
Baseline demographics and clinical characteristics (full-analysis set)
| Characteristics | Hydromorphone group (n=86) | Oxycodone group (n=92) | Total (n=178) |
|---|---|---|---|
| Age (years) | |||
| Mean | 70.1 | 68.4 | 69.2 |
| SD | 10.19 | 9.17 | 9.69 |
| Sex, n (%) | |||
| Male | 47 (54.7) | 61 (66.3) | 108 (60.7) |
| Female | 39 (45.3) | 31 (33.7) | 70 (39.3) |
| Body weight (kg) | |||
| Mean | 51.85 | 54.65 | 53.3 |
| SD | 11.069 | 12.469 | 11.863 |
| Body-mass index (kg/m2), n (%) | |||
| <25 kg/m2 | 72 (83.7) | 83 (90.2) | 155 (87.1) |
| ≥25 kg/m2 | 14 (16.3) | 9 (9.8) | 23 (12.9) |
| Underlying disease (tumor type), n (%) | |||
| Lung | 31 (36) | 25 (27.2) | 56 (31.5) |
| Breast | 6 (7) | 6 (6.5) | 12 (6.7) |
| Gastrointestinal | 26 (30.2) | 39 (42.4) | 65 (36.5) |
| Hepatic–biliary–pancreatic | 12 (14) | 15 (16.3) | 27 (15.2) |
| Urogenital | 10 (11.6) | 6 (6.5) | 16 (9) |
| Others | 1 (1.2) | 1 (1.1) | 2 (1.1) |
| ECOG performance status, n (%) | |||
| 0 | 17 (19.8) | 21 (22.8) | 38 (21.3) |
| 1 | 41 (47.7) | 50 (54.3) | 91 (51.1) |
| 2 | 21 (24.4) | 14 (15.2) | 35 (19.7) |
| 3 | 7 (8.1) | 7 (7.6) | 14 (7.9) |
| 4 | 0 | 0 | 0 |
| Visual analog scale (mm) | |||
| Mean | 53.5 | 52.1 | 52.8 |
| SD | 14.53 | 12.81 | 13.65 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Mean (SD) VAS scores before the start of study treatment in the FAS were 53.5 (14.53) mm in the hydromorphone group and 52.1 (12.81) mm in the oxycodone group, with no notable difference between these two groups. Most patients – 77.9% (67/86) in the hydromorphone group and 70.7% (65/92) in the oxycodone group – completed or discontinued the study with no increase in initial dose. There was no notable between-group difference. Study-drug dose was increased twice or more for four patients in the hydromorphone group and five patients in the oxycodone group. The mean number of rescue-medication doses per day was less than one on all evaluation days for both groups, with no between-group difference.
Efficacy
Table 3 shows the analysis of changes in VAS score in the FAS. Mean (SD) VAS scores at baseline and completion/discontinuation of treatment were 53.5 (14.53) and 23 (17.91) mm, respectively, in the hydromorphone group and 52.1 (12.81) and 23.2 (18.83) mm, respectively, in the oxycodone group, showing a decrease from baseline in both groups. Mean changes in VAS score (SD) at completion/discontinuation of treatment were similar in both groups: −30.5 (20.8) mm in the hydromorphone group and −29.1 (21.46) mm in the oxycodone group.
Table 3.
Changes in visual analog-scale scores (full-analysis set)
| Parameters | Hydromorphone group, n=86 | Oxycodone group, n=92 |
|---|---|---|
| Baseline, mm | ||
| Mean | 53.5 | 52.1 |
| SD | 14.53 | 12.81 |
| Minimum | 36 | 35 |
| Median | 49 | 49 |
| Maximum | 97 | 95 |
| At completion/discontinuation of treatment, mm | ||
| Mean | 23 | 23.2 |
| SD | 17.91 | 18.83 |
| Minimum | 0 | 0 |
| Median | 19 | 20 |
| Maximum | 66 | 90 |
| Change, mm | ||
| Mean | −30.5 | −29.1 |
| SD | 20.8 | 21.46 |
| Minimum | −87 | −73 |
| Median | −31.5 | −31 |
| Maximum | 19 | 42 |
| Least squares meana | −30 | −29.6 |
| Differencea | −0.4 | |
| 95% CI | −5.9 to 5 | |
| P-value | 0.8732 | |
Notes:
Hydromorphone group – oxycodone group. Analysis of covariance (explanatory variable: baseline visual analog-scale score, groups).
The between-group (hydromorphone group − oxycodone group) difference (95% CI) in least squares mean changes in VAS score from baseline to completion/discontinuation of treatment was −0.4 mm (−5.9 to 5 mm). The upper limit of the 95% CI was below 10 mm, the noninferiority limit determined at the time of planning. This verified the noninferiority of hydromorphone to oxycodone.
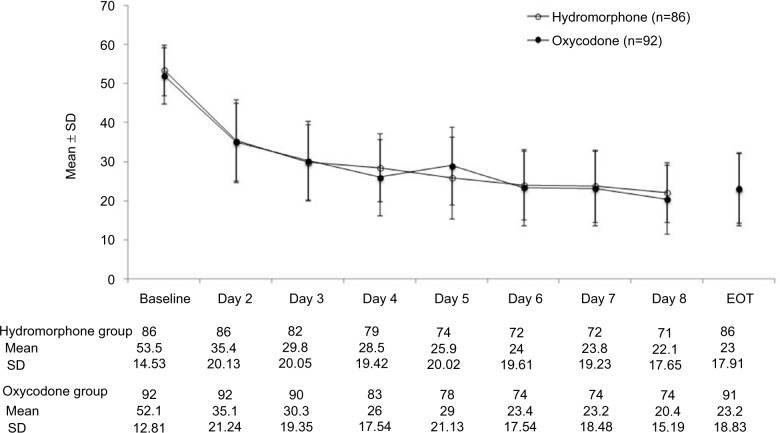
Figure 2 shows VAS scores at baseline and on each evaluation day, as well as time-course changes (mean ± SD) in VAS score measured on each evaluation day from baseline. In both groups, mean VAS score decreased by day 2, with a trend toward increase in mean change with increasing duration of treatment. Comparison with the time-course changes in VAS score with the use of oxycodone extended-release tablets showed a similar decrease over time.
Figure 2.
Changes in VAS scores in the full-analysis set.
Note: Compared with baseline VAS scores, significant changes were observed in VAS scores for assessments on days 2–8 (P<0.0001) and at EOT (P<0.0001).
Abbreviations: VAS, visual analog scale; EOT, end of treatment (or withdrawal from treatment).
A paired t-test was conducted for VAS scores at baseline and on each evaluation day (days 2–8 and at completion/discontinuation of treatment). Two-sided 95% CIs calculated for differences in means are shown in Figure 2. Compared with baseline, a significant difference in VAS score was observed for all evaluation days, daily assessments on days 2–8 (P<0.0001), and at completion/discontinuation of treatment (P<0.0001). Table 4 shows sleep evaluation in the FAS. Many patients in both groups showed an improvement in sleep at completion/discontinuation of treatment compared with baseline, and there was a significant difference by Wilcoxon signed-rank test (P<0.0001 for both groups).
Table 4.
Sleep evaluation (cross-frequency table, full-analysis set)
| Group | Baseline | At completion/discontinuation of treatment
|
||||
|---|---|---|---|---|---|---|
| 0. Very unsatisfactory/did not sleep at all | 1. Markedly unsatisfactory | 2. Slightly unsatisfactory | 3. Satisfactory | Total, n (%) | ||
| Hydromorphone group (n=86) | 0. Very unsatisfactory/did not sleep at all | 0 | 2 | 6 | 4 | 12 (14) |
| 1. Markedly unsatisfactory | 0 | 5 | 9 | 6 | 20 (23.3) | |
| 2. Slightly unsatisfactory | 1 | 6 | 21 | 14 | 42 (48.8) | |
| 3. Satisfactory | 0 | 1 | 6 | 5 | 12 (14) | |
| Total, n (%) | 1 (1.2) | 14 (16.3) | 42 (48.8) | 29 (33.7) | 86 | |
| Oxycodone group (n=92) | 0. Very unsatisfactory/did not sleep at all | 0 | 3 | 2 | 1 | 6 (6.5) |
| 1. Markedly unsatisfactory | 1 | 9 | 16 | 7 | 33 (35.9) | |
| 2. Slightly unsatisfactory | 1 | 6 | 17 | 21 | 45 (48.9) | |
| 3. Satisfactory | 0 | 0 | 0 | 8 | 8 (8.7) | |
| Total, n (%) | 2 (2.2) | 18 (19.6) | 35 (38.0) | 37 (40.2) | 92 | |
Notes: Compared with baseline evaluations, change in sleep in both groups was significantly different (P<0.0001) at end of treatment (Wilcoxon signed-rank test). However, there were no significant (P=0.7683) between-group differences in sleep evaluations (Wilcoxon rank sum test).
Safety and tolerability
Table 5 shows a list of AEs with an incidence rate ≥5%. Of all the 181 randomized patients, one in the oxycodone group was excluded from the safety analysis population, owing to a major deviation. The incidence of AEs was 80.7% (71 of 88) in the hydromorphone group and 83.7% (77 of 92) in the oxycodone group, and no significant between-group difference was observed. The most common AEs (incidence rate ≥10%) were nausea, vomiting, somnolence, diarrhea, and constipation. No respiratory depression/lowered breathing was reported in either group. The incidence of severe AEs was 8% (seven of 88) in the hydromorphone group and 10.9% (ten of 92) in the oxycodone group. Severe AEs were observed in one subject (appetite loss) in the hydromorphone group and two subjects (dizziness and malaise in one patient each) in the oxycodone group (Table 5).
Table 5.
List of adverse events with incidence of ≥5% (safety analysis set)
| Preferred terma | Hydromorphone group (n=88) n (%) |
Oxycodone group (n=92)* n (%) |
Total (n=180 n (%) |
|---|---|---|---|
| Patients with adverse event/s | 71(80.7) | 77 (83.7) | 148 (82.2) |
| Nausea | 36 (40.9) | 21 (22.8) | 57 (31.7) |
| Vomiting | 32 (36.4) | 16 (17.4) | 48 (26.7) |
| Somnolence | 23 (26.1) | 18 (19.6) | 41 (22.8) |
| Diarrhea# 16 (18.2) | 17 (18.5) | 33 (18.3) | |
| Constipation | 11 (12.5) | 14 (15.2) | 25 (13.9) |
| Fever | 7 (8) | 5 (5.4) | 12 (6.7) |
| Dizziness | 6 (6.8) | 5 (5.4) | 11 (6.1) |
| Appetite loss | 7 (8) | 3 (3.3) | 10 (5.6) |
| Malaise | 3 (3.4) | 6 (6.5) | 9 (5) |
Notes:
As per MedDRA (Medical Dictionary for Regulatory Activities) version 18.1;
major protocol deviation in one patient;
the reason for high diarrhea incidence was not clear and may have been the use of prophylactic laxatives.
The incidence rate of serious AEs (including death) was 12.5% (eleven of 88) in the hydromorphone group and 15.2% (14 of 92) in the oxycodone group, and there was no significant between-group difference. Of these, events considered related to study drugs were observed in four patients (hypercalcemia, appetite loss, somnolence, and vomiting in one patient each) in the hydromorphone group and six patients (ileus in two patients, and decreased level of consciousness, respiratory failure, vomiting, asthenia, and malaise in one patient each [respiratory failure and malaise in a single patient]) in the oxycodone group. The outcome was classified as “not resolved” for appetite loss and somnolence, occurring in one patient each, in the hydromorphone group, and for ileus, respiratory failure, and malaise, occurring in one patient each (respiratory failure and malaise in a single patient), in the oxycodone group. Other events were resolved or improved by discontinuation of study treatment or with interventions. There were no pronounced changes in laboratory values or vital signs. A 12-lead electrocardiogram showed no clinically significant QT prolongation.
Discussion
This double-blind, double-dummy study compared the efficacy of hydromorphone extended-release tablets with an oxycodone extended-release tablet formulation. In order to minimize potential bias of carryover effects of opioid analgesics, we included only opioid-naïve cancer patients. The initial dose of oxycodone extended-release tablets was determined as 10 mg/day, instead of the 20 mg/day dose recommended overseas, to allow assessment of the efficacy and safety of the formulation at a lower dose. With the verified noninferiority of hydromorphone extended-release tablets to the oxycodone extended-release formulation, the efficacy of the once daily hydromorphone extended-release tablets, designed using an original technique for extended-release formulation development ([GWATab®] Gelling WAter-soluble polymer-matrix Tablet; Daiichi Sankyo Company, Limited, Tokyo, Japan),13 has been confirmed to be equivalent to that of hydromorphone extended-release tablets currently used in many countries. In addition, the magnitude of decrease in VAS score in the present study was similar to that in a preceding study (JAPICCTI-132338), which compared hydromorphone immediate-release tablets and an oxycodone immediate-release powder formulation in Japanese opioid-naïve patients with cancer pain. This suggests that we have reproduced the efficacy results from the preceding study, demonstrating hydromorphone’s comparable efficacy with oxycodone in both immediate- and extended-release formulations.
Other clinical studies of oxycodone in opioid-naïve patients have reported similar baseline values of pain and similar percentages of decrease. This suggests that the efficacy results from those studies were reproduced in the present study. In addition, the most common AEs identified and their incidence rates did not greatly differ from previous reports.17–21
With regard to safety, incidence rates of nausea and vomiting were higher in the hydromorphone group than in the oxycodone group. However, the severity of nausea and vomiting was mild or moderate in all these patients (mild in approximately 70% of these patients). Of AEs that led to discontinuation of study treatment, nausea and/or vomiting were observed in six patients each in the hydromorphone and oxycodone groups, with similar frequency in both groups. These results suggested that there would be no problems with the tolerability of hydromorphone extended-release tablets, as in the case of the oxycodone extended-release formulation. It is known that although nausea and vomiting occur in 10%–40% of patients in the early stage of opioid treatment, most patients develop tolerance to these AEs.22
This study has some limitations. Because it was conducted in opioid-naïve patients, the analgesic effect was investigated only in patients receiving relatively low doses. Therefore, the efficacy and safety of the hydromorphone extended-release tablets administered once daily were not confirmed in patients who were switched from other opioids and in patients requiring high-dose opioids. Further studies need to be conducted on these issues. In addition, all subjects in this study were required to take laxatives and antiemetics to allow a strict comparison between groups in incidence rates of nausea/vomiting and constipation, which are common adverse reactions to opioids.23 The impact of such prophylactic treatment on safety evaluation is unknown, and the incidence rates and severity of nausea/vomiting and constipation observed in this study cannot be compared with those in other studies that did not require prophylactic medication with antiemetics and laxatives. Another limitation of this study is that drug interactions with cancer chemotherapy, radiotherapy, and other concomitant medications and withdrawal effects for opioids were not assessed.
Conclusion
We confirmed the noninferiority of hydromorphone extended-release tablets to oxycodone extended-release formulation in opioid-naïve Japanese cancer patients. The hydromorphone extended-release tablet formulation poses no significant safety concerns.
Supplementary material
Hirosaki National Hospital
Mito Red Cross Hospital
Mito Medical Center
Yuai Memorial Hospital
Ibaraki Prefectural Central Hospital
Tsuchiura Kyodo General Hospital
Tsukuba Medical Center Hospital
Tochigi Cancer Center
Gunma Prefectural Cancer Center
Takasaki General Medical Center
Ageo Central General Hospital
National Hospital Organization Saitama National Hospital
National Hospital Organization Chiba Medical Center
Chiba Cancer Center
Funabashi Municipal Medical Center
Chiba Tokushukai Hospital
Kanto Central Hospital of the Mutual Aid Association of Public School Teachers
Tokyo Metropolitan Health and Medical Treatment Corporation Toshima Hospital
Kanagawa Cancer Center
Kawasaki Municipal Hospital
Shonan Kamakura General Hospital
Aizawa Hospital
Japanese Red Cross Society Suwa Hospital, Suwa Red Cross Hospital
Japanese Red Cross Shizuoka Hospital
Yaizu City Hospital
Shizuoka Prefectural Hospital Organization, Shizuoka General Hospital
Shizuoka Cancer Center
Aichi Cancer Center Hospital
Toyohashi Medical Center
Matsusaka City Hospital
Kyoto Okamoto Memorial Hospital
Osaka General Medical Center
Rinku General Medical Center
National Hospital Organization Kinki-Chuo Chest Medical Center
Saiseikai Noe Hospital
Sano Hospital
Meiwa Hospital
Japanese Red Cross Society Himeji Hospital
Institute of Biomedical Research and Innovation Hospital
Okayama Medical Center
National Hospital Organization Kure Medical Center and Chugoku Cancer Center
Iwakuni Clinical Center
Japan Community Health Care Organization, Shimonoseki Medical Center
Saiseikai Imabari Hospital
Ehime Prefectural Central Hospital
Saiseikai Fukuoka General Hospital
Japan Community Health Care Organization, Kyushu Hospital
Beppu Medical Center
Miyazaki Prefectural Miyazaki Hospital
Acknowledgments
This study and preparation of this report were funded by Daiichi Sankyo Co Ltd. The authors wish to thank all of the investigators at the participating centers (see Box S1) for their contribution to this study. We would also like to thank Marion Barnett of Edanz Medical Writing for providing editorial support.
Footnotes
Disclosure
SI, AI, and YK are employees of Daiichi Sankyo. YS participated in this study as a medical specialist, and ST and EA functioned as safety assessment advisors. YS has received personal fees from Daiichi Sankyo. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organization . Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva: WHO; 1996. [Google Scholar]
- 2.Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63(1):65–76. doi: 10.1016/0304-3959(95)00017-M. [DOI] [PubMed] [Google Scholar]
- 3.International Narcotics Control Board . Report of the International Narcotics Control Board for 2013. Vienna: United Nations; 2014. [Google Scholar]
- 4.Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84(5):587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 6.Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol. 2012;23(Suppl 7):vii139–vii154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Adult cancer pain. Version 2. 2012. [Accessed July 21, 2017]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 8.Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. 2007. [Accessed July 21, 2017]. Available from: http://paincommunity.org/blog/wp-content/uploads/Opioids-Renal-Hepatic-Dysfunction.pdf.
- 9.Lussier D, Richarz U, Finco G. Use of hydromorphone, with particular reference to the OROS formulation, in the elderly. Drugs Aging. 2010;27(4):327–335. doi: 10.2165/11318320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Davison SN, Mayo PR. Pain management in chronic kidney disease: the pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag. 2008;4(6):335–336. 339–344. [PubMed] [Google Scholar]
- 11.Quigley C, Wiffen P. A systematic review of hydromorphone in acute and chronic pain. J Pain Symptom Manage. 2003;25(2):169–178. doi: 10.1016/s0885-3924(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 12.Toyama K, Uchuda N, Ishizuka H, et al. Single-dose evaluation of safety, tolerability and pharmacokinetics of newly formulated hydromorphone immediate-release and hydrophilic matrix extended-release tablets in healthy Japanese subjects without co-administration of an opioid antagonist. J Clin Pharmacol. 2015;55(9):975–984. doi: 10.1002/jcph.501. [DOI] [PubMed] [Google Scholar]
- 13.Fukui S, Yano H, Yada S, Mikkaichi T, Minami H. Design and evaluation of an extended-release matrix tablet formulation; the combination of hypromellose acetate succinate and hydroxypropylcellulose. Asian J Pharm Sci. 2017;12(2):149–156. doi: 10.1016/j.ajps.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra AM, Preuper HR, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155(12):2545–2550. doi: 10.1016/j.pain.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Moore RA, Straube S, Aldington D. Pain measures and cut-offs: ‘no worse than mild pain’ as a simple, universal outcome. Anaesthesia. 2013;68(4):400–412. doi: 10.1111/anae.12148. [DOI] [PubMed] [Google Scholar]
- 17.Dekel BG, Tomasi M, Vasarri A, et al. Opioid titration with sustained-release oxycodone and immediate-release morphine for moderate/severe cancer pain: a pilot assessment of the CoDem protocol. J Opioid Manag. 2014;10(1):29–38. doi: 10.5055/jom.2014.0189. [DOI] [PubMed] [Google Scholar]
- 18.Lazzari M, Greco MT, Marcassa C, Finocchi S, Caldarulo C, Corli O. Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: a propensity analysis. Drug Des Devel Ther. 2015;9:5863–5872. doi: 10.2147/DDDT.S92998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koizumi W, Toma H, Watanabe K, et al. Efficacy and tolerability of cancer pain management with controlled-release oxycodone tablets in opioid-naive cancer pain patients, starting with 5 mg tablets. Jpn J Clin Oncol. 2004;34(10):608–614. doi: 10.1093/jjco/hyh104. [DOI] [PubMed] [Google Scholar]
- 20.Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin. 2013;29(10):1399–1409. doi: 10.1185/03007995.2013.831816. [DOI] [PubMed] [Google Scholar]
- 21.Mercadante S, Porzio G, Ferrera P, et al. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin. 2012;28(11):1775–1779. doi: 10.1185/03007995.2012.739151. [DOI] [PubMed] [Google Scholar]
- 22.Fallon M, Cherny NI, Hanks G. Opioid analgesic therapy. In: Smith HS, editor. Opioid Therapy in the 21st Century. 2nd ed. New York: Oxford University Press; 2014. pp. 661–698. [Google Scholar]
- 23.Vignaroli E, Bennett MI, Nekolaichuk C, et al. Strategic pain management: the identification and development of the IAHPC opioid essential prescription package. J Palliat Med. 2012;15(2):186–191. doi: 10.1089/jpm.2011.0296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hirosaki National Hospital
Mito Red Cross Hospital
Mito Medical Center
Yuai Memorial Hospital
Ibaraki Prefectural Central Hospital
Tsuchiura Kyodo General Hospital
Tsukuba Medical Center Hospital
Tochigi Cancer Center
Gunma Prefectural Cancer Center
Takasaki General Medical Center
Ageo Central General Hospital
National Hospital Organization Saitama National Hospital
National Hospital Organization Chiba Medical Center
Chiba Cancer Center
Funabashi Municipal Medical Center
Chiba Tokushukai Hospital
Kanto Central Hospital of the Mutual Aid Association of Public School Teachers
Tokyo Metropolitan Health and Medical Treatment Corporation Toshima Hospital
Kanagawa Cancer Center
Kawasaki Municipal Hospital
Shonan Kamakura General Hospital
Aizawa Hospital
Japanese Red Cross Society Suwa Hospital, Suwa Red Cross Hospital
Japanese Red Cross Shizuoka Hospital
Yaizu City Hospital
Shizuoka Prefectural Hospital Organization, Shizuoka General Hospital
Shizuoka Cancer Center
Aichi Cancer Center Hospital
Toyohashi Medical Center
Matsusaka City Hospital
Kyoto Okamoto Memorial Hospital
Osaka General Medical Center
Rinku General Medical Center
National Hospital Organization Kinki-Chuo Chest Medical Center
Saiseikai Noe Hospital
Sano Hospital
Meiwa Hospital
Japanese Red Cross Society Himeji Hospital
Institute of Biomedical Research and Innovation Hospital
Okayama Medical Center
National Hospital Organization Kure Medical Center and Chugoku Cancer Center
Iwakuni Clinical Center
Japan Community Health Care Organization, Shimonoseki Medical Center
Saiseikai Imabari Hospital
Ehime Prefectural Central Hospital
Saiseikai Fukuoka General Hospital
Japan Community Health Care Organization, Kyushu Hospital
Beppu Medical Center
Miyazaki Prefectural Miyazaki Hospital