Abstract
Post-treatment Lyme disease syndrome (PTLDS) is a pain disorder for which there remains no gold standard treatment option. Here, we report a case of PTLDS in a female patient whose pain was refractory to treatment options such as radiofrequency ablation, vitamin infusion therapy, opioid analgesics, and other pharmacotherapies. The patient commenced an experimental intravenous ketamine infusion therapy at the Florida Spine Institute (Clearwater, FL, USA) and achieved relief from her chronic pain, an improved quality of life, reduced depression and suicidal ideation, and reduced opioid consumption.
Keywords: chronic Lyme, late Lyme, pain, analgesic, suicidality, depression
Video abstract
Introduction
Ketamine is an anesthetic that was first synthesized in the early 1960s.1 Except in certain cases, its use as an anesthetic has declined since its approval by United States Food and Drug Administration (FDA) in 1970. However, in recent times, it has garnered the attention of scientists and clinicians as new uses for this off-patent drug are discovered. At the time of writing, there are 180 open studies registered as clinical trials that involve ketamine.2 Ketamine has proven successful in placebo-controlled clinical trials for the treatment of depression,3,4 suicidal ideations,5 and pain.6
Predominantly known as an N-methyl-d-aspartate (NMDA) receptor antagonist, ketamine also exhibits anti-inflammatory and immunomodulatory actions.7,8 Recently, ketamine has been utilized off-label as an effective option for treating certain neuropathic pain conditions that currently do not have gold standard treatment options such as complex regional pain syndrome (CRPS) and fibromyalgia.9 Takahashi et al10 first described a clinical case of CRPS that was responsive to low-dose ketamine. The scientific evidence supporting the analgesic properties of ketamine continues to grow. If efficacy is established in Phase III clinical trials, ketamine could be widely adopted as a first-line therapy for certain types of pain, especially given the opioid epidemic that the world currently faces.11
Post-treatment Lyme disease syndrome (PTLDS) occurs secondary to Lyme disease (LD) in 10–15% of patients who were ideally treated with antibiotics.12 It is characterized by persistent or recurrent symptoms of fatigue, musculoskeletal pain, and cognitive complaints leading to functional decline. Although the etiology of the disease is not fully known, several lines of evidence suggest that immune mechanisms may be involved. Bockenstedt et al13 showed that spirochete remnants can persist even after successful antibiotic treatment to eliminate infectious Borrelia burgdorferi in mice. Moreover, they showed that these remnants contain immunogenic material that induces immunoglobulin G (IgG) responses and stimulates macrophages to produce tumor necrosis factor (TNF)-α in vitro. In a clinical study, the synovial tissue from patients with severe LD was compared with that from patients with the known autoimmune disease, rheumatoid arthritis (RA).14 These authors found that the synovial lesions of LD and RA patients were very similar. Importantly, they showed that HLA-DR and HLA-DQ expression was intense throughout the lesions. Both of these major histocompatibility complex (MHC) class II cell surface receptors are involved in other autoimmune diseases including RA and celiac disease. Thus, immunomodulatory drugs may be useful in the treatment of PTLDS.
In the clinical case described herein, we show the clinical course of a PTLDS patient who was treated with intravenous (IV) ketamine infusions under the care of Dr. Ashraf F Hanna at the Florida Spine Institute (Clearwater, FL, USA).
Case report
On May 26, 2015, a 31-year-old, 100 lb, female patient reported to the Florida Spine Institute, referred by a general surgeon for the treatment of her chronic pain disorder, potentially related to a previous bout with LD. All pain scores were captured by administering a standard unipolar visual analog scale (VAS) for pain. The patient complained of diffuse body pain (6–7/10), fatigue, headache, and brain fog (7–8/10). The onset of her pain had been gradual, beginning ~3 years prior, a short time after being diagnosed and treated for LD. Given the timing and symptoms that the patient described, a diagnosis of PTLDS was made. The pain was described as persistent, moderate to severe, and gradually worsening. Her pain increased during general activity. Her current treatment regimen included fentanyl transdermal patches, clonazepam, oxycodone hydrochloride, and citalopram hydrobromide. Still, these medications only provided mild relief. She had begun to experience opioid-induced constipation (OIC) and was seeking more adequate pain relief without this side effect. The patient had previously tried physical therapy and IV vitamin infusions, which resulted in no relief. She also underwent trigger point injections and a radiofrequency ablation procedure that resulted in inadequate pain reduction.
In certain clinical settings, ketamine has been shown to be useful in treating pain that does not respond well to other treatments. Thus, IV ketamine therapy was discussed with the patient over several follow-up visits to the clinic. Given that other numerous treatment modalities failed to achieve adequate pain relief, she opted to try IV ketamine. She was scheduled for her first infusion on July 28, 2015.
During follow-up visits leading up to initiation of IV ketamine therapy, the patient underwent several cognitive tests. The GAD-7 (Generalized Anxiety Disorder) survey was administered, indicating severe anxiety (score 18/21). The patient also took the Center for Epidemiologic Studies Depression-Revised (CESD-R) test, indicating Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), Major Depressive Episode (score 50/60). Furthermore, her answers indicated suicidal ideation.
The patient reported for her first ketamine infusion on July 28, 2015. Her pain levels were unchanged since her previous visit (score 7/10). Ketamine HCl was infused with 200 mg ketamine, 200 mg lidocaine, and 6 mg midazolam in 100 mL bag of saline over a 4-hour period. Midazolam was used to reduce or prevent psychotomimetic side effects that ketamine can cause in some patients. The ketamine dosage was increased by 200 mg each day until 800 mg was reached. The patient received IV ketamine for 10 days. She did not demonstrate any complications. VAS pain scores were recorded on every visit to the clinic except for two interim infusion days. During the initial 10-day infusion period, the patient reported little change in her level of pain (scores ranged from 6 to 8).
The patient reported for a follow-up visit from her ketamine infusions on September 8, 2015. Her pain levels were drastically lower (score 1/10) following her 10-day ketamine infusion therapy. The decision was made to reduce the dosage of fentanyl patches from 125 µg to 75 µg every 48 hours. Although she reported very low pain at this time, over the course of several follow-up interactions she commented that her pain seemed to be slowly returning. During a follow-up visit on October 1, 2015, the patient reported that her pain levels remained low (score 3/10) but seemed to be becoming more severe than just after her last ketamine infusion. Her transdermal fentanyl dosage was kept at 75 µg every 48 hours. During another follow-up visit on November 4, 2015, the patient’s pain had returned to baseline (score 7–8/10). She was scheduled for a 4-day IV ketamine booster infusion to commence the following week. On November 9, the patient reported for her first ketamine booster infusion. Prior to the infusion of 800 mg of ketamine, her pain score was taken (score 7/10). Prior to the second infusion, the patients pain score had improved (score 4/10). Her pain score remained unchanged during the third and fourth ketamine infusions.
Approximately 1 month later, the patient’s pain had returned to baseline so she underwent another 3-day IV ketamine infusion session. During this infusion, her pain score was reduced from a 7 to 2/10. During a follow-up interview, the patient stated that she was very pleased with the outcome of her IV ketamine therapy. She indicated that she no longer felt depressed nor had suicidal ideations. Moreover, the patient reported that her brain fog had improved. However, 2 months later, the patient returned to the clinic with a pain flare up. She underwent another 3-day IV ketamine infusion with similar results (score 2/10).
No ketamine-related side effects or physical dependence was observed during treatment. Written informed consent was obtained from the patient for publication of this case report.
Discussion and conclusion
A recent double-blind, randomized, placebo-controlled clinical trial was conducted to evaluate the efficacy of IV ketamine in patients with treatment-resistant depression.15 These investigators reported that IV ketamine was effective at reducing depressive symptoms in this patient population. The results of our case report and evidence from similar studies and preceding case reports substantiate the antidepressant efficacy of ketamine.4,16–18 To our knowledge, there have been no other reports in the literature that have assessed the effects of IV ketamine in a patient with PTLDS until now.
In this patient with whole-body chronic pain associated with PTLDS, IV ketamine drastically reduced pain levels (Figure 1). The patient’s depression and suicidal ideations were also eliminated post-ketamine infusion. Although we19,20 and others21,22 have reported the efficacy of IV ketamine for the treatment of chronic pain, this patient’s results were not typical. In this case, the drastic reductions in pain levels (VAS reduced from 7 to 2) were followed by an eventual return to baseline. However, when the patient’s pain did return, shorter booster infusions were sufficient to maintain analgesia for months at a time. This is in contrast to, for example, our previous documentation of a patient with fibromyalgia who achieved complete remission for more than a year post-infusion without additional treatment.19
Figure 1.
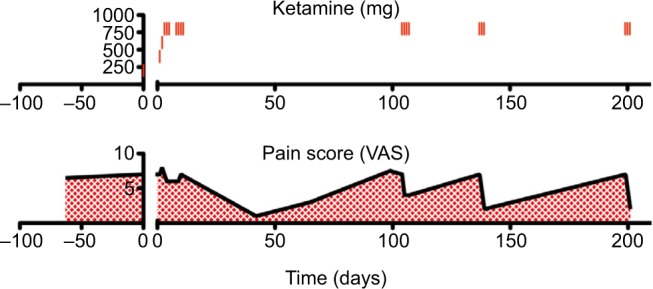
The summarization of the clinical course.
Abbreviation: VAS, visual analog scale
The current PTLDS patient’s pain levels were adequately controlled using IV ketamine infusions for a duration of ~1–2 months from the last infusion session. Given that the elimination half-life of IV ketamine is only 2.5 hours,23,24 this means that the duration of analgesia produced in this case did not require the sustained pharmacological action of ketamine, since it would have been eliminated within the first day following the last infusion. This observation supports theories that chronic pain associated with PTLDS may be neuropathic in nature25 and that ketamine’s analgesic efficacy is centrally mediated.
“Central sensitization” has been coined to describe numerous neuropathic pain conditions resulting from a nociceptive insult that triggers a prolonged but reversible increase in the excitability and synaptic efficacy of neurons in central nociceptive pathways.26 Ketamine is thought to de-sensitize centrally mediated pain via repeated NMDA receptor blockade.27 However, it is likely that ketamine acts via multiple mechanisms to produce analgesia in neuropathic pain conditions. Neuropathic pain has been associated with increased glial activation and subsequent release of pro-inflammatory cytokines. Interestingly, ketamine produces pharmacological effects that reduce cell excitotoxicity via NMDA antagonism and reduce inflammation by suppressing the hyperactivation of microglia.28 Moreover, ketamine produces immunomodulatory actions that may also be uniquely beneficial to conditions that may have an autoimmune component, such as PTLDS. Thus, ketamine appears to produce a robust polypharmacological “entourage effect” that is highly effective in treating neuropathic pain conditions – which are notoriously difficult to treat with more conventional analgesic drugs.
In conclusion, the pain relief (~71% decrease) described in this case report was achieved without the use of increasing doses of opioid analgesics and, in fact, afforded the patient’s fentanyl dosage to be reduced from 125 µg to 75 µg (40% decrease) every 48 hours. Opioid-sparing therapies, such as ketamine, should be used more frequently for the management of chronic pain. This is especially important given the frequency of opioid dependence and abuse, which has reached such severity to be widely regarded as an “epidemic”.29 Future studies should be conducted to optimize ketamine for the management of chronic pain conditions without the use of opioids, where appropriate.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;(182):313–333. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- 2.U.S. National Institutes of Health [homepage on the Internet] [Accessed May 22, 2016]. Available from: https://clinicaltrials.gov/
- 3.Loo CK, Galvez V, O’Keefe E, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134(1):48–56. doi: 10.1111/acps.12572. [DOI] [PubMed] [Google Scholar]
- 4.Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016;46(3):623–635. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- 5.Price RB, Iosifescu DV, Murrough JW, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31(4):335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miziara LE, Simoni RF, Esteves LO, Cangiani LH, Grillo-Filho GF, Paula AG. Efficacy of continuous S(+)-ketamine infusion for postoperative pain control: a randomized placebo-controlled trial. Anesthesiol Res Pract. 2016;2016:6918327. doi: 10.1155/2016/6918327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62(1):47–58. [PubMed] [Google Scholar]
- 8.Zilberstein G, Levy R, Rachinsky M, et al. Ketamine attenuates neutrophil activation after cardiopulmonary bypass. Anesth Analg. 2002;95(3):531–536. doi: 10.1097/00000539-200209000-00005. table of contents. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen J, Bengtsson A, Backman E, Henriksson KG, Bengtsson M. Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol. 1995;24(6):360–365. doi: 10.3109/03009749509095181. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Miyazaki M, Nanbu T, Yanagida H, Morita S. The NMDA-receptor antagonist ketamine abolishes neuropathic pain after epidural administration in a clinical case. Pain. 1998;75(2–3):391–394. doi: 10.1016/s0304-3959(97)00189-9. [DOI] [PubMed] [Google Scholar]
- 11.Olsen Y. The CDC guideline on opioid prescribing: rising to the challenge. JAMA. 2016;315(15):1577–1579. doi: 10.1001/jama.2016.1910. [DOI] [PubMed] [Google Scholar]
- 12.Aucott JN, Crowder LA, Kortte KB. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J Infect Dis. 2013;17(6):e443–e449. doi: 10.1016/j.ijid.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. 2012;122(7):2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steere AC, Duray PH, Butcher EC. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31(4):487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 15.Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 16.Li CT, Chen MH, Lin WC, et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: a randomized controlled study. Hum Brain Mapp. 2016;37(3):1080–1090. doi: 10.1002/hbm.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvez V, O’Keefe E, Cotiga L, et al. Long-lasting effects of a single subcutaneous dose of ketamine for treating melancholic depression: a case report. Biol Psychiatry. 2014;76(3):e1–e2. doi: 10.1016/j.biopsych.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Clark P. Treatment-refractory depression: a case of successful treatment with intranasal ketamine 10% Ann Clin Psychiatry. 2014;26(2):145. [PubMed] [Google Scholar]
- 19.Hanna AF, Smith AJ. Intravenous ketamine produces long-term pain relief in a patient with fibromyalgia. Fibromyalgia. 2016;1(1):104. [Google Scholar]
- 20.Hanna AF, Armstrong JS, Smith AJ. Effects of intravenous ketamine infusions in a neuropathic pain patient with lichen sclerosus et atrophicus. Case Rep Dermatol. 2016;8(2):164–170. doi: 10.1159/000446528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147(1–3):107–115. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Kiefer RT, Rohr P, Ploppa A, Altemeyer KH, Schwartzman RJ. Complete recovery from intractable complex regional pain syndrome, CRPS-type I, following anesthetic ketamine and midazolam. Pain Pract. 2007;7(2):147–150. doi: 10.1111/j.1533-2500.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 23.Wieber J, Gugler R, Hengstmann JH, Dengler HJ. Pharmacokinetics of ketamine in man. Anaesthesist. 1975;24(6):260–263. [PubMed] [Google Scholar]
- 24.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53(1):27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Weissenbacher S, Ring J, Hofmann H. Gabapentin for the symptomatic treatment of chronic neuropathic pain in patients with late-stage Lyme borreliosis: a pilot study. Dermatology. 2005;211(2):123–127. doi: 10.1159/000086441. [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson J, Axelsson G, Hallin RG, Gustafsson LL. Beneficial effects of ketamine in a chronic pain state with allodynia, possibly due to central sensitization. Pain. 1995;60(2):217–222. doi: 10.1016/0304-3959(94)00139-6. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Y, Kawaji K, Sun L, et al. Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci. 2011;31(48):17370–17382. doi: 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34(2):e1–e23. doi: 10.1016/j.emc.2015.11.002. [DOI] [PubMed] [Google Scholar]


