Abstract
Metastatic papillary thyroid carcinoma without an identifiable primary tumor despite extensive microscopic examination of the thyroid gland is a rare but true phenomenon. We retrieved seven of such cases and described in details the clinical and pathologic features of these tumors. BRAF V600E immunohistochemistry and Sequenom molecular profile were conducted in selected cases. All patients harbored metastatic disease in the central (n = 3), lateral (n = 3), or both neck compartments (n = 1). The histotype of the metastatic disease was papillary thyroid carcinoma (PTC, n = 5), poorly differentiated thyroid carcinoma (PDTC) in association with a PTC columnar variant (n = 1), and anaplastic thyroid carcinoma (ATC) in association with a PTC tall cell variant (n = 1). Fibrosis was present in the thyroid of five patients. All patients with PTC were alive without evidence of recurrence. The 76-year-old patient with PDTC did not recur and died of unknown causes. Finally, the patient with ATC was alive with distant metastasis at last follow up. The median follow up for this cohort was 2.2 years (range 0.8 – 17). BRAF V600E was detected in 4 of 6 cases by immunohistochemistry. In conclusion, metastatic nodal disease without identifiable thyroid primary is a rare but real phenomenon of unknown mechanisms. Although most tumors are low grade and well differentiated, aggressive behavior due to poorly differentiated or anaplastic carcinoma can happen. Most cases are BRAF V600E positive thyroid tumors. A papillary carcinoma phenotype is found in all reported cases.
Keywords: Papillary thyroid carcinoma, occult primary thyroid carcinoma, lymph node metastasis
Introduction
Papillary thyroid carcinoma (PTC) with clinically apparent nodal or distant metastasis but lacking a primary cancer in the thyroid on pre-operative ultrasonographic examination is referred by some as occult PTC (1–3). In the majority of cases, the primary carcinomas are identified after careful pathologic examination of the total thyroidectomy specimen, mostly in the form of papillary microcarcinoma (i.e. tumors of < 1 cm in size) (1, 2). However, we have encountered rare cases where the primary tumors were not detected even after thorough and complete pathologic examination of the thyroid. To date, only two well documented cases with entire microscopic examination of the thyroid gland but without mutation analysis have been reported (2, 4). The clinical presentation, pathologic features, molecular findings and outcome of these metastatic thyroid carcinomas without detectable primary have not been well characterized. In this study, we gathered seven of such cases and provided a detailed clinico-pathologic review of these tumors, including an effort to detect BRAF mutation on selected cases using BRAF immunohistochemistry and molecular profiling, aiming to shed some lights on this rare phenomenon in thyroid carcinoma.
Material and Methods
Study cohort
The pathology database of Memorial Sloan Kettering Cancer center (MSKCC) was searched for candidate patients who underwent total thyroidectomy with histologically confirmed nodal metastasis of thyroid carcinoma but no detectable primary tumors in the thyroidectomy between 2000 and 2016. All cases were reviewed by at least two endocrine pathologists (BX and RG) to confirm the histotype of the metastases and the absence of primary tumor. One additional case was contributed from Cornell University Medical Center by one of the authors (TS). Cases were included in the study if they displayed 1) histologically confirmed metastatic thyroid carcinoma in lymph nodes with 2) absence of thyroid primary after 3) histopathologic examination of the entire gland. A total of seven cases fulfilling the above criteria were included in the present study. All seven cases lacked any suspicious or definite area(s) of papillary thyroid carcinoma in the thyroid gland proper after through histologic examination of the entirely submitted thyroid, and exhibited diagnostic nuclear features of papillary thyroid carcinoma in the nodal metastasis (e.g. nuclear crowding, nuclear enlargement, nuclear membrane irregularity and chromatin clearing), allowing definite diagnosis of metastasis and distinguishing them from benign mimickers (e.g. benign thyroid inclusion within a lymph node). The study was approved by the institutional review board of MSKCC.
Clinical and pathologic review
The patients’ charts were reviewed to record the following clinical parameters: age at diagnosis, sex, radiation exposure, pre and post-operative thyroglobulin (TG) and anti-TG levels, status of postoperative radioactive iodine ablation, duration of clinical follow up (FU), and clinical outcome. The following pathologic parameters of the nodal metastases were documented: total number of lymph nodes sampled; number of lymph nodes with metastatic thyroid carcinoma; location of the positive lymph nodes (central vs. lateral neck compartment); size of the largest involved lymph nodes and largest metastatic focus; extranodal extension, and histotype of the metastatic carcinoma. Poorly differentiated carcinoma (PDTC) was defined using the MSKCC criteria (5). In brief, a diagnosis of PDTC was rendered when a tumor exhibited tumoral necrosis or elevated mitotic index of ≥ 5 mitotic figures per 10 high power fields (HPFs, 400X) regardless of the growth pattern and nuclear features.
The pathologic features of the thyroidectomy, including benign thyroid diseases (e.g. chronic lymphocytic thyroiditis or nodular hyperplasia) and the presence and extent of fibrosis, were collected.
BRAF immunohistochemistry
Six cases (Cases #1, 2, 3, 4, 6, and 7) with available material were subjected to immunohistochemistry analysis using the VE1 antibody directed against the BRAF V600E mutated protein (Spring Bioscience, Pleasanton, California, 1:50 dilution) in the nodal metastasis. The procedure was performed using the Ventana semiautomated staining system (Ventana Medical Systems, Inc., Tucson, Arizona). Staining intensity was scored as 0 (no staining), 1+ (faint), 2+ (moderate), and 3+ (strong) as previously described (6). A positive control consisted of a melanoma positive for BRAFV600E mutation at the DNA and protein level and negative external and internal controls (including background lymph node, thyroid, and tonsillar tissue) were used with each run. In brief, a tumor was considered immunopositive for BRAF V600E if it displayed a staining intensity of 3+.
Sequenom mutation profiling
One case harboring anaplastic thyroid carcinoma (ATC) in the lymph node (case #7) was profiled using a MassARRAY system (Sequenom) based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Tumor sample DNA was used to interrogate for the presence of single nucleotide variant (SNV) in 91 hot-spots in 8 oncogenes: EGFR, KRAS, BRAF, PIK3CA, AKT1, NRAS, MEK1 (MAPK1), and ERBB2, as previously described (7).
Results
Clinical presentation and outcome
The clinico-pathologic characteristics of the study cohort are summarized in Table 1. The median age of presentation was 55 (range 30 – 76). There was near equal gender distribution with a female to male ratio of 1.3:1. None of the patients had prior radiation exposure to the head and neck region. Three patients in our cohorts underwent total thyroidectomy for benign conditions (e.g. thyroid nodules or Grave’s disease; patients #2, 4, and 5) and were found to have lymph node metastases in the central neck compartment; three (patients #1, 6, and 7) presented with lateral neck mass with a diagnosis of metastatic carcinoma confirmed on fine needle aspiration cytology; and one (patient #3) was found to have incidental nodal metastatic papillary thyroid carcinoma (pN1a) in a resection specimen for laryngeal squamous cell carcinoma. Three patients did not have interpretable post-operative serum TG levels because of high anti-thyroglobulin antibodies (patients #1 and #7), or simply because the postoperative serum TG assay was not performed (patient #3). The remaining four patients had low postoperative (i.e. < 0.3 ng/ml) TG values. Pre-operative serum TG was normal (15 ng/ml) in patient #3, and was not available in all other cases. Three patients received post-operative radioactive iodine treatment: one with PDTC and a large nodal metastasis of 4.5 cm in size (patient #6); one PTC with 5 positive lymph nodes (patient #1); and another PTC with only two of thirty-six lymph nodes containing low volume (0.05 cm) metastatic disease (patient #4).
Table 1.
Clinicopathologic features of the patients with metastatic thyroid carcinoma and undetectable primary tumor: the study cohort and the reported cases in the literature.
| Clinical Features and outcome | Thyroid | Lymph node (LN) metastasis | BRAF V600 EIHC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/ sex |
Presenting symptoms |
Post- operative |
Status (y)b |
Recurrence | Benign thyroid disease |
Fibros is in thyroid |
Positive LN/LN sampled (N) |
Size of largest metastatic focus/ largest involved LN (cm) |
LN cpt |
Histotype | ENE | |||
| TG (ng/ml) |
RAI/ RT |
|||||||||||||
| 1 | 49/F | Lateral neck mass with history of Hashimoto’s thyroiditis | NA | RAI | NED (17.0) | No | CLT | Multifocal a/w CLT | 5/11 | 1.9/2.0 | Lateral (pN1b) | PTC, classical | Absent | Positive a |
| 2 | 53/M | Bilateral thyroid nodules | <0.3 | None | NED (2.4) | No | NH | Multifocal a/w NH | 1/5 | 1.7/1.7 | Central (pN 1a) | PTC, classical | Absent | Positive |
| 3 | 55/M | Incidental pN1 detected on neck dissection for laryngeal SCC | <0.3 | RT for laryngeal SCC, No RAI | NED (2.2) | No | DQT, CCH | Multifocal a/w DQT | 2/65 | 0.66/0.85 | Lateral and central (pN1b) | PTC, follicular | Absent | Negative |
| 4 | 74/F | Thyroid nodule detected on US for hyperthyroidism | <0.3 | RAI | NED (0.8) | No | NH | Multifocal a/w NH | 2/36 | 0.05/0.15 | Central (pN1a) | PTC, follicular | Absent | Negative |
| 5 | 30/F | Grave's disease, post-methimazole treatment | <0.3 | None | NED (0.8) | No | CLT | Absent | 1/3 | 0.1/0.5 | Central (pN1a) | PTC, oncocytic solid | Absent | NA |
| 6 | 76/F | Lateral neck mass | <0.3 | RAI | DUC (3.7) | No | CLT, NH | Unifocal (0.2cm) | 1/13 | 4.5/4.5 | Lateral (pN1b) | PDTC | Present | Positive |
| 7 | 68/M | Lateral neck mass | NA | RT | AWD (1.9) | Yes, lung/spine | None | Absent | 6/86 | 5.5/5.5 | Lateral (pN1b) | ATC | Present | Positive c |
| Ref [4] | 29/F | lateral neck mass | NA | NA | NA | NA | NA | Present | NA | 1.5 | NA (pN1) | PTC classical | NA | NA |
| Ref [2] | 51/M | Bone metastasis | NA | NA | NA | NA | CLT | Present | NA | NA | Bone (pM1) | PTC | NA | NA |
Ref: reference, US: ultrasound, SCC: squamous cell carcinoma, CLT: chronic lymphocytic thyroiditis, NH: nodular hyperplasia; CCH: c cell hyperplasia; DQT: De Quervain thyroiditis; a/w: associated with; cpt: compartment, PDTC: poorly differentiated thyroid carcinoma; PTC: papillary thyroid carcinoma, ATC: anaplastic thyroid carcinoma, RAI: radioactive iodine, RT: radiation therapy, ENE: extranodal extension, NA: not available, DUC: dead of unknown cause, NED: no evidence of disease, AWD: alive with disease, IHC: immunohistochemistry.
A positive BRAF V600E immunostain was defined as strong (3+) homogenous immunolabeling in at least 60% of tumor cells.
At last follow-up (FU period, years).
BRAFV600E mutation also detected at the DNA level.
The median follow up of our cohort was 2.2 years (range: 0.8 to 17 years). All patients with a diagnosis of metastatic PTC (patients #1 to #5) were alive without evidence of disease or recurrence at the last follow up. The patient with metastatic PDTC did not have documented recurrence and died of unknown causes 3.7 years after diagnosis. The 68-year-old patient with metastatic ATC developed distant metastasis to mediastinum, lung, and spine within 1.6 years, and was alive with disease at the last follow up (1.9 years).
Histopathologic features
The thyroid was sampled in its entirety in all cases (15 – 29 thyroid sections per case, median = 18). A pathology review involving at least two endocrine pathologists (RG, BX) was conducted for each case and confirmed the absence of neoplastic process within the thyroid. None of the seven cases contained psammoma bodies in the thyroid gland. Benign thyroid diseases, including chronic lymphocytic thyroiditis (n = 3), De Quervain thyroiditis (i.e. granulomatous thyroiditis, n = 1), nodular hyperplasia (n = 3), and/or bilateral C cell hyperplasia (n = 1), were noted in five patients. Fibrosis within the thyroid, either unifocal (n = 1) or multifocal (n = 4), was present in five patients. The multifocal fibrosis was associated with benign thyroid diseases, e.g. chronic lymphocytic thyroiditis (n = 1), nodular hyperplasia (n = 2) and De Quervain thyroiditis (n = 1). Chronic inflammatory infiltrate was noted within the fibrotic areas in the two patients with chronic lymphocytic thyroiditis (case #1) and De Quervain thyroiditis (case #3). None of the patients exhibited features of multifocal fibrosing thyroiditis as defined and described previously (8). One of the seven patients (patient #6) displayed in his thyroid a 0.2 cm unifocal stellate scar-like fibrosis associated with non-psammomatous calcification. The other 4 cases with fibrosis had fibrotic areas ranging from 0.1 to 0.6 cm in size.
All patients harbored metastatic disease in the central (n = 3), lateral (n = 3), or both neck compartments (n = 1), involving at least one (up to six) lymph nodes. The histotype of the metastatic disease was as follows: PTC (n = 5), PDTC with an elevated mitotic index of 6 mitotic figures per 10 high power fields in association with a PTC columnar variant (n =1), and ATC in association with a PTC tall cell variant (n = 1, Figures 1–3). The architectural patterns of the five pure PTC metastasis were classical (n = 2), follicular (n = 2), and solid (n = 1). Extranodal extension was noted only in the metastatic PDTC and ATC. None of the seven patients contained benign thyroid inclusions in the lymph nodes sampled.
Figure 1.
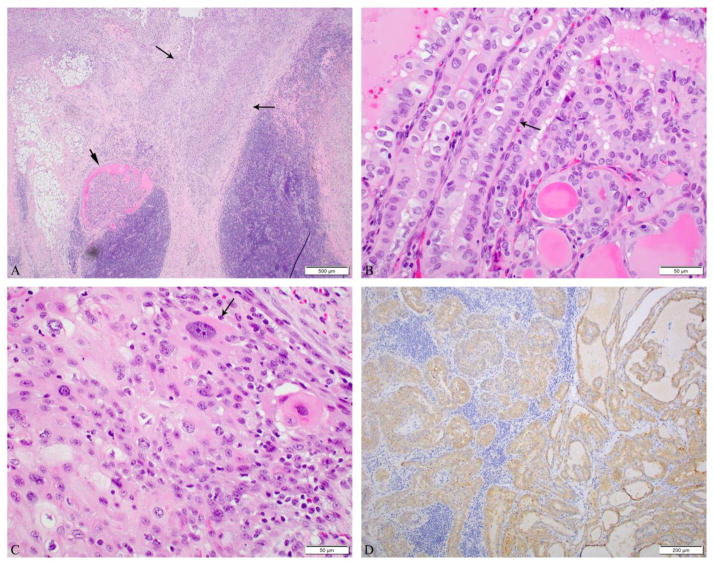
Microscopic pictures of an anaplastic thyroid carcinoma (ATC) in association with a papillary thyroid carcinoma, tall cell variant (PTC-TCV) in a 5.5 cm lateral neck lymph node of a 68-year-old male patient. No carcinoma was found in the thyroidectomy. A: The PTC component (short arrow) is seen adjacent to the anaplastic carcinoma (long arrows). B: PTC-TCV component made of elongated follicles lined by tumor cells (arrow) whose height is at least twice their width with an oncocytic cytoplasm and the characteristic nuclear features of PTC.C: The ATC component has a squamoid appearance with cells having highly pleomorphic and very large nuclei (arrow). D: BRAF V600E immunostain was positive in the tumor. Genotypic analysis of tumor DNA revealed a BRAF V600E mutation.
Figure 3.
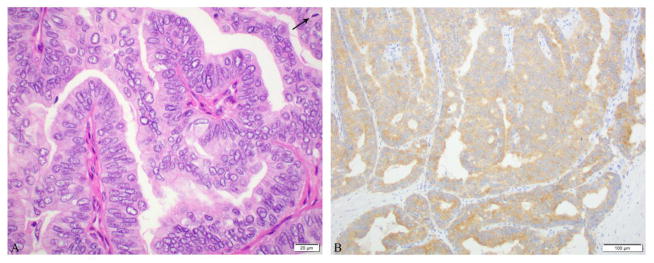
Microscopic pictures of a papillary thyroid carcinoma (PTC), classical type in a 1.2 cm central compartment lymph node from a 49-year-old female. No carcinoma was found in the thyroidectomy. A: PTC with papillae (arrow). Inset shows the characteristic nuclear features of PTC. B: BRAF V600E immunostain was positive in the tumor.
BRAF V600E status
BRAF V600E immunohistochemistry studies were performed in six metastatic tumors (cases #1–4 PTC, #6 PDTC, and #7 ATC). Four of six cases tested (cases #1, 2, 6, and 7) showed strong (3+) staining including the PDTC and ATC (Table 1 and Figures 1–3).
Sequenom mutation profiling study of the ATC confirmed the presence of BRAF V600E mutation. In addition, a PIK3CA pE542K mutation was also detected.
Discussion
In this study, we report seven patients with metastatic thyroid cancer and no detectable primary tumor despite entire histopathologic examination of the thyroid gland. To date, a total of two such patients have been previously reported in the literature (2, 4). The clinical and pathologic features of the current and previous cases are summarized in Table 1. Two additional studies have reported six patients with nodal metastasis in the lateral neck compartment without identifiable primary tumor in the thyroid. However, as it is unclear whether the thyroid was entirely sampled for histologic examination, these cases were not included in the literature review (1, 9). Among the nine well-documented cases with thyroid entirely submitted, eight had nodal metastases, while the remaining patient developed distant metastasis to bone. The nodal metastases could not represent benign thyroid inclusions since they all displayed a PTC phenotype at the cytoarchitectural level (e.g. clear irregular nuclei and/or true papillary formations). Indeed, the stringent criteria for benign thyroid inclusions require the absence of the cytoarchitectural features of PTC, the presence of thyroid follicles in only one node in a subcapsular location and a small size of the thyroid inclusion (10). Among the eight reported patients with nodal metastases and undetected primary thyroid carcinoma, the most common presenting symptom was a large lateral neck mass (4 of 8 patients), while the remaining four patients underwent thyroidectomy for various reasons (one for laryngeal squamous cell carcinoma, one for Grave’s disease, one for nodular hyperplasia with bilateral thyroid nodules, and one for thyroid nodule detected on ultrasound examination for hyperthyroidism). All nine reported cases had at least a component of papillary thyroid carcinoma in the metastases. The nodal metastases of two patients (#6 and #7) contained a component of papillary thyroid carcinoma and a component of PDTC/ATC, likely reflecting disease progression from a well-differentiated thyroid carcinoma to a poorly differentiated/anaplastic carcinoma in the metastatic site, a phenomenon that has been reported previously (10–12).
Several possible hypotheses might explain this rare phenomenon of metastatic disease without identifiable primary tumor within the thyroid. First, although the thyroid was entirely submitted for examination in our cohort and in two of the previously reported cases (2, 4), typically only a 4-micron histologic section was prepared from a 3-mm-thick tissue block. In other words, only approximately 0.1% of the actual tissue was prepared as hematoxylin and eosin slides and examined microscopically. Hence, a microcarcinoma of < 3 mm in size, which has the potential to develop metastases (12–14), might not be detected under routine pathologic examination despite the fact that the entire thyroid is submitted. The other possibility is tumor regression. Spontaneous tumor regression, defined as diminution of a malignant neoplasm as a consequence of host response, is a common phenomenon affecting 10–35% cutaneous melanoma (15, 16), and has also been reported in certain carcinomas, e.g. Merkel cell carcinoma (17), hepatocellular carcinoma (18), and renal cell carcinoma (19). The histologic features of melanoma regression are fibrosis, often in association with lymphocytes or macrophages (16). In our cohort, five of seven patients had evidence of fibrosis in their thyroid: four in association with benign thyroid disease while the other displayed a 0.2 cm unifocal stellate fibrosis. Similarly, Monchik et al. have also noted an area of fibrosis in the thyroid from a 29-year-old female patient who had nodal metastatic PTC without a primary thyroid carcinoma in an entirely submitted thyroid gland (4). Singh et al. reported a patient with metastatic PTC involving bone and a thyroid with chronic lymphocytic thyroiditis and extensive fibrosis (2). Additionally, several prior studies have demonstrated that intratumoral fibrosis might be present in certain papillary thyroid carcinoma, and was associated with increased incidences of lymph node metastasis and extrathyroidal invasion (20), as well as the development of distant metastases and death (12, 21). Taken together, it is tempting to speculate that fibrosis might be a sign of complete or partial tumor regression in thyroid carcinoma and might be a cause for this rare phenomenon of metastases without identifiable primary tumors.
In 4 of 6 cases tested, the metastases showed strong (3+) BRAF V600E immunostain, which has been shown to be highly specific for BRAF V600E mutation in papillary, poorly differentiated, and anaplastic thyroid carcinoma (6), Additionally, the presence of BRAF V600E mutation was confirmed at the DNA level in the metastatic anaplastic thyroid carcinoma using Sequenom mutation profiling assay. The above data shows that BRAF V600E mutation is a highly prevalent event in this cohort of metastatic thyroid carcinoma with unknown primary. The Cancer Genome Atlas (TCGA) has shown that BRAF V600E mutation was the most common genetic alteration in PTC, in particular the classical and tall cell variants (22), whereas follicular variant of PTC frequently harbored RAS alteration. Additionally, Landa et al. has shown that poorly differentiated thyroid carcinoma with BRAF mutation had a tendency to metastasize to lymph nodes, while RAS-mutated tumors were more inclined to metastasize distantly (23). Taken together, the positive BRAF immunohistochemistry in two thirds of cases tested, the existence of a PTC component in all cases, and the presence of nodal metastasis in the majority of these patients (8/9) strongly suggested that BRAF mutation was the main driver for these rare tumors. One should therefore be cautious in attributing metastatic thyroid carcinoma in the neck lymph nodes to encapsulated follicular-patterned lesions in the thyroid, e.g. follicular carcinomas, encapsulated follicular variant of papillary thyroid carcinomas with invasion, or a noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP), as these lesions are almost always associated with RAS rather than BRAF mutations (22, 24–26), and have a propensity for distant rather than locoregional spread (21). In the event of nodal metastasis in a thyroid harboring an encapsulated follicular patterned tumor (without apparent associated PTC), it is much more likely that a BRAF-related undetected or regressed papillary microcarcinoma is the source of the nodal disease. The absence of BRAF mutation in such a scenario does not automatically imply that the encapsulated follicular patterned carcinoma is the origin of the nodal disease. Indeed, classical PTC and infiltrative follicular variant PTC might lack BRAF mutation in a significant proportion of cases (27).
Two patients in our cohort harbored poorly differentiated or anaplastic thyroid carcinoma in the nodal metastasis. The presence of aggressive histology in the neck nodes or distant sites in the setting of apparently indolent very small thyroid primary has been previously reported. Piana et al. and Xu et al. described 4 cases of subcentimeter papillary microcarcinoma (0.4 to 0.8 cm in greatest dimension) with metastatic poorly differentiated thyroid carcinoma to neck lymph nodes (11, 12). One of those patients died of thyroid carcinoma. In another publication, a patient with a 0.2 cm papillary microcarcinoma, infiltrative follicular variant was found to have distant metastasis to bone at presentation (21). Therefore, it is plausible for a patient devoid of primary thyroid carcinoma to develop a poorly differentiated thyroid carcinoma or anaplastic thyroid carcinoma in the metastatic site.
In conclusion, metastatic thyroid carcinoma without primary tumor after extensive thyroid sampling is a rare but real phenomenon that might be encountered in daily practice. Its mechanism is unknown. Although most tumors are low grade and well differentiated, aggressive behavior due to a poorly differentiated or anaplastic carcinoma can happen. Two thirds of cases are BRAF V600E positive thyroid tumors with a papillary carcinoma phenotype present in all reported cases. Lymph node metastasis in the setting of an encapsulated follicular patterned tumor (without apparent associated separate PTC foci) could have originated from BRAF-related undetected or regressed papillary microcarcinoma. In that case scenario, genotyping or immunstaining the metastasis for BRAFV600E could help direct the pathologist to the source of the metastasis.
Figure 2.
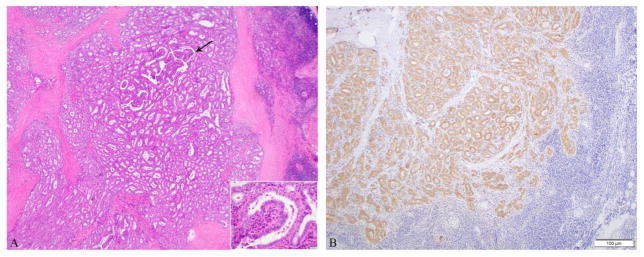
Microscopic pictures of a poorly differentiated thyroid carcinoma composed of a papillary thyroid carcinoma, columnar cell phenotype with high mitotic rate (6 mitosis/10 high power fields, 400x) presenting as a 4.5 cm left neck mass in a 76 year old female. No carcinoma was found in the thyroidectomy A: Papillae lined by columnar cells with pseudostratified nuclei associated with mitosis (arrow). B: BRAF V600E immunostain was positive in the tumor.
Highlights.
Metastatic thyroid carcinoma without identifiable primary in thyroid does occur.
Two thirds of these metastases are BRAF V600E positive.
All reported cases have a papillary carcinoma component.
Although most metastatic tumors are low grade, higher grade ones can be found.
The mechanism of this rare phenomenon is unknown.
Acknowledgments
Funding: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No competing financial interests exist for all contributory authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito Y, Hirokawa M, Fukushima M, Inoue H, Yabuta T, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Occult papillary thyroid carcinoma: diagnostic and clinical implications in the era of routine ultrasonography. World journal of surgery. 2008;32:1955–1960. doi: 10.1007/s00268-008-9614-9. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa M, Toyoda N, Yonemoto T, Fujiyama A, Ogawa Y, Tokoro T, Sakaguchi N, Yoshimura M, Yoshikawa N, Tabata S, Kumazawa H, Yamashita T, Sakaida N, Okamura A, Kasagi K, Inada M. Occult papillary thyroid carcinoma in Hashimoto's thyroiditis presenting as a metastatic bone tumor. Endocrine journal. 1998;45:111–116. doi: 10.1507/endocrj.45.111. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Lv L, Yang K. Occult thyroid carcinoma: a rare case report and review of literature. Int J Clin Exp Pathol. 2014;7:5210–5214. [PMC free article] [PubMed] [Google Scholar]
- 4.Monchik JM, De Petris G, De Crea C. Occult papillary carcinoma of the thyroid presenting as a cervical cyst. Surgery. 2001;129:429–432. doi: 10.1067/msy.2001.112965. [DOI] [PubMed] [Google Scholar]
- 5.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 6.Ghossein RA, Katabi N, Fagin JA. Immunohistochemical detection of mutated BRAF V600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. The Journal of clinical endocrinology and metabolism. 2013;98:E1414–1421. doi: 10.1210/jc.2013-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. The Journal of molecular diagnostics : JMD. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellegara G, Rosai J. Multifocal fibrosing thyroiditis: report of 55 cases of a poorly recognized entity. The American journal of surgical pathology. 2015;39:416–424. doi: 10.1097/PAS.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Butuc R, Lopez R. Metastatic papillary thyroid carcinoma with absence of tumor focus in thyroid gland. Am J Case Rep. 2013;14:73–75. doi: 10.12659/AJCR.883834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, GT . Tumor of the thyroid and parathyroid gland (AFIP atlas of tumor pathology series 4) Silver Spring, MD: American Registry of Pathology Press; 2015. [Google Scholar]
- 11.Xu B, Ibrahimpasic T, Wang L, Sabra MM, Migliacci JC, Tuttle RM, Ganly I, Ghossein R. Clinicopathologic Features of Fatal Non-Anaplastic Follicular Cell-Derived Thyroid Carcinomas. Thyroid : official journal of the American Thyroid Association. 2016;26:1588–1597. doi: 10.1089/thy.2016.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, Rosai J. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Human pathology. 2013;44:556–565. doi: 10.1016/j.humpath.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid : official journal of the American Thyroid Association. 2014;24:245–253. doi: 10.1089/thy.2012.0645. [DOI] [PubMed] [Google Scholar]
- 14.Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, McCabe C, Boelaert K, Franklyn JA. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. The Journal of clinical endocrinology and metabolism. 2014;99:2834–2843. doi: 10.1210/jc.2013-2118. [DOI] [PubMed] [Google Scholar]
- 15.Ribero S, Gualano MR, Osella-Abate S, Scaioli G, Bert F, Sanlorenzo M, Balagna E, Fierro MT, Macripo G, Sapino A, Siliquini R, Quaglino P. Association of Histologic Regression in Primary Melanoma With Sentinel Lymph Node Status: A Systematic Review and Meta-analysis. JAMA Dermatol. 2015;151:1301–1307. doi: 10.1001/jamadermatol.2015.2235. [DOI] [PubMed] [Google Scholar]
- 16.Ribero S, Moscarella E, Ferrara G, Piana S, Argenziano G, Longo C. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30:2030–2037. doi: 10.1111/jdv.13815. [DOI] [PubMed] [Google Scholar]
- 17.Walsh NM. Complete spontaneous regression of Merkel cell carcinoma (1986–2016): a 30 year perspective. J Cutan Pathol. 2016;43:1150–1154. doi: 10.1111/cup.12812. [DOI] [PubMed] [Google Scholar]
- 18.Pectasides E, Miksad R, Pyatibrat S, Srivastava A, Bullock A. Spontaneous Regression of Hepatocellular Carcinoma with Multiple Lung Metastases: A Case Report and Review of the Literature. Dig Dis Sci. 2016;61:2749–2754. doi: 10.1007/s10620-016-4141-2. [DOI] [PubMed] [Google Scholar]
- 19.Janiszewska AD, Poletajew S, Wasiutynski A. Spontaneous regression of renal cell carcinoma. Contemp Oncol (Pozn) 2013;17:123–127. doi: 10.5114/wo.2013.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda M, Mikami T, Numata Y, Okamoto M, Okayasu I. Papillary thyroid carcinoma with heterotopic ossification is a special subtype with extensive progression. American journal of clinical pathology. 2013;139:587–598. doi: 10.1309/AJCPQZQN50HKIAHA. [DOI] [PubMed] [Google Scholar]
- 21.Xu B, Tuttle RM, Sabra MM, Ganly I, Ghossein R. Primary Thyroid Carcinoma with Low-Risk Histology and Distant Metastases: Clinicopathologic and Molecular Characteristics. Thyroid : official journal of the American Thyroid Association. 2017 doi: 10.1089/thy.2016.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW, 2nd, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. The Journal of clinical endocrinology and metabolism. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 26.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. The American journal of surgical pathology. 2006;30:216–222. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]