Abstract
Purpose
Despite accumulating evidence from experimental animal studies showing that paternal environmental exposures induce genetic and epigenetic alterations in sperm which in turn increase the risk of adverse health outcomes in offspring, there is limited epidemiological data on the effects of human paternal preconception exposures on children’s health. We summarize animal and human studies showing that paternal preconception environmental exposures influence offspring health. We discuss specific approaches and designs for human studies to investigate the health effects of paternal preconception exposures, the specific challenges these studies may face, and how we might address them.
Recent Findings
In animal studies, paternal preconception diet, stress, and chemical exposures have been associated with offspring health and these effects are mediated by epigenetic modifications transmitted through sperm DNA, histones, and RNA. Most epidemiological studies have examined paternal preconception occupational exposures and their effect on the risk of birth defects and childhood cancer; few have examined the effects of low-level general population exposure to environmental toxicants. While the design and execution of epidemiological studies of paternal preconception exposures face challenges, particularly with regard to selection bias and recruitment, we believe these are tractable and that preconception studies are feasible.
Summary
New or augmented prospective cohort studies would be the optimal method to address the critical knowledge gaps on the effect of paternal preconception exposures on prevalent childhood health outcomes. Determining if this period of life represents a window of heightened vulnerability would improve our understanding of modifiable risk factors for children’s health and wellbeing.
Keywords: Children’s health, chemical exposures, epidemiology, epigenetics, paternal, preconception, prenatal
Introduction
Homer Simpson, patriarch of the animated television show, The Simpsons, once said, “I never thought of fatherhood as something that could affect a kid.” We believe this quote aptly describes why there are limited epidemiological studies focused on the potential for paternal environmental exposures to affect children’s health. The notion of male-mediated developmental toxicity was first described over two decades ago by Olshan and Faustman,1 but the field has advanced little since then. Consequently, there is limited human data on the health effects of paternal preconception exposures, despite older literature showing that environmental exposures can induce sperm DNA mutations and growing evidence from recent experimental animal studies demonstrating that environmental exposures to the father may affect offspring health via epigenetic alterations transmitted through sperm.2–7
Given the potential for paternal environmental exposures to adversely affect the health of subsequent generations, there is a critical need for epidemiological studies to investigate this understudied and underappreciated contribution to children’s health. Existing cohorts must be augmented or new cohorts must be established to address hypotheses that will help achieve a greater understanding of the role that father’s preconception exposure plays in child health. We believe the opportunity is ripe as many established and mature cohorts examining the health effects of maternal exposures during pregnancy are reaching the end of their childhood follow-up and the majority of these studies did not assess paternal exposures and the extent they may affect child health outcomes.
In this commentary, we briefly summarize some relevant accumulating experimental studies in animals and the sparse epidemiological studies that have examined paternal preconception exposures and child health to provide a rationale for studying this potentially important window of exposure. We then discuss how we can use epidemiological studies to investigate paternal preconception exposures, the specific challenges these studies face, and how we might address them.
Paternal Preconception Exposures and Offspring Health
Recent experimental studies in animals and observational studies in humans challenge two traditional paradigms in developmental programming: 1) the in utero and early life environment is the primary determinant of child and subsequent adult health and 2) paternal factors can only influence child health via Mendelian inheritance. This first assumption is challenged by recent animal and human studies showing that paternal preconception stress, diet, and exposure to environmental toxicants are associated with hypothalamic-pituitary-adrenal (HPA) axis function, birth defects, childhood cancers, growth, obesity, and cardiometabolic risk markers in offspring.
Experimental studies in animals have challenged the second assumption by showing that epigenetic modifications encoded in sperm (and oocytes) are heritable and influence offspring phenotypes. The origins of this hypothesis likely lies in the history of investigating the impact of environmental chemical exposures on male fertility. Indeed, there are suggestions that exposure to lead, phthalates, and some pesticides may have detrimental effects on male fertility, including reduced semen quality and decreased time to pregnancy.8,9
While sperm has traditionally been thought of as merely a vehicle for transporting 23 chromosomes, it also carries an epigenetic cargo consisting of methylated DNA, non-coding RNAs, protamines, and histones that are critical to fertilization and programming early embryonic development.10 For instance, despite each sperm carrying only 5–10 fg of RNA, compared to 1 ng of maternal RNA in the oocyte,11 there is evidence that paternally-derived RNAs play an important role in the development of obesity, metabolic disorders, and stress responses in animals.12–15 Thus, environmental chemical exposures may affect the sperm epigenome by altering DNA methyltransferase or histone deacetylase activity, interfering with hormonal regulation of sperm development, or inducing oxidative stress that results in mitochondrial or nuclear DNA damage.
In light of mounting evidence, it is plausible that some preconception environmental exposures could increase the risk of disease via epigenetic modifications of the germline. Evolutionarily, it makes sense for information about the fathers’ (and mothers’) preconception experiences to be passed onto their offspring to improve fitness and survival. Below we describe how paternal exposure to three broad classes of environmental stressors – psychosocial stress, diet, and environmental chemicals – impacts offspring health in animal or epidemiological studies.
Psychosocial Stress
In a series of animal studies, paternal stress before conception was associated with changes in sires’ sperm miRNAs, decreased HPA axis response in offspring following an acute stressor, and increased expression of glucocorticoid-responsive genes in the brain of the offspring.16 In a follow-up study, the offspring phenotypes were recapitulated by injecting sperm miRNAs from stress-exposed fathers into zygotes created through in vitro fertilization (IVF) using control mice.17 In another study, male mice were conditioned to fear the scent of acetophenone.18 Both F1 and F2 male offspring feared acetophenone at the initial challenge, despite never having smelled the chemical directly. Changes in the olfactory system neuroanatomy specific to acetophenone accompanied these phenotypic changes in the offspring, as well as hypomethylation of genes in the offspring’s sperm specific to this pathway. In experiments using IVF to create embryos with sperm from male mice conditioned to fear the scent of acetophenone, the authors confirmed paternal transmission of these effects to offspring.
In a series of epidemiologic studies among adults born to Holocaust survivors, post-traumatic stress disorder (PTSD) in mothers and fathers was associated with increased risk of PTSD, lower levels of urinary cortisol, increased glucocorticoid sensitivity, and lower methylation of the glucocorticoid receptor gene in offspring.19–24
While the context of environmental stressors experienced by rodents and humans in these studies differs, stress has been associated with epigenetic alterations in both rodents and humans, thus providing evidence that paternal environmental experiences could be transmitted through the sperm epigenome.
Diet
Several human and rodent studies have examined the effect of paternal preconception diet on offspring adiposity, obesity or related cardiometabolic outcomes. Ng and colleagues showed that female offspring sired by males fed a high fat diet had impaired glucose tolerance and insulin secretion during a glucose tolerance test.25 These same females had reduced beta cell islet areas and altered expression of genes involved in regulatory pathways associated with insulin and glucose metabolism.26 Others have reported that a high fat diet during postnatal life modifies some of the metabolic derangements associated with a paternal preconception high fat diet.27 At the other end of the nutritional spectrum, paternal preconception fasting was associated with alterations in offspring serum glucose levels.28 Furthermore, Carone and colleagues showed that sires fed a low protein diet had offspring with elevated hepatic expression of genes involved in lipid and cholesterol synthesis and decreased cholesterol ester concentrations in the liver.29 In a follow-up study, they injected sperm or tsRNA purified from sperm obtained from sires fed the low protein diet into control oocytes or zygotes created using IVF and showed that a low protein paternal diet affected preimplantation embryo gene regulation.30
Altered metabolic states in the father before conception can also affect offspring disease susceptibility in animal models. Wei and colleagues showed that paternal pre-diabetes caused impaired glucose tolerance and insulin resistance in offspring and altered methylation and expression of genes involved in glucose-insulin homeostasis in the pancreatic islet cells of these offspring.31 Many of these same genes were hyper- or hypomethylated in the sperm DNA of pre-diabetic fathers, suggesting that DNA methylation may be one mechanism that paternal preconception environmental exposures programs offspring phenotypes.
In humans, two studies from the same cohort examined food availability in paternal grandparents and the risk of mortality in grandchildren.32,33 The authors reported sex-specific, grandparent of origin effects, where sufficient food availability during the grandfather or grandmother’s childhood was associated with increased mortality in the grandson or granddaughter, respectively. There was an absence of association of the grandfather’s food availability with granddaughter’s mortality, and vice versa, suggesting sex-specific, parent-of-origin transmission.
Environmental Toxicants
Numerous epidemiologic studies over the last several decades have examined the risk of birth defects, childhood cancers, and adverse neonatal health outcomes associated with paternal occupational exposures before or during pregnancy.1,34–37 Some studies suggest an increased risk of male genital malformations among infants born to fathers with occupational exposure to polychlorinated compounds and heavy metals.38,39 In a nested case-control study, Pierik and colleagues reported that paternal exposure to pesticides was associated with nearly 4-fold increased risk of cryptorchidism in male infants, while maternal exposure was not.40 In a retrospective follow-up of 71 New Zealand Malaysian veterans, Carran and Shaw reported that veterans who had applied di-n-butyl phthalate as an insecticide during military operations were more likely to have sons with cryptorchidism or hypospadias compared to the general population.41 Several case-control studies have reported increased risk of several types of childhood cancer among fathers with occupational exposure to benzene, aniline, creosote, diesel fuel, turpentine, lacquer thinner, insecticides, fungicides, and herbicides.42–44 Pooled- and meta-analyses show that the risk of childhood leukemia is elevated among children born to fathers with preconception benzene and pesticide exposures.45,46 Finally, there is some evidence that paternal occupational exposure to radiation, chromium, and benzene may be associated with increased risk of preterm delivery, low birthweight, or being small for gestational age.47,48
Despite a large body of epidemiological evidence examining paternal occupational exposures and birth defects or childhood cancers, there are very few prospective studies examining non-occupational general population environmental exposures in fathers and offspring health. Investigators from the LIFE study assembled a cohort of couples recruited before conception to examine the relationship of preconception persistent and non-persistent chemical exposures with time-to-pregnancy and neonatal anthropometry.49,50 Men’s preconception exposure to some phthalates, polychlorinated biphenyls (PCBs), and lead was associated with increased time to pregnancy, even after adjusting for the female partner’s exposure to these chemicals.50 Paternal preconception serum concentrations of PCBs, polybrominated diphenyl ethers, and dichlorodiphenyltrichloroethane were associated with reduced birth weight in this cohort as was paternal preconception urinary monoethylhexyl phthalate concentrations.49,51 Several paternal chemical exposures were associated with altered secondary sex ratio in this same cohort.52
Considerations of the Current Literature
The current literature has several limitations that impede our understanding of the human health impacts of paternal preconception environmental exposures. First, virtually no studies have examined the potential effects of paternal exposures on neurodevelopment, asthma/allergy, adiposity, or cardiometabolic function; instead most focused on birth defects, childhood cancer, and neonatal outcomes. A second limitation is that most prior research has examined the effects of only occupational exposures and few studies have examined exposure to the multitude of environmental toxicants that the general population of men are exposed to on a daily basis, typically at low-levels.53 Recent advances in analytic chemistry techniques have revolutionized the study of low-level environmental chemical exposures and allow investigators to quantify concentrations in a variety of biospecimens with great sensitivity and specificity.54 Another factor to consider is the difficulty disentangling the effect of paternal preconception exposures from maternal preconception or prenatal exposures. Often, environmental exposures are correlated within a couple because of shared residence, diet, and lifestyle.55 While animal studies can address this by mating exposed males with an unexposed dam, it is important to consider the impact of such co-exposure in human studies where both partners may share similar environments. Therefore, this necessitates epidemiological studies that measure, consider, and adjust for both partners’ exposures.
Integral to the study of paternal preconception exposure is the need to expand our understanding of windows of heightened vulnerability within the context of spermatogenesis. Epidemiological (and animal) studies need to carefully consider timing in the design and analysis of paternal exposure. To date, epidemiological studies have predominately examined exposures occurring shortly before conception and assumed that their levels and effects during spermatogenesis are constant. This is a justifiable starting point for identifying preconception paternal exposures that influence child health, but additional studies with more extensive preconception exposure assessment may be required to identify discrete periods of susceptibility during spermatogenesis. Furthermore, it is also important to consider exposures occurring months or years earlier, including prenatal or pubertal development.
Finally, renewed efforts to study paternal preconception exposures should not come at the expense of continued examination of maternal prenatal and preconception exposures. Indeed, they should (and could) be studied in parallel and complement each other. Just as maternal exposures may confound paternal exposures, the reverse may also be true. We believe that paternal exposures are critical to study because their potential health effects are largely unknown despite growing evidence in animal studies demonstrating their importance. In addition, the effects and biological mechanisms of paternal preconception exposures may be easier to isolate than maternal preconception exposures in epidemiological studies for reasons described below.
Epidemiological Studies of Paternal Preconception Exposures
Study Design
Most epidemiological studies have focused on paternal preconception occupational exposures with only a limited number of neonatal or child health outcomes. These studies are further limited by case-control designs and are restricted by the number and type of exposures that can be accurately assessed using questionnaires or other available data. Below we describe five study designs that could be used to advance our understanding of the impact that paternal preconception exposures have offspring health (see Table 1 for summary).
Table 1.
Description of, strengths, and limitations of epidemiological study designs to examine the association between paternal preconception environmental exposures and childhood health
| Design/Method | Description | Strengths | Limitations |
|---|---|---|---|
| Population-based prospective cohort | Enroll couples planning or trying to become pregnant; prospective exposure and covariate assessment; continued follow-up during before conception and during gestation, infancy, and childhood. |
Prospective exposure assessment; ability to choose exposure(s), outcome(s), and covariates; ability to collect biological intermediates (i.e., semen). |
Potential for selection bias; loss to follow-up; can be logistically intensive to enroll and follow-up couples trying to become pregnant; potential for exposure misclassification of episodic/non-persistent exposures. |
| Clinic-based prospective cohort | Enroll couples who are seeking treatment for infertility; prospective exposure and covariate assessment; continued follow-up during fertility treatment cycles, pregnancy, and infancy/childhood. |
Prospective exposure assessment; ability to choose exposure(s), outcome(s), and covariates; clinically-defined population can make identifying, enrolling, and following participants easier to accomplish; ability to collect biological intermediates (i.e., semen). |
Potential for selection bias; loss to follow-up; potential for infertility and fertility treatments to be associated with paternal exposures and child health; potential for exposure misclassification of episodic/non-persistent exposures. |
| Case-control | Enroll cases and controls of specific fetal, neonatal, infant, or child disease; retrospectively assess exposure(s). |
Ability to study clinical diseases; feasible and efficient design in clinic and population settings; ability to follow children for assessment of other health outcomes. |
Limited to studying a single disease; limited number and types of exposures that can be adequately characterized retrospectively (e.g., self- reported occupation vs. biomarkers of low-level exposures); exposure misclassification (e.g., recall bias). |
| Recruit subsequent siblings in existing cohorts | Enroll fathers from existing prospective birth cohorts; assess exposure(s) after birth of index child; follow subsequent infants/children. |
Ability to recruit from defined population; high likelihood of subsequent pregnancies; prospective exposure assessment; ability to choose exposure(s), outcome(s), and covariates; ability to collect biological intermediates (i.e., semen). |
Potential for selection bias; logistically intensive for couples with long latency between pregnancies; potential for exposure misclassification of episodic/non-persistent exposures; cannot examine first pregnancies. |
| Retrospective exposure assessment in existing cohorts |
Conduct follow-up on children from existing cohort or case- control studies; retrospectively assess paternal exposure using questionnaires, mathematical modeling, stored biospecimens, etc. |
Feasible and efficient design, especially if infant/child follow-up has begun; defined population; ability to choose outcome(s). |
Limited number and types of exposures that can be adequately characterized retrospectively (e.g., self- reported occupation vs. biomarkers of low-level exposures); potential for exposure misclassification of episodic/non-persistent exposures; availability and assessment of important paternal covariates before conception. |
Population-Based Prospective Cohort Study
We believe that prospective cohort studies with preconception enrollment are the optimal method for quantifying the potential effects of paternal preconception exposure on prevalent health outcomes in children since investigators can prospectively collect biospecimens, measure potential confounders and covariates, and assess maternal preconception and prenatal environmental exposures. There has been mixed experiences with executing prospective preconception studies in the field as we discuss below.
The single largest effort to study maternal and paternal preconception exposures came during the development and implementation of the National Children’s Study (NCS) in the early 2000s. The NCS planned to enroll a nationally-representative prospective cohort of pregnant women, with a subset of several thousand couples enrolled before pregnancy.56 While the NCS was ultimately abandoned, the pilot work revealed that while preconception enrollment was possible, it required intensive efforts for enrollment and follow-up and most women did not complete baseline exposure assessment within 30 days of conception.57
In contrast to the NCS experience, numerous investigators have successfully enrolled women or couples before conception to study the impact of preconception exposures on fertility, pregnancy loss, and in some cases, neonatal outcomes. In 2004, Buck identified 15 studies with preconception enrollment of women or couples that were followed until pregnancy or up to 12 menstrual cycles.58 Subsequently, Buck-Louis and colleagues initiated the LIFE Study and demonstrated that it was possible to identify and enroll 501 Michigan and Texas couples who were trying to conceive by screening and recruiting couples listed in commercial and government databases. However, they had to contact and screen >400,000 individuals to identify 1,184 eligible couples.59 More recently, internet-based advertising for recruiting and web-based platforms for questionnaire administration have emerged as technologies to establish preconception cohorts. This design has the benefit of not having to screen and identify eligible participants since targeted advertisements can be tailored towards couples trying to conceive on specific websites (e.g., The Bump) or through social media (e.g., Facebook). Indeed, Wise and colleagues used this method in the PRESTO study and enrolled 2,421 of 3,805 screened women who were trying to conceive.60 Of those who enrolled, 1,384 invited their male partner to participate and 693 of these men agreed and completed a questionnaire related to demographics, medical history, lifestyle, and other factors that might influence fertility. In both PRESTO and the LIFE Study, the investigators also demonstrated that participants are willing and able to provide biological specimens at designated laboratories (PRESTO) or at home when given supplies and instructions (LIFE).
Additional limitations and challenges of prospective cohorts include selection bias, maintaining sufficient statistical power over the course of follow-up during preconception, pregnancy, and childhood, and accurate exposure assessment during lengthier preconception periods, especially for non-persistent chemicals.
Clinic-Based Prospective Cohort Study
An alternative to traditional population-based designs is recruiting couples seeking fertility treatments and attempting conception at a clinical facility. One major advantage of this design is that it reduces the need to invest resources into identifying couples at risk of becoming pregnant. Given than >70,000 children in the United States were born following conception with assisted reproductive technology (ART) in 2014 (~1.6% of all births),61 this clinical population remains a feasible target for preconception exposure studies. Using the EARTH Study, a prospective preconception cohort of couples, we have previously shown that both men and women are very willing to actively participate in research by providing biospecimens during fertility treatments, and continue follow-up over the course of pregnancy.55,62 Among EARTH Study couples who have a live-born infant, most were willing to continue participating in follow up of their children. In a pilot study of 257 families from the EARTH Study, 201 (78%) agreed to participate and to date 138 (54%) completed questionnaires that were returned by mail.
This design has the same strengths and limitations of population-based prospective cohort studies and the additional limitation that underlying infertility itself or its treatments may be related to both paternal preconception exposures and child health.
Prospective Cohort of Subsequent Siblings
Enrolling subsequent siblings from ongoing prospective pregnancy and birth cohort studies is an alternative to starting an entirely new preconception cohort.56 In this design, assessment of paternal exposure could begin after enrollment of the mother during pregnancy or after delivery of the index child and continue until the birth of a subsequent child. This design has the advantages of following fertile couples who have an above average likelihood of having another child and being able to conduct prospective exposure assessment. Limitations are similar to prospective designs and an additional limitation includes the inability to examine first pregnancies in couples.
Retrospective Assessment of Paternal Exposures in Existing Cohorts
A fourth design could retrospectively assess paternal exposures in existing case-control or cohort studies. This method is both feasible and efficient, especially if child follow-up has already begun. However, the number and types of exposures that can be examined presents a major challenge to this design. Questionnaires or existing records can be used to retrospectively assess some environmental exposures, but these may lack sensitivity or specificity for some low-level and/or non-persistent environmental chemical exposures.
Mathematical or pharmacokinetic modeling are promising new alternatives to assess paternal preconception exposures. Many persistent chemicals, such as polybrominated diphenyl ethers, have long biological half-lives (i.e., years). Thus, paternal exposure after conception may be informative of paternal preconception exposure. Some investigators have employed predictive or pharmacokinetic modeling techniques to reconstruct prenatal or childhood exposure to persistent environmental pollutants nearly a decade after measurement of the initial exposure.63,64 For instance, Verner and colleagues used Super Learner, a machine-learning algorithm, to explain 95% of the variance in mother’s p,p’-dichlorodiphenyltrichloroethylene (p,p’-DDE) concentrations during pregnancy using maternal-level covariates (e.g., parity) and children’s p,p’-DDE concentrations at 9 years of age. Thus, it may be possible to reconstruct father’s preconception exposure to some persistent environmental chemicals in existing case-control and cohort studies of child health.
New Follow-up of Existing Male Fertility Cohorts
Finally, many existing studies have examined male reproductive health outcomes.58,65,66 Most of these studies did not continue following participants after conception or delivery. Thus, new child cohorts could be established to investigate the impact of paternal preconception exposures by leveraging existing biospecimens collected from fathers and conducting new follow-up on children born to participants. Limitations are similar to prospective designs; an additional limitation includes the possibility of low response rates, especially if there has been a long latency between study inception and child follow-up.
Limitations and Challenges in Epidemiological Studies
Epidemiological studies of paternal (and maternal) preconception exposure face several unique challenges related to recruitment, follow-up, selection bias, and generalizability.
Enrolling couples at risk of becoming pregnant into population-based prospective preconception cohort studies can be logistically challenging because about 10% of reproductive age women in the United States become pregnant each year and about 63% of these are intended pregnancies.67,68 With a relatively small proportion of couples actively trying to conceive at a given time, large source populations may be needed to ensure that a sufficiently large enough number of participants can be enrolled before conception. In addition, studies trying to recruit fathers face unique challenges such as questions surrounding paternity and confirming that they are the biological father. Furthermore, recruiting men of reproductive age is challenging due to their lack of interest in participating in research studies. Despite this, prior studies described above demonstrate the feasibility of enrolling fathers in research studies.
Furthermore, only those couples who conceive, maintain their pregnancy, and have a live birth will be eligible for any child follow-up and censoring at these times could reduce the study’s sample size or induce selection bias. Thus, investigators should design preconception cohort studies to account for this censoring and reduction in sample size before offspring are born. Additionally, sample size calculations should consider loss to follow-up over the course of childhood.
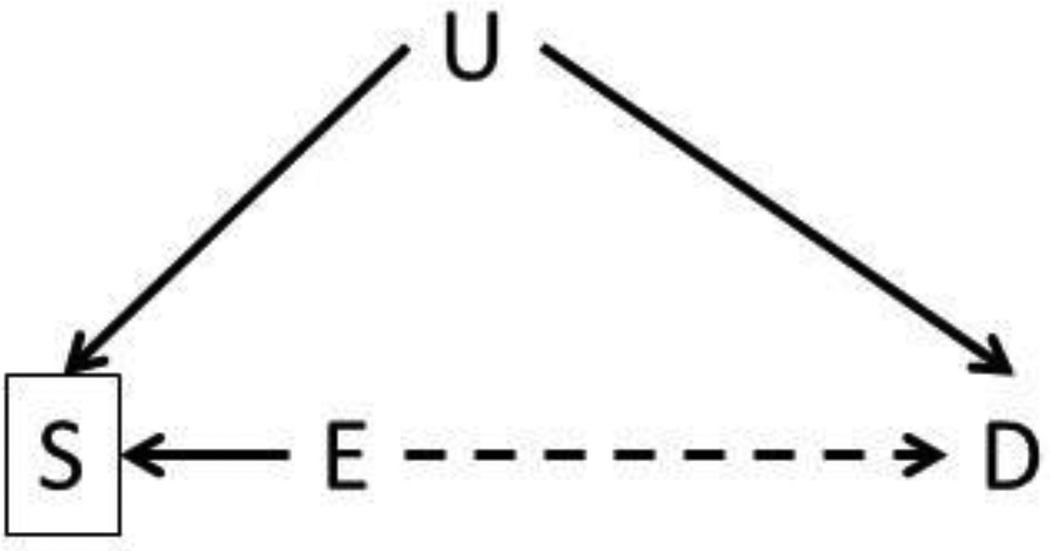
The interpretation of associations between preconception exposures and child health outcomes also need to consider the potential for selection bias. As discussed by Hatch and colleagues, selection bias in preconception studies may arise when conditioning on a selection factor that is indirectly or directly caused by exposure and other measured or measured factors associated with child health (Figure 1).69 For instance, selection bias may be present when recruiting couples who intend to get pregnant because they may be different than couples who unintentionally conceive in terms of socioeconomic factors associated with both environmental exposures and child health. Reassuringly, three groups of investigators used Scandinavian cohorts and corresponding registry data to show that selection biases do not influence the association between a multitude of peri- or prenatal risk factors and maternal or child health outcomes in studies with preconceptional or prenatal enrollment.69–71
Figure 1. Example illustrating the potential for selection bias to influence studies of paternal preconception environmental exposures and child health.
In this figure, E is the exposure of interest (e.g., paternal smoking), S is some selection factor of study eligibility, D is a child health outcome, and U is and unknown or unmeasured factor that causes both selection and the child health outcome.
Selection bias may also affect studies recruiting couples from fertility clinics if their infertility diagnosis, infertility treatments, or factors predictive of these are associated with both child health and preconception environmental exposures. Adjusting for these factors or restricting to couples who receive less intensive fertility treatments (e.g., intrauterine insemination) may reduce these potential biases.
Consideration of live-birth bias may also be necessary in studies of preconception exposures. For example, associations between environmental exposures and child health might appear protective if the exposure is associated with reduced fecundability or fetal loss.72 However, the relevance of such considerations is questionable since the population at risk is live-born children and the fetuses-at-risk approach can produce biased estimates for postnatal outcomes.73,74
While statistical generalizability of preconception cohorts may be a concern, we do not believe this should take precedence over internal validity.75 By analogy, Doll and Hill examined the mortality of British doctors by smoking habits and the results were scientifically generalizable despite being conducted on a group of male physicians from the United Kingdom.76 Indeed, recruiting couples trying to conceive may offer some advantages since the higher degree of parental investment may increase willingness to participate in studies and decrease the variability of socioeconomic factors associated with child health and development, thus reducing one potential source of confounding.
Finally, studying children conceived with ART is of public health importance because there is concern that certain fertility treatments might be associated with neurodevelopmental disorders like autism spectrum disorder.77,78 Children conceived with ART represent a large and growing segment of the population with over 4 million babies born worldwide since 1978. In the United States, these children account for about 1.6% of births per year.61,77 Thus, studies of preconception exposures among children conceived with fertility treatments are needed to address this potentially sensitive subgroup.
Future Opportunities
The study of paternal preconception exposure to environmental toxicants offers some unique opportunities that are not possible in studies of maternal preconception or prenatal exposures. First, maternal preconception environmental exposures are often difficult to separate from prenatal exposure, particularly for persistent chemicals that have long biological half-lives (e.g., PCBs). Thus, it would be challenging, if not impossible, to determine whether maternal preconception or prenatal exposure impacts the offspring since serum levels of these chemicals will be very stable before conception and during pregnancy.
Second, in many instances, investigators can collect preconception semen samples, whereas oocyte collection is precluded apart from the setting of a fertility clinic. A fertility clinic study could collect a portion of the semen sample used for insemination/fertilization that led to the livebirth. This could allow for studies of sperm epigenetic mechanisms that might be impacted by environmental exposures and in turn would directly link paternal preconception environmental exposures with child health. Examples of informative sperm epigenetic mechanisms include DNA methylation, different types of RNAs, and sperm proteins.79,80
Third, the short duration of spermatogenesis, ~70 days, offers the opportunity to focus on a narrow window of exposure, much like studies of prenatal exposures. However, it is important to consider that other relevant paternal preconception exposures may be outside this window.
Finally, this review should serve as a call for more collaborative efforts between epidemiologists, toxicologists, and basic scientists to identify candidate toxicants that should be investigated, relevant models of exposure and child health outcomes, and windows of heightened susceptibility.
Conclusion
Despite compelling experimental and limited epidemiologic data demonstrating paternal preconception occupational exposures, diet, and stress affect offspring health, there are almost no epidemiological studies examining the health effects of environmental toxicants during this unique period of potentially heightened developmental susceptibility. We believe that new or augmented prospective cohort studies would be the optimal method to address this critical knowledge gap. Furthermore, a fuller understanding of fathers’ contribution to their children’s health will be relevant to healthcare providers advising couples on lifestyle decisions prior to conception.
Acknowledgments
NIEHS grants R00 ES020346, R01 ES024381, R01 ES025214, R01 ES022955, P01 ES000002, and R01 ES009718. We thank David Savitz for his helpful feedback on an earlier version of this commentary.
Footnotes
Conflict of Interest
Joseph M. Braun, Carmen Messerlian, and Russ Hauser each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Olshan AF, Faustman EM. Male-mediated developmental toxicity. Annual review of public health. 1993;14:159–181. doi: 10.1146/annurev.pu.14.050193.001111. [DOI] [PubMed] [Google Scholar]
- 2.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nature reviews Genetics. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Twinn DS, Constancia M, Ozanne SE. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Semin Cell Dev Biol. 2015;43:85–95. doi: 10.1016/j.semcdb.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends in molecular medicine. 2015;21:134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puri D, Dhawan J, Mishra RK. The paternal hidden agenda: Epigenetic inheritance through sperm chromatin. Epigenetics. 2010;5:386–391. doi: 10.4161/epi.5.5.12005. [DOI] [PubMed] [Google Scholar]
- 7.Krawetz SA. Paternal contribution: new insights and future challenges. Nature reviews Genetics. 2005;6:633–642. doi: 10.1038/nrg1654. [DOI] [PubMed] [Google Scholar]
- 8.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol. 2014;44:467–498. doi: 10.3109/10408444.2013.875983. [DOI] [PubMed] [Google Scholar]
- 9.Snijder CA, te Velde E, Roeleveld N, Burdorf A. Occupational exposure to chemical substances and time to pregnancy: a systematic review. Hum Reprod Update. 2012;18:284–300. doi: 10.1093/humupd/dms005. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction. 2012;143:727–734. doi: 10.1530/REP-11-0450. [DOI] [PubMed] [Google Scholar]
- 11.Krawetz SA, Kruger A, Lalancette C, et al. A survey of small RNAs in human sperm. Human reproduction (Oxford, England) 2011;26:3401–3412. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HB, Chen HY, Su PH, et al. Fetal and Childhood Exposure to Phthalate Diesters and Cognitive Function in Children Up to 12 Years of Age: Taiwanese Maternal and Infant Cohort Study. PloS one. 2015;10:e0131910. doi: 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gapp K, Jawaid A, Sarkies P, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandjean V, Fourre S, De Abreu DA, Derieppe MA, Remy JJ, Rassoulzadegan M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep. 2015;5:18193. doi: 10.1038/srep18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen O, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nature Reviews: Genetics. 2016 doi: 10.1038/nrg.2016.106. A review of the biological mechanisms that paternal exposures might be transmitted to offspring via sperm.
- 16. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. Animal study showing that paternal preconception stress exposure can induce epigenetic changes in father’s sperm and phenotypic changes in offspring.
- 17. Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1508347112. Animal study showing that offspring phenotypic changes observed in response to paternal stress could be created by injecting sperm miRNAs from stressed fathers into zygotes created from control animals.
- 18. Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. This series of elegant experiments in animals showed that paternal fear of a specific scent could be transmitted to offspring via father’s sperm.
- 19.Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of psychiatric research. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehrner A, Bierer LM, Passarelli V, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierer LM, Bader HN, Daskalakis NP, et al. Elevation of 11beta-hydroxysteroid dehydrogenase type 2 activity in Holocaust survivor offspring: evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology. 2014;48:1–10. doi: 10.1016/j.psyneuen.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehuda R, Halligan SL, Bierer LM. Cortisol levels in adult offspring of Holocaust survivors: relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology. 2002;27:171–180. doi: 10.1016/s0306-4530(01)00043-9. [DOI] [PubMed] [Google Scholar]
- 23.Yehuda R, Daskalakis NP, Lehrner A, et al. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. The American journal of psychiatry. 2014;171:872–880. doi: 10.1176/appi.ajp.2014.13121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, Duvdevani T. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. The American journal of psychiatry. 1998;155:1163–1171. doi: 10.1176/ajp.155.9.1163. [DOI] [PubMed] [Google Scholar]
- 25.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 26.Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2015 doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, Lane M. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an "obesogenic" diet. Physiological reports. 2015;3 doi: 10.14814/phy2.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition. 2006;22:327–331. doi: 10.1016/j.nut.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Carone BR, Fauquier L, Habib N, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science (New York, NY. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y, Yang CR, Wei YP, et al. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci U S A. 2014;111:1873–1878. doi: 10.1073/pnas.1321195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 33.Pembrey ME, Bygren LO, Kaati G, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 34.Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol. 2009;5:17–24. doi: 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chia SE, Shi LM. Review of recent epidemiological studies on paternal occupations and birth defects. Occupational and environmental medicine. 2002;59:149–155. doi: 10.1136/oem.59.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olshan AF, van Wijngaarden E. Paternal occupation and childhood cancer. Advances in experimental medicine and biology. 2003;518:147–161. doi: 10.1007/978-1-4419-9190-4_12. [DOI] [PubMed] [Google Scholar]
- 37.Anderson D, Schmid TE, Baumgartner A. Male-mediated developmental toxicity. Asian J Androl. 2014;16:81–88. doi: 10.4103/1008-682X.122342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nassar N, Abeywardana P, Barker A, Bower C. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occupational and environmental medicine. 2010;67:585–589. doi: 10.1136/oem.2009.048272. [DOI] [PubMed] [Google Scholar]
- 39.Morales-Suarez-Varela MM, Toft GV, Jensen MS, et al. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish National Birth Cohort study. Environ Health. 2011;10:3. doi: 10.1186/1476-069X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environmental health perspectives. 2004;112:1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carran M, Shaw IC. New Zealand Malayan war veterans' exposure to dibutylphthalate is associated with an increased incidence of cryptorchidism, hypospadias and breast cancer in their children. N Z Med J. 2012;125:52–63. [PubMed] [Google Scholar]
- 42.De Roos AJ, Olshan AF, Teschke K, et al. Parental occupational exposures to chemicals and incidence of neuroblastoma in offspring. American journal of epidemiology. 2001;154:106–114. doi: 10.1093/aje/154.2.106. [DOI] [PubMed] [Google Scholar]
- 43.Feingold L, Savitz DA, John EM. Use of a job-exposure matrix to evaluate parental occupation and childhood cancer. Cancer causes & control : CCC. 1992;3:161–169. doi: 10.1007/BF00051656. [DOI] [PubMed] [Google Scholar]
- 44.van Wijngaarden E, Stewart PA, Olshan AF, Savitz DA, Bunin GR. Parental occupational exposure to pesticides and childhood brain cancer. American journal of epidemiology. 2003;157:989–997. doi: 10.1093/aje/kwg082. [DOI] [PubMed] [Google Scholar]
- 45.Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. American journal of epidemiology. 2016;183:1–14. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey HD, Fritschi L, Infante-Rivard C, et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: findings from the childhood leukemia international consortium. Int J Cancer. 2014;135:2157–2172. doi: 10.1002/ijc.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American journal of obstetrics and gynecology. 2000;182:465–472. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitz DA, Whelan EA, Kleckner RC. Effect of parents' occupational exposures on risk of stillbirth, preterm delivery, and small-for-gestational-age infants. American journal of epidemiology. 1989;129:1201–1218. doi: 10.1093/oxfordjournals.aje.a115241. [DOI] [PubMed] [Google Scholar]
- 49.Robledo CA, Yeung E, Mendola P, et al. Preconception Maternal and Paternal Exposure to Persistent Organic Pollutants and Birth Size: The LIFE Study. Environmental health perspectives. 2014 doi: 10.1289/ehp.1308016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buck Louis GM, Barr DB, Kannan K, Chen Z, Kim S, Sundaram R. Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE study. Andrology. 2016 doi: 10.1111/andr.12171. A population-based prospective cohort of couples enrolled before conception with extensive collection of questionnaires, fertility/pregnancy outcomes, and biospecimens, including semen.
- 51.Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GM. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ Health. 2015;14:73. doi: 10.1186/s12940-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graversen L, Sorensen TI, Gerds TA, et al. Prediction of adolescent and adult adiposity outcomes from early life anthropometrics. Obesity (Silver Spring, Md. 2015;23:162–169. doi: 10.1002/oby.20921. [DOI] [PubMed] [Google Scholar]
- 53.Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. 2012 at http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Feb2012.pdf.
- 54.Needham LL, Calafat AM, Barr DB. Assessing developmental toxicant exposures via biomonitoring. Basic & clinical pharmacology & toxicology. 2008;102:100–108. doi: 10.1111/j.1742-7843.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 55.Smith KW, Braun JM, Williams PL, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environmental health perspectives. 2012;120:1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selevan SG, Stanford JB. Workshop recommendations for the preconception cohort of the National Children's Study. Paediatric and perinatal epidemiology. 2006;20(Suppl 1):60–65. doi: 10.1111/j.1365-3016.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 57.Stanford JB, Brenner R, Fetterer D, Palmer L, Schoendorf KC Study USNCs. Impact of preconception enrollment on birth enrollment and timing of exposure assessment in the initial vanguard cohort of the U.S. National Children's Study. BMC medical research methodology. 2015;15:75. doi: 10.1186/s12874-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buck GM, Lynch CD, Stanford JB, et al. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environmental health perspectives. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development--the LIFE Study. Paediatric and perinatal epidemiology. 2011;25:413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatric and perinatal epidemiology. 2015;29:360–371. doi: 10.1111/ppe.12201. A large epidemiological study using internet based enrollment and data collection to study preconception risk factors in couples.
- 61.CDC. Fertility Clinic Success Rate Report: 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 62. Braun JM, Smith KW, Williams PL, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012;120:739–745. doi: 10.1289/ehp.1104139. A clinic-based prospective cohort of couples enrolled before conception with extensive collection of questionnaires, fertility/pregnancy outcomes, child healht outcomes, and biospecimens, including semen.
- 63. Verner MA, Gaspar FW, Chevrier J, et al. Increasing sample size in prospective birth cohorts: back-extrapolating prenatal levels of persistent organic pollutants in newly enrolled children. Environmental science & technology. 2015;49:3940–3948. doi: 10.1021/acs.est.5b00322. An epidemiological study demonstrating that prenatal exposure to persistent pollutants can be accurately estimated using children’s levels measured up to 9 years later.
- 64.Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA. Measured Prenatal and Estimated Postnatal Levels of Polychlorinated Biphenyls (PCBs) and ADHD-Related Behaviors in 8-Year-Old Children. Environmental health perspectives. 2015;123:888–894. doi: 10.1289/ehp.1408084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olshan AF, Perreault SD, Bradley L, et al. The healthy men study: design and recruitment considerations for environmental epidemiologic studies in male reproductive health. Fertil Steril. 2007;87:554–564. doi: 10.1016/j.fertnstert.2006.07.1517. [DOI] [PubMed] [Google Scholar]
- 66.Bonde JP, Hjollund NH, Jensen TK, et al. A follow-up study of environmental and biologic determinants of fertility among 430 Danish first-pregnancy planners: design and methods. Reproductive toxicology. 1998;12:19–27. doi: 10.1016/s0890-6238(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 67.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS data brief. 2013:1–8. [PubMed] [Google Scholar]
- 68.Mosher WD, Jones J, Abma JC. Intended and unintended births in the United States: 1982–2010. National health statistics reports. 2012:1–28. [PubMed] [Google Scholar]
- 69. Hatch EE, Hahn KA, Wise LA, et al. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology (Cambridge, Mass. 2016;27:98–104. doi: 10.1097/EDE.0000000000000400. This epidemiological analysis shows that selection bias does not greatly influence the results of well-established associations between perinatal risk factors and maternal/neonatal health in a Danish Cohort.
- 70.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology (Cambridge, Mass. 2006;17:413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 71.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and perinatal epidemiology. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 72.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. International journal of epidemiology. 2015 doi: 10.1093/ije/dyu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werler MM, Parker SE. Bias from conditioning on live-births in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants (Liew et al. 2015) International journal of epidemiology. 2015;44:1079–1080. doi: 10.1093/ije/dyv139. [DOI] [PubMed] [Google Scholar]
- 74.Basso O. Implications of Using a Fetuses-at-Risk Approach When Fetuses Are Not at Risk. Paediatric and perinatal epidemiology. 2016;30:3–10. doi: 10.1111/ppe.12254. [DOI] [PubMed] [Google Scholar]
- 75.Rothman KJ. Six persistent research misconceptions. Journal of general internal medicine. 2014;29:1060–1064. doi: 10.1007/s11606-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. British medical journal. 1954;1:1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu YH, Wang N, Jin F. Long-term follow-up of children conceived through assisted reproductive technology. J Zhejiang Univ Sci B. 2013;14:359–371. doi: 10.1631/jzus.B1200348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA. 2013;310:75–84. doi: 10.1001/jama.2013.7222. [DOI] [PubMed] [Google Scholar]
- 79.Jodar M, Sendler E, Krawetz SA. The protein and transcript profiles of human semen. Cell and tissue research. 2016;363:85–96. doi: 10.1007/s00441-015-2237-1. [DOI] [PubMed] [Google Scholar]
- 80.Wu H, Hauser R, Krawetz SA, Pilsner JR. Environmental Susceptibility of the Sperm Epigenome During Windows of Male Germ Cell Development. Curr Environ Health Rep. 2015;2:356–366. doi: 10.1007/s40572-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]