Abstract
Objective:
To evaluate the response of the vaginal mucosa with TX-004HR and its correlation with vulvar and vaginal atrophy (VVA) symptoms, and whether visual examination is a useful measure for assessing VVA.
Methods:
REJOICE was a 12-week, phase 3, multicenter, randomized, double-blind, placebo-controlled study of a vaginal, muco-adhesive, 17β-estradiol softgel capsule (TX-004HR 4, 10, and 25 μg) in postmenopausal women with VVA and moderate-to-severe dyspareunia. Treatments were self-administered vaginally once per day for 2 weeks, then twice per week for 10 weeks. The vagina was visually examined at baseline and at weeks 2, 6, 8, and 12; changes were evaluated using a 4-item scale for vaginal color, vaginal epithelial integrity, vaginal epithelial surface thickness, and vaginal secretions.
Results:
Significant improvements were observed with all three TX-004HR doses versus placebo in vaginal color (least square mean score changes of −0.96 to −1.06 for TX-004HR doses vs −0.60 for placebo at week 12), epithelial integrity (−0.97 to −1.07 vs −0.60), epithelial surface thickness (−0.94 to −1.03 vs −0.61), and secretions (−1.01 to −1.06 vs −0.64) (P < 0.001 for all comparisons at all time points). Both Pearson's correlations and logistic regression receiver-operating characteristic curve analyses significantly correlated the sum of the individual visual assessment scores with dyspareunia (P < 0.0001) and vaginal dryness (P < 0.0001) at 12 weeks.
Conclusions:
Greater improvements in the vaginal mucosa of postmenopausal women with VVA and moderate-to-severe dyspareunia were observed with TX-004HR versus placebo, and vaginal mucosa assessment scores correlated with vaginal symptoms of dyspareunia and dryness. Visual vaginal assessment by healthcare professionals is a useful measure for diagnosing VVA and assessing response to treatment.
Keywords: Dyspareunia, Estradiol, Estrogen therapy, Menopause, Vaginal atrophy, Visual examination
Depletion of estrogens during menopause can lead to symptoms of vulvar and vaginal atrophy (VVA) in women. VVA is a component of the genitourinary syndrome of menopause (GSM), and collectively includes changes to the labia majora/minora, clitoris, vestibule/introitus, vagina, urethra, and bladder.1,2 A review of surveys reported up to two-thirds of women have physical evidence of VVA,3 and approximately 50% of postmenopausal women have VVA symptoms.4 These symptoms may include vaginal dryness, irritation, itching, dysuria, and/or pain or bleeding with sexual activity.5,6 Symptoms of VVA are not limited to sexually active women,5 can range from mild to debilitating,4 and can negatively affect quality of life.3,7 Unfortunately, VVA is unlikely to improve over time without treatment,8 and if treatment is discontinued, the signs and symptoms of VVA tend to recur.
Clinically, a variety of objective and subjective tools are available to practitioners to determine the extent of VVA in postmenopausal women, based mostly on patient history and/or physical examination.9-12 The most common objective measures used to assess VVA include measuring vaginal pH13 and vaginal maturation index (VMI).14 A vaginal pH ≤ 4.5 is considered normal, whereas a pH ≥ 4.6 is supportive of VVA, as long as the patient does not have bacterial vaginosis.14,15 Superficial cells above 15% on a vaginal smear is considered normal, whereas ≤5% is typical for postmenopausal women with VVA.14 Whereas VMI is the standard for VVA confirmation, it is generally not used in clinical practice,14 because vaginal cytological smears need to be collected and sent for analysis.
Symptoms of VVA can be subjectively assessed using patient questionnaires evaluating the severity of symptoms, such as dyspareunia, vaginal dryness, soreness, irritation, or discharge. These are reflected in quality-of-life measures. VVA can also be objectively measured with a visual examination of the vagina using assessment tools such as the Vaginal Atrophy Index (VAI),16 the Genital Health Clinical Evaluation (GHCE),17 the Vaginal Physical Examination Scale,18 the Vaginal Health Index (VHI),19 or the Global Atrophy Score.20 However, no clear consensus has been reached as to which tool is best when visually evaluating VVA. An accepted method of visually assessing the vagina of postmenopausal women could aid clinicians in opening dialog about VVA with their patients, accurately diagnosing VVA, and ultimately determining their patients’ optimal treatment and response to treatment.
TherapeuticsMD Inc (Boca Raton, FL) has developed a vaginal, muco-adhesive, softgel capsule that contains solubilized 17β-estradiol (TX-004HR) designed for the treatment of postmenopausal VVA and moderate-to-severe dyspareunia. Each softgel capsule is self-administered without an applicator for ease of use, and rapidly dissolves leading to the release of the micronized estradiol with minimal messy vaginal leakage. The REJOICE trial has recently demonstrated that TX-004HR (4, 10, and 25 μg) is safe and effective for the treatment of vaginal changes and various self-reported symptoms of VVA in postmenopausal women.21 TX-004HR provided significant improvements in the percentage of vaginal superficial and parabasal cells, vaginal pH, and severity of dyspareunia compared with placebo as early as week 2, with improvements maintained through week 12.21 Treatment with TX-004HR also significantly improved symptomatic vaginal dryness and vulvar and/or vaginal itching/irritation compared with placebo.21 Moreover, these improvements were achieved with negligible to very low estrogen absorption.22
This study reports the effects of TX-004HR (4, 10, and 25 μg) versus placebo on the vaginal mucosa of postmenopausal women using a visual examination evaluating vaginal color, vaginal epithelial integrity, vaginal epithelial surface thickness, and vaginal secretions. Also reported are correlations between the vaginal visual assessment scores and symptoms of vaginal dryness and dyspareunia. It is proposed that the visual examination may be a meaningful and useful tool for documenting VVA, potentially predicting VVA symptoms, and evaluating women's response to treatment.
METHODS
Study design and population
The REJOICE trial was a 12-week, double-blind, randomized, placebo-controlled, phase 3 trial conducted at 89 sites in Canada and the United States (NCT02253173) between October 2014 and October 2015. Women eligible for the study were randomized to either TX-004HR 4, 10, or 25 μg softgel capsules or matching placebo. One capsule was inserted vaginally daily for 2 weeks, and then once every 3 to 4 days for 10 weeks (twice a week). The study was conducted in accordance to the Declaration of Helsinki and complied with the ethical principles of Good Clinical Practice. An independent institutional review board (IRB) approved the study protocol and informed consent form. All participants gave written informed consent before any study-related activities.
Inclusion and exclusion criteria of the REJOICE trial have been previously described in detail.21 Briefly, postmenopausal women (40-75 years of age) were eligible if they had ≤5% superficial cells on vaginal cytological smear, vaginal pH > 5.0, menopausal onset of moderate-to-severe dyspareunia as self-reported most bothersome symptom (MBS), body mass index ≤38 kg/m2, and were currently sexually active (with vaginal penetration). Women could not participate in the study if they had a history or active presence of clinically important medical disease that might confound the study or be detrimental to their health. Additionally, study participants could not have used oral, transdermal, vaginal, intrauterine, implants/injectables drugs that contained either estrogens, progestins, androgens, or selective estrogen receptor modulators (SERMs) before the study (a washout period between 4 weeks and 6 months was required depending on the specific medication used). Use of prescription and nonprescription medications and remedies known to treat VVA, including vaginal lubricants and moisturizers, was not allowed during the study.
The secondary endpoint of visually evaluating the vaginal epithelium was performed during the gynecological examination at screening and at weeks 2, 6, 8, and 12. A 4-point scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe) was used to assess parameters of vaginal color, vaginal epithelial integrity, vaginal epithelial surface thickness, and vaginal secretions (Table 1). VVA symptoms, including dyspareunia, vaginal dryness, and vaginal itching and/or irritation, were also self-assessed using a 4-point scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe). The visual examination assessment tool was consistent with vaginal parameters used in the GHCE,17 the Vaginal Physical Examination Scale,18 and the VHI.19 The 4-item visual examination worksheet can be found in the Supplemental Digital Content 1.
TABLE 1.
Vaginal mucosa assessment scale
| Severity | ||||
| Assessment criteria | No atrophy (0) | Mild (1) | Moderate (2) | Severe (3) |
| Vaginal color | Pink | Lighter in color | Pale in color | Transparent, either no color or inflamed |
| Vaginal epithelial integrity | Normal | Vaginal surface bleeds with scraping | Vaginal surface bleeds with light contact | Vaginal surface has petechiae before contact and bleeds with light contact |
| Vaginal epithelial surface thickness | Rugation and elasticity of vault | Poor rugation with some elasticity noted of vaginal vault | Smooth, some elasticity of vaginal vault | Smooth, no elasticity, constriction of the upper one third of vagina or loss of vaginal tone (cystocele and rectocele) |
| Vaginal secretions | Normal clear secretions noted on vaginal walls | Superficial coating of secretions, difficulty with speculum insertion | Scant not covering the entire vaginal vault, may need lubrication with speculum insertion to prevent pain | None, inflamed, ulceration noted, need lubrication with speculum insertion to prevent pain |
Statistical analysis
The intent-to-treat (ITT) population included all women who were randomized and received at least one dose of treatment (N = 764). The modified intent-to-treat (MITT) population (n = 747), which was used for the primary efficacy analyses, included all women in the ITT population who had baseline values for all co-primary efficacy variables (parabasal and superficial cells, vaginal pH, dyspareunia severity) and with at least one postbaseline value for any of the co-primary variables.
Change from baseline in the visual examination of the vaginal epithelium for each TX-004HR dose, as prespecified, was compared with placebo at weeks 2, 6, 8, and 12 using a mixed-effects repeated measures (MMRM) model with the MITT population. The MMRM analysis used participant and intercept as random effects, and treatment group and visit as fixed effects, with an interaction term for visit and treatment, and baseline values as a covariate.
Correlations between the total sum of the visual examination individual scores and the severity of dyspareunia and dryness at week 12 were evaluated by Pearson's r in the entire ITT population, independent of treatment groups. Receiver-operating characteristic (ROC) curves based on logistic regression models were used to determine whether total visual assessment scores had good sensitivity and specificity to detect moderate-to-severe dryness or dyspareunia at week 12. The c-statistic was used to assess the significance of the ROC curve. Sensitivity and specificity, and visual summary score threshold were determined by the standard method of precisely where the line with slope = −1 intersects the tangent line to the ROC curve.
RESULTS
Participant disposition and demographics
Of the 2,183 women screened, 764 were enrolled and randomized to TX-004HR 4 μg (n = 191), 10 μg (n = 191), 25 μg (n = 190), or placebo (n = 192). Of these, 92% (704/764) of the ITT participants and 94% (703/747) of the MITT participants completed the study. Reasons for discontinuation within the MITT population were similar between groups and included adverse events (n = 9), lack of efficacy (n = 4), investigator decision (n = 2), lost to follow up (n = 9), protocol violation (n = 2), and consent withdrawal (n = 18).
Demographic and baseline characteristics of the MITT population were similar among groups (Table 2). The majority of the women were white, and had a mean age of 59 years and a mean BMI of 27 kg/m2.
TABLE 2.
Participant demographic and baseline characteristics (MITT population)
| Characteristic | TX-004HR 4 μg (n = 186) | TX-004HR 10 μg (n = 188) | TX-004HR 25 μg (n = 186) | Placebo (n = 187) |
| Participants | ||||
| Age, yrs | 59.8 ± 6.0 | 58.6 ± 6.3 | 58.8 ± 6.2 | 59.4 ± 6.0 |
| Race, n (%) | ||||
| White | 162 (87.1) | 165 (87.8) | 161 (86.6) | 160 (85.6) |
| Black or African American | 20 (10.8) | 21 (11.2) | 24 (12.9) | 21 (11.2) |
| Asian | 3 (1.6) | 2 (1.1) | 1 (0.5) | 1 (0.5) |
| BMI, kg/m2 | 26.6 ± 4.9 | 26.8 ± 4.7 | 26.8 ± 4.8 | 26.6 ± 4.6 |
| Natural menopause, n (%) | 111 (59.7) | 114 (60.6) | 118 (63.4) | 124 (66.3) |
| Hysterectomized, n (%) | 87 (46.8) | 86 (45.7) | 85 (45.7) | 73 (39.0) |
| Years since menopause, yrs | 14.2 ± 8.9 | 14.3 ± 9.4 | 13.8 ± 9.4 | 13.9 ± 9.4 |
| Baseline characteristics | ||||
| Superficial cells, % | 1.3 ± 1.2 | 1.2 ± 1.2 | 1.3 ± 1.2 | 1.3 ± 1.3 |
| Parabasal cells, % | 52.3 ± 39.2 | 51.3 ± 38.0 | 53.5 ± 38.3 | 52.0 ± 39.2 |
| Vaginal pH | 6.3 ± 0.9 | 6.3 ± 0.8 | 6.3 ± 0.9 | 6.3 ± 1.0 |
| Dyspareunia, severity score | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.4 | 2.7 ± 0.5 |
| Dryness, severity score | 2.3 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.7 |
| Vaginal color | ||||
| Mean ± SD | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.8 ± 0.6 | 1.8 ± 0.6 |
| Moderate-to-severe, n (%) | 139 (74.7) | 133 (70.7) | 131 (70.4) | 130 (69.5) |
| Vaginal epithelial integrity | ||||
| Mean ± SD | 1.6 ± 0.9 | 1.4 ± 0.9 | 1.5 ± 0.8 | 1.5 ± 0.8 |
| Moderate-to-severe, % | 101 (54.3) | 98 (52.1) | 93 (50.0) | 95 (50.8) |
| Vaginal epithelial surface thickness | ||||
| Mean ± SD | 1.9 ± 0.7 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.6 |
| Moderate-to-severe, % | 146 (78.5) | 136 (72.3) | 144 (77.4) | 145 (77.5) |
| Vaginal secretions | ||||
| Mean ± SD | 1.7 ± 0.7 | 1.7 ± 0.7 | 1.7 ± 0.6 | 1.8 ± 0.6 |
| Moderate-to-severe, % | 128 (68.8) | 126 (67.0) | 125 (67.2) | 137 (73.3) |
Data are represented as mean ± SD unless stated otherwise.
MITT, modified intent-to-treat.
Baseline vaginal characteristics and visual examination scores are shown in Table 2. Most of the women (50%-79%) had the visual inspection parameters rated as moderate-to-severe at baseline. At baseline, all women had moderate-to-severe dyspareunia with a mean severity score of 2.7; 93.4% reported moderate-to-severe dryness with a mean severity score of 2.4.
Vaginal assessment
Vaginal color, vaginal epithelial integrity, vaginal epithelial surface thickness, and vaginal secretions significantly improved from baseline at all time points for all TX-004HR groups compared with placebo (P < 0.0001 for all, except P < 0.001 for vaginal secretions at weeks 2 and 6 for TX-004HR 4 μg; Table 3). Specifically at 12 weeks, severity scores significantly decreased by −0.96 to −1.06 for the TX-004HR doses compared with −0.60 for the placebo for vaginal color, by −0.97 to −1.07 vs −0.60 for epithelial integrity, by −0.94 to −1.03 vs −0.61 for epithelial surface thickness, and by −1.01 to −1.06 vs −0.64 for vaginal secretions. Significant improvements in all four visual inspection parameters were noted as early as 2 weeks and were maintained throughout the 12 weeks of the study with all three doses of TX-004HR. No dose-specific effects were observed.
TABLE 3.
Change from baseline to weeks 2, 6, 8, and week 12 in vaginal parameters in the modified intent-to-treat (MITT) population
| Vaginal parameters | TX-004HR 4 μg (n = 186) | TX-004HR 10 μg (n = 188) | TX-004HR 25 μg (n = 186) | Placebo (n = 187) | ||||
| Vaginal color | ||||||||
| Baseline score, mean ± SD | 1.8 ± 0.61 | 1.7 ± 0.59 | 1.8 ± 0.60 | 1.7 ± 0.64 | ||||
| LS mean (SE) | n | n | n | n | ||||
| Wk 2 | 185 | −0.69 (0.05)a | 187 | −0.77 (0.05)a | 184 | −0.78 (0.05)a | 186 | −0.40 (0.05) |
| Wk 6 | 172 | −0.82 (0.05)a | 170 | −0.93 (0.05)a | 173 | −0.89 (0.05)a | 176 | −0.50 (0.05) |
| Wk 8 | 164 | −0.98 (0.05)a | 165 | −1.04 (0.05)a | 166 | −0.99 (0.05)a | 167 | −0.50 (0.05) |
| Wk 12 | 171 | −0.97 (0.05)a | 173 | −1.06 (0.05)a | 175 | −0.96 (0.05)a | 175 | −0.60 (0.05) |
| Vaginal epithelial integrity | ||||||||
| Baseline score, mean ± SD | 1.6 ± 0.84 | 1.4 ± 0.83 | 1.5 ± 0.77 | 1.5 ± 0.84 | ||||
| LS mean (SE) | n | n | n | n | ||||
| Wk 2 | 185 | −0.85 (0.05)a | 187 | −0.87 (0.05)a | 184 | −0.93 (0.05)a | 186 | −0.53 (0.05) |
| Wk 6 | 172 | −0.97 (0.05)a | 170 | −1.02 (0.05)a | 173 | −1.01 (0.05)a | 176 | −0.61 (0.05) |
| Wk 8 | 164 | −1.03 (0.05)a | 165 | −1.08 (0.05)a | 166 | −1.08 (0.05)a | 167 | −0.66 (0.05) |
| Wk 12 | 171 | −0.97 (0.05)a | 173 | −1.07 (0.05)a | 175 | −1.01 (0.05)a | 175 | −0.60 (0.05) |
| Vaginal epithelial surface thickness | ||||||||
| Baseline score, mean ± SD | 1.9 ± 0.67 | 1.8 ± 0.63 | 1.9 ± 0.59 | 1.9 ± 0.65 | ||||
| LS mean (SE) | n | n | n | n | ||||
| Wk 2 | 185 | −0.76 (0.05)a | 187 | −0.76 (0.05)a | 184 | −0.76 (0.05)a | 186 | −0.40 (0.05) |
| Wk 6 | 172 | −0.85 (0.05)a | 170 | −0.93 (0.05)a | 173 | −0.90 (0.05)a | 176 | −0.53 (0.05) |
| Wk 8 | 164 | −0.96 (0.05)a | 165 | −1.04 (0.05)a | 166 | −0.99 (0.05)a | 167 | −0.59 (0.05) |
| Wk 12 | 171 | −0.98 (0.05)a | 173 | −1.03 (0.05)a | 175 | −0.94 (0.05)a | 175 | −0.61 (0.05) |
| Vaginal secretions | ||||||||
| Baseline score, mean ± SD | 1.8 ± 0.68 | 1.7 ± 0.66 | 1.7 ± 0.63 | 1.8 ± 0.63 | ||||
| LS mean (SE) | n | n | n | n | ||||
| Wk 2 | 185 | −0.79 (0.05)b | 187 | −0.83 (0.05)a | 184 | −0.86 (0.05)a | 186 | −0.54 (0.05) |
| Wk 6 | 172 | −0.90 (0.05)b | 170 | −0.95 (0.05)a | 173 | −0.97 (0.05)a | 176 | −0.60 (0.05) |
| Wk 8 | 164 | −1.00 (0.05)a | 165 | −1.04 (0.05)a | 166 | −1.06 (0.05)a | 167 | −0.63 (0.05) |
| Wk 12 | 171 | −1.01 (0.05)a | 173 | −1.06 (0.05)a | 175 | −1.04 (0.05)a | 175 | −0.64 (0.05) |
Data are mean ± SD unless otherwise noted.
LS, least square; SD, standard deviation; SE, standard error.
aP < 0.0001 versus placebo; bP < 0.001.
Significantly more women in the TX-004HR groups than in the placebo group had a none-to-mild severity rating by week 2, which was maintained through week 12 for vaginal color (week 2: TX-004HR 74%-80% vs placebo 57%; P ≤ 0.0001 for each dose; week 12: 79%-84% vs 65%; P < 0.0001 for each dose), vaginal epithelial integrity (week 2: 87%-80% vs 72%; P < 0.001 for each dose; week 12: 83%-89% vs 71%; P ≤ 0.0001 for each dose), vaginal epithelial surface thickness (week 2: 70%-74% vs 50%; P < 0.0001 for each dose; week 12: 78%-83% vs 55%; P ≤ 0.0001 for each dose), and vaginal secretions (week 2: 78%-82% vs 61%; P < 0.001 for each dose; week 12: 80%-83% vs 61%; P < 0.0001 for each dose).
Changes in severity scores at 12 weeks from baseline were similar across each visual assessment item (Fig. 1A-D). Approximately 63% to 71% women treated with TX-004HR had a reduction (improvement) in their visual examination severity scores (vs 47%-55% with placebo), whereas 20% to 27% had no improvement (vs 28% to 42% with placebo). Less than 5% of women receiving TX-004HR had an increase in their severity scores at week 12 compared with less than 12% receiving placebo.
FIG. 1.
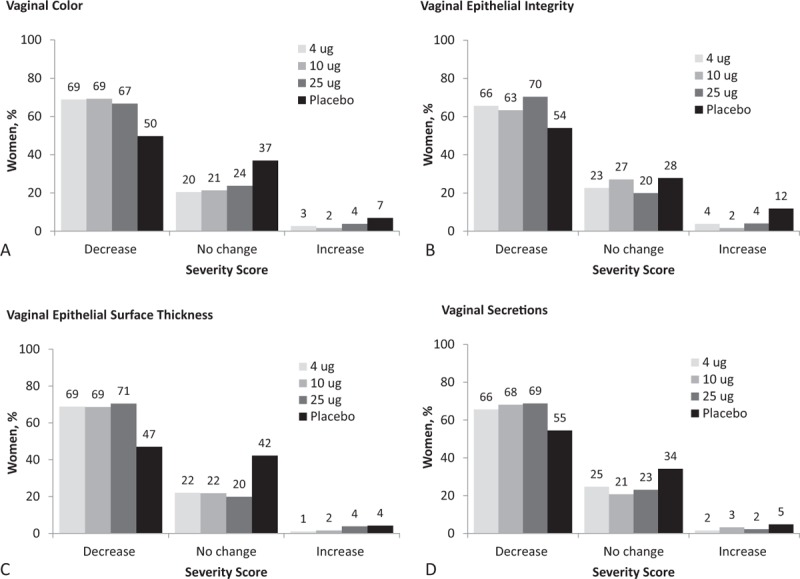
Change in severity of visual assessments from baseline to week 12 in (A) vaginal color, (B) vaginal epithelial integrity, (C) vaginal epithelial thickness, (D) vaginal secretions in MITT population. MITT, modified intent-to-treat.
Sensitivity and specificity of visual examination score
A simple linear correlation was observed between the total sum of the individual visual examination score and severity of dyspareunia (r = 0.31, P < 0.0001), and the severity of vaginal dryness (r = 0.38, P < 0.0001) at 12 weeks (Fig. 2A and C) when all participants were analyzed independent of treatment.
FIG. 2.
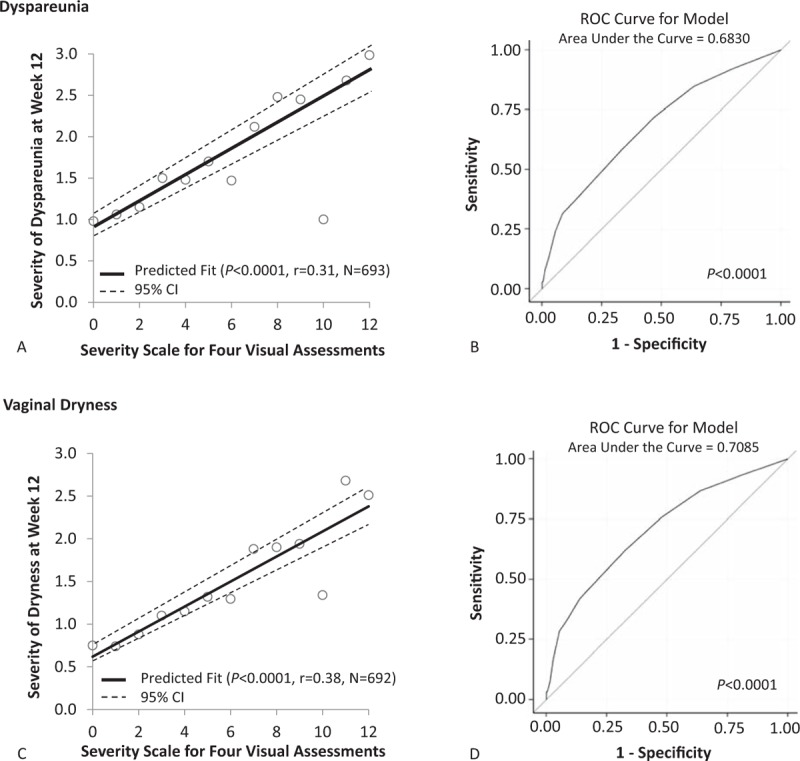
Pearson's correlations and logistic regression ROC curves between the sum of the four individual visual assessment scores and dyspareunia (A and B) or vaginal dryness (C and D) at week 12 in the ITT population. ITT, intention to treat; ROC, receiver-operating characteristic.
Furthermore, ROC analyses for both dyspareunia and vaginal dryness showed a significant visual score threshold. For dyspareunia, ROC analysis revealed an area under the curve (AUC, c-statistic) of 0.68 (P < 0.0001; Fig. 2B) with a significant odds ratio (OR) of 1.34 (95% confidence interval [CI] 1.24-1.44, P < 0.0001). Using an ROC threshold of ≥5, sensitivity and specificity levels were 58% and 67%, respectively. For vaginal dryness, the AUC was 0.71 (P < 0.0001; Fig. 2D) with a significant OR of 1.39 (95% CI 1.29-1.49, P < 0.0001). Sensitivity and specificity levels with an ROC threshold ≥5 were 62% and 67%, respectively. A visual summary score of ≥5 can correspond to many different combinations including a women with a mild score in three of the four components and a moderate score in the fourth component.
DISCUSSION
Visual examination of the vaginal epithelium of postmenopausal women with VVA and moderate-to-severe dyspareunia showed that treatment with TX-004HR clinically and significantly improved vaginal color, vaginal epithelial integrity, vaginal epithelial surface thickness, and vaginal secretions as early as 2 weeks, and maintained those improvements through 12 weeks compared with placebo. Approximately two-thirds of women receiving TX-004HR had improvements in their severity scores for each assessment. Moreover, the total sum of the visual assessments was moderately correlated with dyspareunia and dryness severity scores at 12 weeks.
Interestingly, women treated with placebo also showed some improvements in their scores at week 2, whereas women treated with TX-004HR showed continued improvements through 12 weeks of treatment; such continued improvements were not observed to the same extent with the placebo. Three possible explanations for the improvements observed with the placebo include the potential lubricating effect of the excipient Miglyol, a fractionated coconut oil contained in all softgel capsules, improved appearance based on vaginal lubrication caused by increased sexual activity,23 and/or bias on the part of the physicians performing the examinations as they may anticipate improvement. Nevertheless, TX-004HR still significantly improved evaluated signs and symptoms of VVA better than placebo.
Correlations between objective and subjective measures have been previously evaluated for VVA. For example, a vaginal pH > 6.0 measured at the midvagina was shown to correlate with elevated levels (≥20%) of parabasal cells.13 Visual assessments of vaginal atrophy using the Vaginal Physical Examination Scale also significantly correlated with vaginal pH and the maturation index score.18 Similar correlations were observed using the visual assessment scoring in the phase 2 pilot trial of TX-004HR, which showed significant correlations with the change from baseline to day 15 for percentage of vaginal cells and vaginal pH (r = 0.35, P = 0.02) with the sum of the four visual assessments24; however, these correlations have limited utility in the clinical setting, because measurements of vaginal pH and cells are not often performed.
Beyond simple correlations, the ROC curve analyses also showed that visual assessment summary scores ≥5 had good sensitivity and specificity to detect moderate-to-severe dryness or dyspareunia. Because visual inspection of the vagina with the 4-point assessment tool showed moderate positive correlation with dyspareunia and vaginal dryness in this study, this tool may help healthcare professionals diagnose VVA and assess its treatment, and provide a vehicle for healthcare professionals to initiate discussion with their patients about a sensitive topic. Several large-scale studies have shown that it is difficult for patients to discuss vulvovaginal health openly with their healthcare professionals because they are either embarrassed, uninformed about VVA and its treatments, or believe that the topic is not appropriate for discussion.4 Therefore, of the 50% of postmenopausal women who have symptoms of VVA, far fewer seek treatment.4
Visual examination of the vagina may help practitioners identify women at risk of dyspareunia and vaginal dryness, and allow them to proactively engage women in conversations about VVA symptoms such as dyspareunia and dryness, and discuss available treatment options. Whereas various assessment tools, such as the VAI,16 the GHCE,17 the Vaginal Physical Examination Scale,18 the VHI,19 or the Global Atrophy Score,20 have been described, consensus in the healthcare community on the best tool to assess VVA has not been reached, possibly because patients and clinicians have both been shown to be reluctant to address this issue.25,26 Furthermore, this topic has not been studied extensively or systematically, contributing to the lack of consensus on a method used to evaluate and describe VVA. This is in contrast to other tools that have been adopted by the majority of healthcare professionals to assess other disorders such as obstructive sleep apnea, sexual dysfunction, and pelvic relaxation and/or prolapse.27-30 Although the vaginal mucosa assessment scale reported in this study has not been previously validated, the statistically significant results obtained from the data of 89 different sites across the United States and Canada suggest that the scale may yield consistent and reproducible scores. This visual scale, which is both consistent and reproducible, may be of utility in research to reduce development costs, ease regulatory approval, and be used as a practical tool in clinical settings.
One limitation of this analysis was that VVA measurement using the vaginal mucosa assessment scale was not a primary endpoint of the study, but was included as a secondary endpoint to determine whether visual examinations could be used to determine treatment effects and their correlation with VVA symptoms. Furthermore, the correlations observed in this study may not be applicable to all populations as all women had moderate-to-severe dyspareunia and the majority were white, and in good health. Studies evaluating this vaginal mucosa assessment scale in postmenopausal women with symptoms other than dyspareunia would be useful in validating the scale.
CONCLUSIONS
Improvements observed in the vaginal mucosa assessment scores support the robust efficacy of TX-004HR (4, 10, and 25 μg) observed in the treatment of VVA and moderate-to-severe dyspareunia in postmenopausal women. Overall, visual assessments of the vagina performed by experienced healthcare professionals appear to be a useful measure to diagnose VVA and assess response to treatment. In an era of increasing dependence on objective measures of clinical changes, even for VVA, knowing that an experienced clinician's judgment correlates strongly with objective measures is reassuring and can be self-empowering.
Supplementary Material
Supplementary Material
Acknowledgments
The authors would like to thank the investigators of the REJOICE Study Group (see document, Supplemental Digital Content 2, for an Appendix listing the investigators). The authors would also like to acknowledge Harvey Kushner, PhD, for statistical analysis and manuscript review, and the medical writing assistance of Dominique Verlaan, PhD, of Precise Publications, LLC, which was supported by TherapeuticsMD.
Footnotes
Source(s) of the work or study: TherapeuticsMD.
Funding/support: TherapeuticsMD sponsored the study and funded the medical writing support provided by Dominique Verlaan, PhD of Precise Publications, LLC.
Financial disclosure/conflicts of interest: Dr Simon has served (within the last year) or is currently serving as a consultant to or on the advisory boards of AbbVie, Inc, Allergan, Plc, AMAG Pharmaceuticals Inc, Amgen Inc, Apotex, Inc, Ascend Therapeutics, Azure Biotech, Inc, JDS Therapeutics, LLC, Merck & Co, Inc, Millendo Therapeutics, Inc, Noven Pharmaceuticals, Inc, Novo Nordisk, Nuelle, Inc, Perrigo Company, PLC, Radius Health, Inc, Regeneron Pharmaceuticals, Inc, Roivant Sciences, Inc, Sanofi S.A., Sebela Pharmaceuticals, Inc, Sermonix Pharmaceuticals, Inc, Shionogi Inc, Sprout Pharmaceuticals, Symbiotec Pharmalab, TherapeuticsMD, and Valeant Pharmaceuticals; and has received Iin the last year) or is currently receiving grant/research support from AbbVie, Inc, Actavis, PLC, Agile Therapeutics, Bayer Healthcare LLC, GlaxoSmithKline, New England Research Institute, Inc, Novo Nordisk, Palatin Technologies, Symbio Research, Inc, and TherapeuticsMD; and has also served (within the last year) or is currently serving on the speaker's bureaus of Amgen Inc, Eisai, Inc, Merck, Noven Pharmaceuticals, Inc, Novo Nordisk, Shionogi Inc, and Valeant Pharmaceuticals; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. Dr Archer (within the past 3 years) has received research support from Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Bayer Healthcare, Endoceutics, Glenmark, Merck (previously Schering Plough, Organon), Radius Health, Shionogi Inc, and TherapeuticsMD; has served as consultant to Abbvie (previously Abbott Laboratories), Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis (previously CHEMO), InnovaGyn, Merck (previously Schering Plough, Organon), Pfizer, Radius Health, Sermonix Pharmceuticals, Shionogi, Inc, Teva Women's Healthcare, and TherapeuticsMD. Dr Kagan is a consultant to Allergan, AMAG, Amgen, Azure, Heptares, Juniper, Noven Pharmaceuticals, Palatin, Pfizer, Shionogi, Sprout, Valeant, and Merck; and has received research grants and support from TherapeuticsMD, but paid to Sutter Health. Dr Constantine consults to multiple pharmaceutical companies including but not limited to TherapeuticsMD, and has stock options from TherapeuticsMD. Dr Bernick is a board member and an employee of TherapeuticsMD with stock/stock options. Dr Graham and Dr Mirkin are employees of TherapeuticsMD with stock/stock options.
REFERENCES
- 1.Lev-Sagie A. Vulvar and vaginal atrophy: physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol 2015; 58:476–491. [DOI] [PubMed] [Google Scholar]
- 2.Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and The North American Menopause Society. Menopause 2014; 21:1063–1068. [DOI] [PubMed] [Google Scholar]
- 3.Gass ML, Cochrane BB, Larson JC, et al. Patterns and predictors of sexual activity among women in the hormone therapy trials of the Women's Health Initiative. Menopause 2011; 18:1160–1171. [DOI] [PubMed] [Google Scholar]
- 4.Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013; 5:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009; 6:2133–2142. [DOI] [PubMed] [Google Scholar]
- 6.Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the Vaginal Health: Insights: Views and Attitudes survey. Menopause 2013; 20:1043–1048. [DOI] [PubMed] [Google Scholar]
- 7.Thomas HM, Bryce CL, Ness RB, Hess R. Dyspareunia is associated with decreased frequency of intercourse in the menopausal transition. Menopause 2011; 18:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol 2000; 96:351–358. [DOI] [PubMed] [Google Scholar]
- 9.North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. [DOI] [PubMed] [Google Scholar]
- 10.Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010; 13:509–522. [DOI] [PubMed] [Google Scholar]
- 11.Management of menopausal symptoms. Practice Bulletin No. 141. American College of Obstetricians and Gynecologists. Obstet Gynecol 2014; 123:202–216. [DOI] [PubMed] [Google Scholar]
- 12.Society of Obstetricians Gynaecologists of Canada. The detection and management of vaginal atrophy. Number 145, May 2004. Int J Gynaecol Obstet 2005; 88:222–228. [DOI] [PubMed] [Google Scholar]
- 13.Brizzolara S, Killeen J, Severino R. Vaginal pH and parabasal cells in postmenopausal women. Obstet Gynecol 1999; 94:700–703. [DOI] [PubMed] [Google Scholar]
- 14.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, Caillouette JC, Roy T, Faden JS. Vaginal pH is similar to follicle-stimulating hormone for menopause diagnosis. Am J Obstet Gynecol 2004; 190:1272–1277. [DOI] [PubMed] [Google Scholar]
- 16.Leiblum S, Bachmann G, Kemmann E, Colburn D, Swartzman L. Vaginal atrophy in the postmenopausal woman. The importance of sexual activity and hormones. JAMA 1983; 249:2195–2198. [PubMed] [Google Scholar]
- 17.Raymundo N, Yu-cheng B, Zi-yan H, et al. Treatment of atrophic vaginitis with topical conjugated equine estrogens in postmenopausal Asian women. Climacteric 2004; 7:312–318. [DOI] [PubMed] [Google Scholar]
- 18.Greendale GA, Zibecchi L, Petersen L, Ouslander JG, Kahn B, Ganz PA. Development and validation of a physical examination scale to assess vaginal atrophy and inflammation. Climacteric 1999; 2:197–204. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann GA, Ebert GA, Burd ID. Lobo RA. Vulvovaginal complaints. Treatment of the Postmenopausal Woman. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. 195–201. [Google Scholar]
- 20.Pinkerton JV, Shifren JL, La VJ, Rosen A, Roesinger M, Siddhanti S. Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause 2003; 10:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Constantine G, Simon JA, Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol softgel capsule for symptomatic vulvar and vaginal atrophy (VVA). Menopause 2017; 24:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archer DF, Constantine G, Kushner H, et al. TX-004HR vaginal estradiol effectively treats vulvar and vaginal atrophy (VVA) with negligible to low systemic absorption of estradiol. Poster presented at the Endocrine Society's 98th Annual Meeting and Expo. Boston, MA; 2016.
- 23.Association of Professors of Gynecology and Obstetrics. Contemporary Clinical Management of Menopause. Available at: https://www.apgo.org/wp-content/uploads/2016/05/meno.pdf. Accessed January 9, 2017.
- 24.Constantine GD, Kushner H, Bernick B, Graham S, Mirkin S. Vaginal physical examination correlates with vaginal epithelial cells and pH and can be used to assess treatment efficacy. Presented at the Annual Meeting of the Endocrine Society, March 5-8, 2015, San Diego, CA.
- 25.Nappi RE, Palacios S, Panay N, Particco M, Krychman ML. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey. Climacteric 2016; 19:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (Real Women's Views of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10:1790–1799. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res 2000; 9:5–11. [DOI] [PubMed] [Google Scholar]
- 28.Hardinge FM, Pitson DJ, Stradling JR. Use of the Epworth Sleepiness Scale to demonstrate response to treatment with nasal continuous positive airways pressure in patients with obstructive sleep apnoea. Respir Med 1995; 89:617–620. [DOI] [PubMed] [Google Scholar]
- 29.Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q): a new era in pelvic prolapse staging. J Med Life 2011; 4:75–81. [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000; 26:191–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


