Abstract
Background and aim
Worldwide 1,470,900 women are diagnosed yearly with a gynaecological malignancy; 21,000 in the UK. Some patients treated with pelvic radiotherapy develop chronic changes in their bowel function. This systematic review summarises current research on the impact of cancer treatment on the gut and vaginal microbiome in women with a gynaecological malignancy.
Methods
The PRISMA guidelines for systematic reviews were used to ensure transparent and complete reporting. Quantitative studies exploring the gut or vaginal microbiome in this patient cohort were included. Animal studies were excluded. There were no language restrictions.
Results
No studies examined the possible effects of surgery or chemotherapy for gynaecological cancers on the gut or vaginal microbiome.
Three prospective cohort studies were identified using sequencing of changes in the gut microbiome reporting on a total of 23 women with treated for gynaecological cancer. All studies included patients treated with radiotherapy with a dosage ranging from 43.0 to 54.0 Gy. Two studies assessed gastrointestinal toxicity formally; 8 women (57%) developed grade 2 or 3 diarrhoea during radiotherapy. The outcomes suggest a correlation between changes in the intestinal microbiome and receiving radiotherapy and showed a decrease in abundance and diversity of the intestinal bacterial species. Prior to radiotherapy, those who developed diarrhoea had an increased abundance of Bacteroides, Dialister, Veillonella (p<0.01), and a decreased abundance of Clostridium XI and XVIII, Faecalibacterium, Oscillibacter, Parabacteroides, Prevotella and unclassified bacteria (p<0.05).
Conclusion
The limited evidence to date implies that larger studies including both the vaginal and gut microbiome in women treated for a gynaecological malignancy are warranted to explore the impact of cancer treatments on the microbiome and its relation to developing long-term GI toxicity. This may lead to new avenues to stratify those at risk and explore personalised treatment options and prevention of gastrointestinal consequences of cancer treatments.
Keywords: gut microbiome, vaginal microbiome, gynaecology, malignancy, cancer, cancer treatment, gastrointestinal toxicity
Introduction
Over 14 million people worldwide are diagnosed with a new cancer annually.1 Yearly, 1,470,900 women are diagnosed with gynaecological malignancy, 21,000 in the UK.
Treatment for gynaecological cancers includes surgery, radiotherapy and chemotherapy. Radiation delivered by external beam radiotherapy or brachytherapy frequently causes acute gastrointestinal toxicity and up to half of all patients experience chronic change in bowel function. Few data are available with regard to long-term gastrointestinal toxicity following surgery and chemotherapy.2
Gastrointestinal symptoms following cancer treatment may include diarrhoea, abdominal pain, bowel frequency, faecal incontinence, borborygmi, tenesmus and flatulence.3 These symptoms cause fatigue, affect well-being, relationships and socio-economic status. The impact of these symptoms on quality of life and daily activities is often not assessed or addressed.
While progress has been made in defining optimal management of chronic changes in bowel function after cancer treatment3, the ability to predict and risk-stratify in advance those who might develop serious problems as a result of treatment would herald a major advance in outcomes for cancer survivors.
Mechanisms by which gastrointestinal symptoms occur after cancer treatment are starting to be understood and personal parameters which change the risk of side-effects for individuals, identified. Body mass index (BMI), concomitant chemotherapy, use of a statin or ACE inhibitor, diabetes mellitus, connective tissue disorders or HIV disease all alter risk of long-term consequences.4
The gut microbiome, however, may be the key to understanding gastrointestinal toxicity during and following cancer treatment.5 The bacteria within the gastrointestinal tract contribute to health, mood and general well-being by regulating major epithelial and immune functions and feedback to the brain via the vagus nerve and hormone secretion particularly about energy uptake.6–7
In health, microbiomes in the gut and vagina are separated from the host by a multi-level barrier supported by immune cells neutralising pathogens. Failure of this barrier either in gut or vaginal epithelia can cause low-grade chronic inflammation leading to cardiovascular disease, inflammatory bowel disease and cancer.7–9 Cancer itself can cause inflammation leading to dysbiosis, creating a positive feedback loop promoting disease.8,10
The gastrointestinal microbiome is an ecosystem of up to 1,000 bacterial species in any one individual.11 In health, 90% of the total gastrointestinal microbiome is populated by five major bacterial phyla: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Fusobacteria.9 The composition within individuals remains stable for 60% of species over time.12–13
The gut microbiome has a high diversity in healthy people. There is a clear link between gut microbiotic composition and pathological states, eg inflammatory bowel disease5,14–15 The composition of the gut microbiome is less diverse in obese people who have a higher proportion of Firmicutes and fewer Bacteriodes.16–17. There is reduced diversity in people with diarrhoea after pelvic radiotherapy.13,18
A healthy vaginal microbiome is typically populated by aerobic members of the Firmicutes phylum, dominated by Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii.19 Alterations in the vaginal microbiome lead to conditions such as bacterial vaginosis where vaginal lactobacilli are reduced and replaced by anaerobic bacteria.20
Research into the vaginal and cervical microbiome was initially promoted by the desire to prevent and treat radiation fever which was believed to be due to ascending infection by pathogenic vaginal bacteria and could result in delays delivering radiotherapy, dose reduction and poorer treatment outcomes. 21–29 The notion that radiotherapy sterilised the cervix was dispelled by studies analysing aerobic and anaerobic cultures.21–29 These studies however, differed in their conclusions as to the role of the genital tract microbiome in oncogenesis, whether in the presence of gynaecological malignancy it differed from that in health and the significance of changing bacterial composition during and after treatment.
Febrile morbidity in women receiving pelvic radiotherapy is no longer a concern. Prophylactic use of antibiotics before gynaecological procedures, makes risk of infection following biopsies or surgical intervention less common.30 However, even short courses of antibiotics results in reduction in gut microbiota diversity lasting six months to four years.31 The use of antibiotics and its implications for maintaining human health and resilience to disease need to be acknowledged.32
Recent efforts have focused on how the vaginal microbiome impacts on gynaecological malignancy development. Women with vaginal dysbiosis have a higher risk of developing cervical pre-neoplastic changes over time (OR 2.00; 95% CI 1.31-3.05) however, women with Candidiasis do not have (OR 0.42; 95% CI 0.11-1.70).33,34–38 Disturbance of the vaginal flora increases the risk of acquiring oncogenic Human Papillomavirus (HPV)33,38 which plays a causal role in the development of precancerous cervical intra-epithelial neoplasia and invasive cervical cancer.34,38 Although HPV infection is common in sexually-active women and may be indicative of sexual behaviour predisposing to HPV, most infections are transient. Only a small proportion of infected women develop clinically significant pre-invasive lesions or invasive malignant disease. HIV and Human Papilloma Virus (HPV) status is now routinely tested in women with cervical cancer.9,39
The gut microbiome produces estradiol, which may promote oestrogen-driven malignancies such as endometrial cancer.40
The intestinal and vaginal microbiome also plays a significant role in response to oncological treatments and long-term toxicity from those treatments.41–42 The number of people living with and beyond cancer has tripled over the last decade and reducing long term toxicity is a priority.43
Analysing the gastrointestinal and genital tract microbiome
Older methods relied on bacterial culture using gram staining, gas-liquid chromatography or biochemical tests. These approaches are limited by the inability to culture all bacterial species and do not detect changes in bacterial functions.
Modern research uses next generation sequencing methodologies (DNA, rDNA, rRNA) and metagenomics to analyse the DNA of the entire microbial community and to understand how radiotherapy possibly induces changes in microbial composition. However, the virome is rarely included15,44 and these techniques are complex, expensive and mostly do not measure changes in microbial function which may be as important as changes in composition.
Alternative options for analysing the gut microbiome include examining gases produced by the metabolites of the gut microorganisms, Volatile Organic Compounds (VOCs). VOCs are organic chemicals which derive from biological samples such as breath, faeces, sweat, urine or vaginal fluid and are gas phase biomarkers reflecting individuals’ metabolic state.45–47 The gold standard for analysing VOCs is gas chromatography/mass spectrometry (GCMS). However, this technique is expensive and specialised and is not a viable option for day-to-day clinical practice.47
Two alternative techniques can analyse VOCs to draw a unique olfactory signature: electronic sensing (e-nose) or High Field Asymmetric Ion Mobility Spectrometry (FAIMS). These detect specific chemical compounds indicating changes in gut microbiome metabolism involved in fermentation processes.47
This review aims to summarise existing research using modern analysis methodologies on the impact of cancer treatment (surgery, chemotherapy, radiotherapy) on the gut and vaginal microbiome in women with a gynaecological malignancy.
Methods
Literature identification
This systematic review used the PRISMA guidelines to ensure transparent and complete reporting (Appendix 1).48 The review protocol was registered on the International PROSPERO review database: PROSPERO 2016:CRD42016047121 http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016047121.
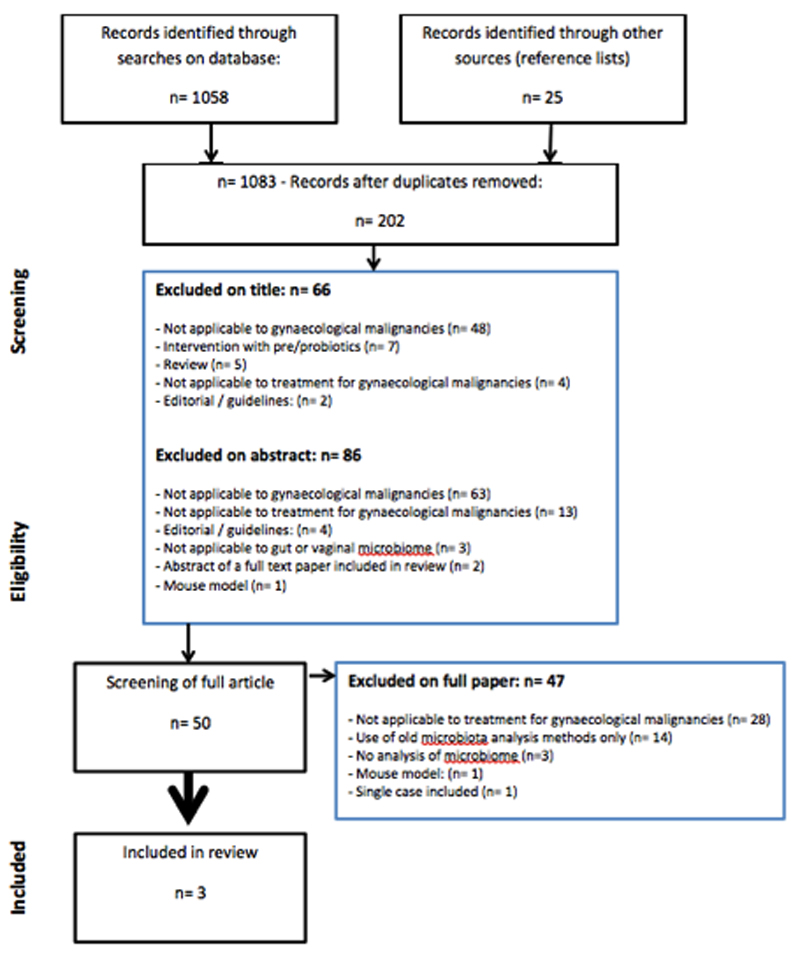
Search strategy (figure 1)
Figure 1.
Flow chart literature search
Relevant studies were identified by one author (ACM) searching the following databases: Embase 1974 to 2016 August 16, Global Health 1973 to 2016 Week 31, HMIC Health Management Information Consortium 1979 to July 2016, Journals@Ovid Full Text August 16, 2016, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present, Ovid Medline database. The PROSPERO database and Cochrane Library were searched for existing similar systematic reviews.
To ensure that the search was comprehensive and studies had not been missed or wrongly excluded, the unpublished and grey literature, general search engines, and reference lists of included papers were checked. Contact was made with the authors of the papers when further information was required. A second reviewer (CN) independently analysed the papers.
The main search terms included: gynaecological cancer, gynaecological malignancy, gynecological cancer, gynecological malignancy, cervix cancer, endometrial cancer, uterine cancer, gut microbiome, intestinal microbiome, vaginal microbiome, vaginal swabs, surgery, chemotherapy, radiotherapy, pelvic radiotherapy, chemoradiation, sequencing, VOC analysis. Appendix 2 presents an example of the full electronic search strategy for the OVID database.
Types of studies included
Quantitative studies exploring the gut or vaginal microbiome in women treated for a gynaecological malignancy were included. There were no language restrictions. Animal studies, case reports or studies including single subjects, expert opinions and consensus statements were excluded. Due to recent advances in treatment (specifically radiotherapy) and new techniques to analyse the microbiome, studies with older conventional culturing techniques were excluded.
As a systematic review protocol assessing the impact of probiotics for the prevention or treatment of chemotherapy or radiotherapy related diarrhoea in cancer patients has been published by the Cochrane collaboration49, studies including the use of pre- or probiotics as an intervention were excluded.
Inclusion criteria were defined using the following components: Patient population (P): women treated for a gynaecological malignancy, exposure of interest (I): cancer treatment: surgery, chemotherapy or radiotherapy, comparator (C): before and after treatment for gynaecological cancer, outcome (O): the change in the gut or vaginal microbiome following treatment for gynaecological cancer and the study designs (S) of interest: randomised controlled trials (RCTs), prospective observational cohort studies and retrospective studies.
Results
No studies were identified examining the possible effects of surgery for gynaecological cancers on the gut microbiome or charting changes in the gut microbiome of women treated with chemotherapy alone. One study included 7 women who received concomitant chemoradiation.53 Analysis of gut microbiotic profiles showed that the number of operational and taxonomic units (OTUs) decreased as well as the richness in bacterial species. However, the impact of chemotherapy alone on the gut microbiome remains unclear.
No studies have examined changes in the vaginal microbiome during treatment for gynaecological malignancies and how this may affect long-term toxicity.
No studies were identified using VOC analysis methods examining the metabolites of gut or vaginal microbiome involving women treated for gynaecological tumours.
Five studies and two abstracts were identified using sequencing to analyse the gut microbiome during treatment with pelvic radiotherapy. One study was excluded50 as it described a single case of a women treated for ovarian cancer in a cohort of 19 patients receiving chemotherapy for a range of malignancies. Both abstracts were excluded as one related to a full publication (table 1) and one described the cohort as ‘cancer patients treated with pelvic radiotherapy’. Contact with the authors revealed this study only included men treated for prostate cancer.51
Table 1.
Studies using pyrosequencing of changes in the gut microbiome in women treated with radiotherapy for a gynaecological malignancy
| Reference | Type of study | Number of patients with gynaecological tumour types |
Number of healthy volunteers as control |
Surgery | Chemotherapy type defined | Radiotherapy dose (Gy) | Analysis technique microbiome | Type of sample | Diarrhoea defined | No of patients reporting diarrhoea |
Before- after design | Number of follow-up time points |
Latest time point after radiotherapy |
Antibiotic use as exclusion ? | Steroid use as exclusion? | Immunotherapy as exclusion? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manichanh et al., 2008 | Prospective cohort | 6 cervix (n=1) uterus (n=1) endometrium (n=4) |
4 | ? | N/A | 43-54 | 16S rRNA sequencing | Stool | Yes CTC v0.2 |
4 | Yes | 4 | 2 weeks | Yes | Yes | Yes |
| Main findings | Patients without diarrhoea maintained their microbiotic profile for 60% during RT Healthy controls and patients with no diarrhoea maintained their bacterial profiles with a similarity near 60% over the 7-wk time course. Patients with diarrhoea showed a significantly modified bacterial profile (P < 0.05) after the beginning of the radiotherapy (49% of similarity, SD = 17), at the end of the radiotherapy (29% similarity, SD = 17), and 2 wk after (35% similarity, SD = 15) with the detection of actinobacteria + increase in fermicutes (bacilli). Patients that develop diarrhoea clustered separately from those that did not develop diarrhoea using the Pearson’s correlation and the unweighted pair-group method using arithmetic averages (UPGMA). |
|||||||||||||||
| Nam et al., 2013 | Prospective cohort | 9 cervix (n=7) endometrium (n=2) |
6 | ? | Yes | 50.4 | 16S rRNA sequencing | Stool | No | - | Yes | 4 | 1-3 months | Yes | Yes | Yes |
| Main findings | Compared to T0, family Eubacteriaceae in each sample at T2, and T3 were significantly decreased (P < 0.032). Fusobacteriaceae significantly increased at T2 and Streptococcaceae significantly increased at T1 compared to T0. Family level taxon Veillonellaceae, Enterococcaceae, Lactobacillales bacterium and Butyrate- producing bacterium at T0, T1, and T2 were not different from each other, but the T0 and T3 samples showed significant differences (P = 0.050). Overall richness in bacterial species decreased - decrease in firmicutes by 10% (p= 0.09) - increase in fusobacteria by 3% (p=0.05) - decrease in bacteriodes during RT but increase after 3 months (no p-value) |
|||||||||||||||
| Wang et al., 2015 | Prospective cohort | 8 cervix (n=8) |
4 | ? | Excl | 44-50 | 16S rRNA sequencing |
Stool | Yes CTCAE v0.3 |
4 | Yes | 2 | 3-5 weeks | Yes | Yes | Yes |
| Main findings | Patients with diarrhoea had lower microbial diversity (p< 0.01) Prior to radiotherapy, compared to patients who did not develop diarrhoea, those who did had increased abundance of Bacteroides, Dialister, Veillonella, and decreased abundance of Clostridium XI and XVIII, Faecalibacterium, Oscillibacter, Parabacteroides, Prevotella and unclassified (Genus: others) |
|||||||||||||||
The remaining studies were included and critically appraised using the Critical Appraisal Skills Programme study checklist for observational cohort studies (Table 1 and 2).52 They reported on a total of 23 women with gynaecological cancer: endometrial cancer (n=7), cervical cancer (n=16). One study included only women with a gynaecological cancer.53 The two other studies also included patients receiving treatment for gastrointestinal cancers.18,54 It was not possible to identify outcomes for the women with gynaecological cancer separately.
Table 2.
Appraisal of studies included using the CASP tool
| Author / year | Study design | Class of evidence | CASP score | Quality grade | Population studied and sample size | Primary outcome measures / results | Author’s conclusions |
|---|---|---|---|---|---|---|---|
| Manichanh et al., 2008 | Prospective cohort | B | 6/11 | III Limited/ weak |
Setting: Spain Sample:
|
Outcome measures: Stability and diversity of faecal microbiota before and after RT using similarity index and cluster analysis Comparison with healthy individuals Sub-analysis of those with and without diarrhoea GI toxicity: CTC v 0.2 Results: Patients that develop diarrhoea clustered separately from those that did not develop diarrhoea using the Pearson’s correlation and the un-weighted pair-group method using arithmetic averages (UPGMA). |
Mixed tumour type + mixed gender cohort, use of different radiotherapy fields, unable to assess whether cohort is representative as no information of eligibility, selection of participants, recruitment period. Results do not include any missing data or drop-out rates which may reflect selective reporting. |
| Nam et al., 2013 | Prospective cohort | B | 7/11 | III Limited/ weak | Setting: South Korea Sample:
|
Outcome measures: Stability and diversity of faecal microbiota before and after RT using ANOVA for difference in OTUs and principal coordinates analysis plots Comparison with healthy individuals GI toxicity: not formally measured, discussion section mentions 8/9 patients developed diarrhoea Results: Overall richness in bacterial species decreased: decrease in firmicutes by 10% (p= 0.09), increase in fusobacteria by 3% (p=0.05), decrease in bacteriodes during radiotherapy but increase after 3 months (no p-value) |
Single tumour type in cohort. Use of chemotherapy in 7/9 patients. Unable to assess whether cohort is representative as no information of eligibility, selection of participants, recruitment period. No formal measurement of GI toxicity but discussion informs 8/9 patients developed a degree of diarrhoea. Results do not include any missing data or drop-out rates which may reflect selective reporting. |
| Wang et al., 2015 | Prospective cohort | B | 7/11 | III Limited/ weak | Setting: China Sample:
|
Outcome measures: Stability and diversity of faecal microbiota before and after RT using difference in OTUs, Shannon Index, Good’s coverage, Chao1 richness estimator. Comparison with healthy individuals Sub-analysis of those with and without diarrhoea GI toxicity: CTCAE v 0.3 Results: Patients with diarrhoea had lower microbial diversity (p< 0.01) |
Mixed tumour type + mixed gender cohort, use of different radiotherapy fields, unable to assess whether cohort is representative as no information of eligibility, selection of participants, recruitment period. 9 patients were excluded from final analysis for various reasons which may reflect selective reporting. |
Outcomes of included studies
All three studies were prospective cohort studies and included patients treated with radiotherapy in a dose of 43.0 to 54.0 Gy but did not provide specific information about radiotherapy fields, the use of boosts or brachytherapy, making comparisons difficult. Whilst all studies acknowledge that radiotherapy may result in toxicity, one study did not report gastrointestinal toxicity.53 In the other studies, the Common Toxicity Criteria (CTC) version 2.018 and the Common terminology Criteria for Adverse Events (CTCAE) version 3.054 assessed bowel function. Where stool consistency was reported, 8 women (57%) developed grade 2 or 3 diarrhoea during radiotherapy. One study also assessed levels of fatigue using the Multidimensional Fatigue Inventory-20 general fatigue score and added measurement of biochemical markers of inflammation from blood samples.54
These studies suggest a correlation between changes in the intestinal microbiome and radiotherapy. All studies showed decrease in abundance and diversity of the intestinal bacterial species. One study identified 10% decrease in firmicutes (p=0.09), 3% increase in fusobacteria (p=0.05) and 9.9% in unclassified bacteria (p=0.04).53 An increase in unspecified bacterial species was seen in those with diarrhoea but patients without diarrhoea maintained their bacterial profiles.18,54 Before radiotherapy, those who developed diarrhoea compared to those who did not, had increased abundance of Bacteroides, Dialister, Veillonella (p<0.01), and a decrease in Clostridium XI and XVIII, Faecalibacterium, Oscillibacter, Parabacteroides, Prevotella and unclassified bacteria (p<0.05).54
Level of evidence is classified as B.55 The quality of the studies had CASP scores of 6/11 and 7/11 (Table 2).18,53–54
Discussion
This review highlights the lack of studies mapping changes in the gut or vaginal microbiomes in women with gynaecological malignancies before, during and following treatment.
In all identified studies the rationale for the study was clearly outlined, namely that radiotherapy can result in serious toxicity and the underlying cause remains unknown. Mainly opinion papers and reviews are used in the background sections of these papers. These studies are essentially feasibility studies which strengthen the rationale for further research. This is especially relevant in view of the emerging studies evaluating probiotics to reduce gastrointestinal toxicity.56–58 These report varying success rates perhaps due to the gap in understanding the impact of multi-modal therapies for gynaecological malignancies on the microbiome. This missing information prevents us offering treatment options targeted to the individual patient.
Two studies included multi-modal cancer therapies which draws attention to the complexity in determining how different treatment interact patho-physiologically with the microbiome in gynaecological malignancy. Whilst receiving chemotherapy was an exclusion criterion for two studies18,54, another53 included seven women receiving a variety of chemotherapeutic agents.
Only two studies assessed acute gastrointestinal toxicity formally, measuring diarrhoea. There was no difference in assessing diarrhoea between the tools used.59 As the last follow-up time-point was two months after radiotherapy, longer term gastrointestinal toxicity which manifests months or years following treatment3 was not assessed and future research needs to incorporate much longer follow-up.
Limitations of the studies
These were single-centred studies. No information regarding how many patients were treated in each centre, how many patients were eligible, how eligible patients were selected or the time scale for recruitment was provided. This may have introduced selection bias. The researchers did not specify guidelines used to stop recruitment. This raises concerns about equity for potential study participants. Information about catchment areas and number of patients treated could have provided additional insight into whether the sample population is representative for the wider population. All studies used healthy volunteers as controls but none described the selection methods or demographic information.
In all studies, no missing data were reported although one study mentions a drop-out rate of 45%. Reporting bias may potentially result if participants only included if they were able to provide all data for inclusion of the final analysis. The inclusion of different tumour types and mixed gender cohorts complicates interpretation of the results as individual patient outcomes could not be separated for analysis. In addition, all three studies were set in different countries and lacked information about other possible confounding factors such as diet, body mass index, medications affecting the gut microbiome or gastrointestinal function, treatment with previous surgery or chemotherapy and co-morbid factors.
The discussion sections addressed a number of key areas; the researchers acknowledge the limitations of 16S rRNA sequencing as not all bacterial species can be adequately identified and support future studies with larger cohorts. Two studies excluded patients treated with antibiotics before radiotherapy whilst all studies excluded those receiving antibiotics during treatment or the use of corticosteroids and immune-suppressants. Whilst the long-term impact of the use of antibiotics resulting in sustained reductions in gut microbial diversity has been acknowledged31, excluding those patients does not reflect clinical oncological management.
Research in the emerging field of the microbiome is currently biased towards the gut microbiome and little is known about interactions with the vaginal microbiome. The viromes and fungal populations have been neglected and may be important. Several studies have examined the vaginal microbiome of women at different stages of malignancy compared to that of women without cancer34–48,60–61 However, no studies reported long-term sequential follow-up data to determine the role of changes in the vaginal microbiome during progression from pre-cancerous lesions to cancer.
The studies to date mostly employ sequencing and did not include metabonomic analysis techniques. The clinical relevance of sequencing used in isolation may be limited. One older prospective study applying electronic sensing to analyse vaginal swabs with a clinical diagnosis of bacterial vaginosis, found the positive predictive value of the test was 61.5%.45 No studies were found using VOC analysis to describe the vaginal microbiome in women with gynaecological cancer nor how treatment such as surgery, chemotherapy and pelvic radiotherapy may impact on the vaginal microbiome of these women. Inclusion of metabonomic analysis techniques offers additional avenues to develop risk stratification pathways and targeted treatment options.
Limitations of the review
This review was limited to prospective cohort studies assessing the gut and vaginal microbiome of women treated for a gynaecological malignancy. No animal studies were included. In view of recent advances in novel, more targeted radiotherapy techniques, and technology available to analyse the microbiome the findings of older studies need to be interpreted cautiously. Due to the small number of studies found and the heterogeneity in study subjects in terms of treatment modalities and reporting methods, meta-analysis was not performed. Registration of this review on the International PROSPERO database reduced the risk of multiple reviews addressing the same question, limited publication bias and provided transparency for updating the review in the future.48
Implications for future research
The limited evidence implies that the role of the vaginal and gut microbiome in women treated for a gynaecological malignancy are relevant and require further study.
Conclusion
The outcomes of these studies support the hypothesis that radiotherapy changes the intestinal microbiome in patients with a decrease in abundance and diversity of the intestinal bacterial species.
Further characterisation of differences and changes in the genital and gastrointestinal microbiome and intestinal function before and after treatment could improve understanding of why some patients develop more severe toxicity and why others remain symptom-free. This may lead to new avenues to stratify those at risk and explore personalised treatment options and prevention of consequences of cancer treatments.
Supplementary Material
Acknowledgements
This paper is independent research arising from a Clinical Doctoral Research Fellowship, Ann Muls, CDRF-2014-05-004 supported by the National Institute for Health Research and Health Education England. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, Health Education England or the Department of Health.
Footnotes
Ethical/Legal Considerations
This manuscript is an original contribution not previously published and is not under consideration for publication elsewhere. Each author has contributed significantly in this systematic review.
References
- 1.Cancer Research UK. Cancer statistics- new cases. 2015 Accessed via: http://www.cancerresearchuk.org/health-professional/cancer-statistics January 2017.
- 2.Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179–92. doi: 10.1136/gutjnl-2011-300563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreyev HJN, Muls A, Norton C, Ralph C, Watson L, Shaw C, Lindsay J. Guidance: The practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol. 2014;0:1–20. doi: 10.1136/flgastro-2014-100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor A, Fernandes A, Kumar P, Muls A. Textbook of the European Gynae Oncology Society. 2016. Acute and chronic toxicity of pelvic radiotherapy. Chapter 138. 12pp. [Google Scholar]
- 5.Ferreira M, Muls A, Dearnaley D, Andreyev HJN. Microbiome and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 2014;15(3):e139–47. doi: 10.1016/S1470-2045(13)70504-7. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff S. Gut health: a new objective in medicine? BMC Medicine. 2011;9:24–38. doi: 10.1186/1741-7015-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch S, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 8.Schwabe R, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/ncr3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase D, Goulder A, Zenhausen F, Monk B, Herbst-Kralovetz M. Review: The vaginal and gastrointestinal microbiomes in gynaecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecologic Oncology. 2015;138:190–200. doi: 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Garrett W, Gallini C, Yatsunenko T, Michaud M, DuBois A, Delaney M, Punit S, Karlsson M, Bry L, Glickman J, Gordon J, et al. Enterobacteriaceae act in concert with the gut microbiome to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekirov I, Russell S, Antunes C, Finlay B. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 12.Guarner F, Malagelada J. Gut flora in health and disease. The Lancet. 2003;360:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 13.Floch M. Fecal Bacteriotherapy, Fecal Transplant, and the Microbiome. Journal of Clinical Gastroenterology. 2010;44(8):529–30. doi: 10.1097/MCG.0b013e3181e1d6e2. [DOI] [PubMed] [Google Scholar]
- 14.Hart AL, Ng SC. Inflammatory Bowel Disease. T.F.M. Publications; 2011. ISBN:9781903378823. [Google Scholar]
- 15.Marchesi J, Adams D, Fava F, Hermes G, Hirshfield G, Hold G, Quraishi M, Kinross J, Smidt H, Tuohy K, Thomas L, et al. The gut microbiota and host health: a new clinical fonrtier. Gut BMJ. 2015;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiBaise J, Zhang H, Crowell M, Krajmalnik-Brown R, Decker G, Rittmann B. Gut microbiome and its possible relationship with obesity. Mayo Clin Proc. 2008;83(4):460–469. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- 17.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiome: the hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manichanh C, Varela E, Martinez C, et al. The gut microbiome predispose to the pathophysiology of acute postradiotherapy diarrhea. Am J Gastroenterol. 2008;103:1754–61. doi: 10.1111/j.1572-0241.2008.01868.x. [DOI] [PubMed] [Google Scholar]
- 19.Ravel J, Gajera P, Abdob Z, Schneiderc GM, Koeniga S, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van de Wijgert J, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mead P. Cervical- vaginal flora of women with invasive cervical cancer. Obstet Gynecol. 1978;52(5):601–604. [PubMed] [Google Scholar]
- 22.Blythe J. Cervical bacterial flora in patients with gynecological malignancies. Am J Obstet Gynecol. 1978;131(4):438–445. doi: 10.1016/0002-9378(78)90420-9. [DOI] [PubMed] [Google Scholar]
- 23.Thadepalli H, Savage E, Rao B. Anaerobic bacteria associated with cervical neoplasma. Gynecologic Oncology. 1982;14:307–312. doi: 10.1016/0090-8258(82)90104-4. [DOI] [PubMed] [Google Scholar]
- 24.Gerstner G, Kucera H, Weghaupt K, Rotter M. Endometrial bacteriology in patients with endometrial cancer before and after primary intracavity irradiation using IR-192 and an afterloading technique. Archives of Gynecology. 1982;231:299–306. doi: 10.1007/BF02111728. [DOI] [PubMed] [Google Scholar]
- 25.Choo Y, Seto W, Ma H. Cervical- vaginal bacterial flora in patients with cervical carcinoma treated with irradiation and febrile morbidity during intracavity radium therapy. Aus NZ J Obstet Gynaec. 1984;24:34–38. doi: 10.1111/j.1479-828x.1984.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 26.Gilstrap L, III, Gibbs R, Michel T, Hauth J. Genital aerobic bacterial flora of women receiving radiotherapy for gynecologic malignancy. Gynecologic Oncology. 1986;23:35–39. doi: 10.1016/0090-8258(86)90112-5. [DOI] [PubMed] [Google Scholar]
- 27.Gordon A, Martens M, LaPread Y, Faro S. Response of lower genital tract flora to external pelvic irradiation. Gynecologic Oncology. 1989;35:233–235. doi: 10.1016/0090-8258(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 28.Guijon F, Paraskevas M, Rand F, Heywood E, Brunham R, McNicol P. Vaginal microbial flora as a cofactor in the pathogenesis if uterine cervical intraepithelial neoplasma. Int J Gynecol Obstet. 1992;37:185–191. doi: 10.1016/0020-7292(92)90379-w. [DOI] [PubMed] [Google Scholar]
- 29.Mikamo H, Sato Y, Hayasaki Y, Kawazoe K, Izumi K, Ito K, Yamada Y, Tamaya T. Intravaginal bacterial flora in patients with uterine cervical cancer. High incidence of detection of Gernerella vaginalis. J Infect Chemother. 1999;5:82–85. doi: 10.1007/s101560050013. [DOI] [PubMed] [Google Scholar]
- 30.Van Eyk N, van Schalkwyk J. Antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can. 2012;34(4):382–391. doi: 10.1016/S1701-2163(16)35222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macfarlane S. Antibiotic treatments and microbes in the gut. Environ Microbiol. 2014;16(4):919–924. doi: 10.1111/1462-2920.12399. [DOI] [PubMed] [Google Scholar]
- 32.Abeles S, Jones M, Santiago-Rodriguez T, Ly M, Klitgord N, Yooseph S, Nelson K, Pride D. Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome. 2016;4(1):39. doi: 10.1186/s40168-016-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engberts M, Verbruggen B, Boon M, van Haaften M, Heintz A. Candida and dysbacteriosis: a cytologic, population-based study of 100,605 asymptomatic women concerning cervical carcinogenesis. Cancer Cytopathology. 2007;111(5):269–274. doi: 10.1002/cncr.22947. [DOI] [PubMed] [Google Scholar]
- 34.Oh H, Kim B, Seo S, Kong J, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21:674.e1–674.e9. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Mitra A, MacIntyre D, Lee Y, Smith A, Marchesi J, Holmes E, Nicholson J, Bennett P, Kyrgiou M. The vaginal microbiome of women with cervical intraepithelial neoplasia. ESGO; International Journal of Gynecological Cancer. Conference: 19th International Meeting of the European Society of Gynaecological Oncology; 2015. pp. 976–977. [DOI] [PubMed] [Google Scholar]
- 36.Kwasniewski W, Kotanski J, Barkzynski B, Warchol W, et al. A metagenomicapproch to characteriztion of the cervix microbiome during HPV dependent carcinogenesis. International Journal of Gynecological Cancer. 2015;25(9 suppl 1):848. [Google Scholar]
- 37.Audriac-Chalfour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE. 2016;11(4):e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piyathilake C, Ollberding N, Kumar R, Macaluso M, Alvarez R, Morrow C. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev Res (Phila) 2016;9(5):357–366. doi: 10.1158/1940-6207.CAPR-15-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillet E, Meys J, Verstraelen H, Verhelst R, De Sutter P, Temmerman M, et al. Association between bacterial vaginosis and cervical intraepithelial neoplasia: systematic review and meta-analysis. PLoS One. 2012;7:e45201. doi: 10.1371/journal.pone.0045201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger N. Obesity and cancer pathogenesis. Annals of the New York Academy of Sciences. 2014;1311(1):57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere M. Systematic review: the role of the gut microbiome in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Alimentary Pharmacology & Therapeutics. 2014;40(5):409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 42.Kyrgiou M, Mitra A, Moscicki A. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2016 doi: 10.1016/j.trsl.2016.07.004. pii: S1931-5244(16)30109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maher J, McConnell H. New pathways of care for cancer survivors: adding the numbers. British Journal of Cancer. 2011;105:S5–10. doi: 10.1038/bjc.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cadwell K. The virome in host health and disease. Immunity. 2015;42(5):805–13. doi: 10.1016/j.immuni.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandiok S, Crawley BA, Oppenheim BA, Chadwick PR, Higgins S, Persaud KC. Screening for bacterial vaginosis: a novel application of artificial nose technology. J Clin Pathol. 1997;50:790–795. doi: 10.1136/jcp.50.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. Biochem. 2011;150(3):257–266. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- 47.Arasaradnam RP, Covington JA, Harmston C, Nwokolo CU. Review article: next generation diagnostic modalities in gastroenterology - gas phase volatile compound biomarker detection. Aliment Pharmacol Ther. 2014;39(8):780–9. doi: 10.1111/apt.12657. [DOI] [PubMed] [Google Scholar]
- 48.Liberati A, Altman D, Tetzlaff J, Mulrow C, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Wetering F, Heus P, Verleye L, van Tienhoven G, et al. Probiotics for the prevention or treatment of chemotherapy or radiotherapy related diarrhoea in cancer patients. Editorial Group: Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group. 2013 doi: 10.1002/14651858.CD008831.pub2. [DOI] [Google Scholar]
- 50.Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, et al. Changes in Human Fecal Microbiota Due to Chemotherapy Analyzed by TaqMan-PCR, 454 Sequencing and PCR-DGGE Fingerprinting. PLoS ONE. 2011;6(12):e28654. doi: 10.1371/journal.pone.0028654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covington J, Wedlake L, Andreyev HJN, Ouaret N. The detection of patients at risk of gastrointestinal toxicity during pelvic radiotherapy, by electronic nose and FAIMS: a pilot study. Sensors. 2012;12:1–9. doi: 10.3390/s121013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critical Appraisal Skills Programme (CASP) Study checklist for observational cohort studies. Critical Appraisal Skills Programme (CASP); Oxford: 2014. Accessed via October 2016 http://media.wix.com/ugd/dded87_e37a4ab637fe46a0869f9f977dacf134.pdf. [Google Scholar]
- 53.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiome of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang A, Ling Z, Yang Z, Kiela PR, Wang T, Wang C, et al. Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot Study. PLoS ONE. 2015;10(5):e0126312. doi: 10.1371/journal.pone.0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handu D, Moloney L, Wolfram T, Ziegler P, Acosta A, Steiber A. Academy of Nutrition and Dietetics Methodology for Conducting Systematic Reviews for the Evidence Analysis Library. Journal of the Academy of Nutrition and Dietetics. 2016;116(2):311–318. doi: 10.1016/j.jand.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13:912–915. doi: 10.3748/wjg.v13.i6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giralt J, Regadera J, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys. 2008;71:1213–1219. doi: 10.1016/j.ijrobp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010;5:31. doi: 10.1186/1748-717X-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 60.Lemen J, Bruner D. E-55. Characterising the vaginal microbiome of gynaecologic cancer patients: an intergrative review. Oncology Nursing Forum. 2016;43(2):61–62. [Google Scholar]
- 61.Verstraelen H. The association between vaginal microbiome dysbiosis with HPV acquisition, HPV persistence, and cervical intraepithelial neoplasia: a systematic review. progress PROSPERO. 2016 CRD42016035620. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.