Abstract
Background
Clinical trials evaluating the benefit of pelvic radiotherapy (PRT) in the radiotherapeutic management of patients with higher-risk prostate cancer have limited the superior field border to the S1/S2 or L5/S1 interspace. However, imaging and surgical series have demonstrated a high frequency of prostatic lymph node (LN) drainage beyond these landmarks.
Objective
To determine the patterns of radiographically defined abdominopelvic LN failures and their potential implications for PRT field design.
Design, setting, and participants
During 1992–2008, 2694 patients with localized prostate cancer were treated with prostate/seminal vesicle–only radiotherapy without PRT. Some 156 patients had their first failure within the abdominopelvic LNs, of whom 60 had isolated failures within the pelvic LNs.
Outcome measurements and statistical analysis
A radiologist reviewed all imaging and mapped each LN failure to a template consisting of 34 abdominopelvic LN stations.
Results and limitations
The median follow-up was 8.9 yr. Of patients who experienced first recurrence in the pelvic LNs (n = 60), the common iliac station was involved in 55% (n = 33), including 10% (n = 6) who had isolated common iliac failures. Use of a PRT field superior border of L5/S1 would fully cover only 42% of the first recurrences among these patients. Extending the field to cover the common iliac stations would increase coverage to 93% of recurrences. The presence of T3/T4 disease and omission of androgen-deprivation therapy both independently conferred an approximate fivefold increase in the likelihood of having a common iliac LN failure. Use of imaging as a surrogate for LN involvement is the primary study limitation.
Conclusions
Pelvic LN failures frequently occur superior to the commonly used L5/S1 landmark for PRT coverage, and use of ADT may be protective of more superior LN failures. The current RTOG 0924 trial is evaluating the benefit of PRT with extended superior coverage to L4/5 when possible, which, according to our data, should significantly improve the coverage of potential sites of failure.
Patient summary
We looked at lymph node recurrence patterns after external beam radiotherapy of the prostate in men who did not have their lymph nodes treated. We found that there was a high incidence of pelvic lymph node recurrences above the internal and external iliac lymph node regions. Therefore, the current field recommendation for pelvic lymph nodes that stops at the superior border of the internal and external iliac vessels provides inadequate coverage of common sites of cancer recurrence, namely the common iliac lymph nodes.
Keywords: Pelvic lymph nodes, Prostate cancer, Metastatic pattern, Radiotherapy
1. Introduction
The use of whole pelvic radiotherapy (WPRT) has not been associated with improvement in outcomes in randomized trials [1–3]. Multiple retrospective series have demonstrated the benefit of WPRT, and the rationale for sterilizing micrometastatic disease and/or altering the microenvironment by prophylactically destroying potential lymph nodes (LNs) that may be routes of tumor spread is sound [4,5]. Proponents of WPRT argue that the lack of benefit demonstrated by the GETUG-01 and RTOG 9413 trials may be due in part to inadequate coverage of the pelvic LNs, given that the respective superior field borders of S1/2 and L5/S1 would not provide full dose coverage to the entire superior pelvic LN basins [6].
There are extensive data from the surgical literature regarding a similar controversy for the extent and use of extended pelvic LN dissection (PLND) [7]. Mattei et al [8] assessed abdominopelvic LN drainage patterns using intraprostatic injection of 99mTc nanocolloid, single-photon emission computed tomography (SPECT)/CT or SPECT/magnetic resonance imaging (MRI), and a superextended PLND. They noted that only 63% of LNs draining the prostate were located in regions surgically evaluated in a traditional extended PLND. Furthermore, 18% (50/277) of pelvic LNs mapped to the common iliac region. Importantly, common iliac LNs are not routinely covered by WPRT.
Although understanding the LN drainage patterns for prostate cancer is important, identifying where patients actually experience recurrence within the pelvis following prostate/seminal vesicle–only radiotherapy is ultimately what should guide our pelvic radiotherapy treatment fields. To investigate this, we analyzed the patterns of radiographically defined abdominopelvic LN failures in a large series of patients treated with definitive dose-escalated radiotherapy without pelvic nodal radiotherapy.
2. Patients and methods
2.1. Patient selection
This study was conducted after approval by the institutional review board. From 1992 to 2008, 2694 consecutive patients with prostate cancer were treated with dose-escalated radiotherapy (75.6–86.4 Gy) at our institution. All patients had pathologic confirmation of prostate cancer and Gleason score by an expert urologic pathologist. All patients had localized prostate cancer as defined by negative pelvic LN imaging with either CT or MRI. Of these patients, 188 experienced a radiographic pelvic or abdominal LN failure as their first site of relapse in the context of biochemical failure. Patients with complete abdominal and pelvic imaging with MRI, CT, and/or fluorodeoxyglucose (FDG) positron emission tomograpy (PET)/CT imaging were included to determine patterns of nodal relapse; 156 men met the inclusion criteria and formed the study cohort.
2.2. Treatment
The radiotherapy techniques utilized have been described previously [9,10]. In brief, radiotherapy was delivered daily, using 42–48 fractions at 1.8 Gy/fraction with 15-MV photons to a total dose of 75.6–86.4 Gy. All patients underwent CT-based simulation with custom immobilization. The entire prostate and seminal vesicles were routinely treated. No patients received elective pelvic LN radiation, as per our institutional policy during this study. Androgen deprivation therapy (ADT) was prescribed at the discretion of the treating radiation oncologist [11]. The median ADT duration was 6 mo (range 3–36 mo), and all patient had neoadjuvant ADT and 80% received additional adjuvant ADT.
2.3. Radiographic LN mapping
A custom nodal location template consisting of 34 abdominal and pelvic LN stations with anatomic boundaries was generated as previously described (Supplementary material) [12]. Multiple adjacent LN stations in the abdomen were combined (pericolic, right colic, middle colic, and left colic were combined as “pericolic/colic”), and for bilateral abdominal LN stations they were merged to one station. In addition, the internal iliac, obturator, and hypogastric LNs were grouped together. Lymph node stations in the chest and inguinal regions were excluded from analysis and were not reviewed by the radiologist (since a previous study revealed that 0.05% of men had isolated thoracic failures, and none had inguinal failures [13]).
The date of abdominal or pelvic failure was recorded according to institutional radiology reporting, and the CT, MR, and/or PET scan(s) corresponding to this time (±3 mo) were re-reviewed by an oncologic radiologist blinded to the clinical details and outcomes at the time of image interpretation. LNs were considered suspicious on imaging if they had a short axis measurement >8 mm in the pelvis or >10 mm in the abdomen (except in the periportal/hepatoduodenal station, for which a threshold of >15 mm was used). Regardless of their size, nodes were also considered suspicious if they had a rounded shape, an irregular outline, a replaced fatty hilum (fat content in the LN hilum is a typical characteristic of benign nodes), or had uptake above background blood-pool activity on FDG-PET/CT. CT imaging of the pelvis and/or abdomen was performed routinely for patients who experienced biochemical failure (prostate-specific antigen [PSA] nadir plus 2 ng/ml), and were ordered thereafter at the discretion of the treating oncologist (usually every 4–6 mo).
The images were reviewed on a picture archiving and communications system (PACS; GE, Waukesha, WI, USA), and the involvement of each LN station was mapped to the custom template. A binary method was used to identify involvement of an LN station (involved or uninvolved) rather than documenting the number of involved LNs within each station.
2.4. Patterns of failure analyses
The location of abdominal and pelvic failures was categorized as three distinct subgroups and a composite total cohort: (1) first failure was limited to the pelvic LNs without bone, visceral, or abdominal LN metastases (n = 60); (2) first failure included the abdominal LNs with or without synchronous pelvic LN involvement, but without bone or visceral metastases (n = 31); (3) abdominal and/or pelvic LN involvement with synchronous bone metastases at the time of first failure (n = 65); and (4) all patients in groups 1–3 whose first site of failure was in the abdomen and/or pelvis.
Coverage of involved LN stations was compared with recommended pelvic LN fields from historical clinical trials and the RTOG consensus contouring atlas based on the superior field border for WPRT [2,14,15]. The superior field border definitions used were S1/S2 for the GETUG trial [8], L5/S1 for RTOG 9413 [14] and the RTOG contouring atlas, and L4/L5 for the ongoing RTOG 0924 trial (NCT01368588).
2.5. Statistical methods
Data are reported as frequency and percentage for categorical variables, and as median with range or interquartile range (IQR) for continuous variables. To determine factors that predict for LNs that arise between L4/L5 and L5/S1, univariate (including radiotherapy dose, age, pre-radiotherapy PSA, Gleason score, T stage, and use of ADT) and multivariate logistical regression analyses (including significant univariate variables) were performed, with results reported as the odds ratio (OR) and 95% confidence interval (CI). ADT use was treated as a dichotomized variable (used or not used). Two-sided p values ≤0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 20 (SPSS, Chicago, IL, USA).
3. Results
3.1. Study cohort
The median follow-up for the cohort was 8.9 yr (IQR 6.2–12.8). The median follow-up for all subgroups (first failure in pelvic LNs, first failure in abdominal LNs, and first failure in abdominal or pelvic LNs with bone metastases) ranged from 8.0 to 9.5 yr. Among all the patients (n = 156), 30.8% had clinical stage ≥T3a disease, 41.0% had a biopsy Gleason score ≥8, 28.8% had PSA >20 ng/dl, 62.8% were considered high risk according to National Comprehensive Cancer Network (NCCN) criteria, and 62.8% received ADT. Baseline characteristics were generally similar for all subgroups analyzed (Table 1). Abdominopelvic imaging was by CT in 75% (n = 117), MRI in 17.3% (n = 27), and PET/CT in 7.7% (n = 12) of patients.
Table 1.
Baseline characteristics
| Total cohort (n = 156) a | First failure
|
|||
|---|---|---|---|---|
| Pelvic LN(s) only (n = 60) | ABD ± pelvic LN(s) (n = 31) | ABD ± pelvic LNs with BMs (n = 65) | ||
| Median age, yr (range) | 69 (45–87) | 67 (45–83) | 69 (49–87) | 68 (46–79) |
| T stage, n (%) | ||||
| ≤T2a | 58 (37.2) | 21 (35.0) | 14 (45.2) | 23 (35.4) |
| T2b,c | 50 (32.1) | 16 (26.7) | 12 (38.7) | 22 (33.8) |
| ≥T3a | 48 (30.8) | 23 (38.3) | 5 (16.1) | 20 (30.8) |
| Gleason score, n (%) | ||||
| ≤6 | 19 (12.2) | 6 (10.0) | 5 (16.1) | 8 (12.3) |
| 7 (3 + 4) | 29 (18.6) | 7 (11.7) | 6 (19.4) | 16 (24.6) |
| 7 (4 + 3) | 44 (28.2) | 25 (41.7) | 3 (9.7) | 16 (24.6) |
| ≥8 | 64 (41.0) | 22 (36.7) | 17 (54.8) | 25 (38.5) |
| Pretreatment PSA, n (%) | ||||
| <10 ng/ml | 64 (41.0) | 22 (36.7) | 12 (38.7) | 30 (46.2) |
| 10–20 ng/ml | 47 (30.1) | 21 (35.0) | 8 (25.8) | 18 (27.7) |
| >20 ng/ml | 45 (28.8) | 17 (28.3) | 11 (35.5) | 17 (26.2) |
| NCCN risk group, n (%) | ||||
| Low | 2 (1.3) | 0 (0.0) | 0 (0.0) | 2 (3.1) |
| Intermediate | 56 (35.9) | 19 (31.7) | 10 (32.3) | 27 (41.5) |
| High | 98 (62.8) | 41 (68.3) | 21 (67.7) | 36 (55.4) |
| Median RT dose, Gy (range) | 81.0 (75.6–86.4) | 81.0 (75.6–86.4) | 86.4 (75.6–86.4) | 81.0 (75.6–86.4) |
| Neoadjuvant ADT use, n (%) | ||||
| Yes | 98 (62.8) | 41 (68.3) | 22 (71.0) | 35 (53.8) |
| No | 58 (37.2) | 19 (31.7) | 9 (29.0) | 30 (46.2) |
| Median follow-up, yr (IQR) | 8.9 (6.2–12.8) | 8.0 (5.3–11.3) | 8.3 (5.5–12.7) | 9.5 (6.7–13.3) |
| Follow-up scan type, n (%) | ||||
| CT | 117.00 (75.0) | |||
| MRI | 27.00 (17.3) | |||
| PET/CT | 12.00 (7.7) | |||
ABD = abdominal; ADT = androgen deprivation therapy; BMs = bone metastases; CT = computed tomography; IQR = interquartile range; LN = lymph node; MRI = magnetic resonance imaging; RT = radiotherapy; PET = positron emission tomography; PSA = prostate-specific antigen; NCCN = National Comprehensive Cancer Network.
Includes all first failures.
3.2. Patterns of failure
Among the entire cohort, 28.2% of patients experienced recurrence in a single LN station. Although recurrence in two (25.6%) or three (12.2%) LN stations simultaneously was relatively common, <10% of patients experienced recurrence in five or more pelvic LN stations simultaneously. These patterns were similar in subgroup 1 (first failure in pelvic LNs) and subgroup 3 (first failure in abdominal or pelvic LNs with bone metastases; Fig. 1). Among the entire cohort, 40.4% did not experience recurrence in the abdomen at the time of first failure, and 9.6% of patients had recurrence in a single abdominal LN station. Patients Recurrence in two abdominal LN stations and three abdominal LN stations occurred in 13.5% and 9.6% of patients, respectively, and approximately 20% of abdominal recurrences occurred in five or more abdominal LN stations simultaneously.
Fig. 1.
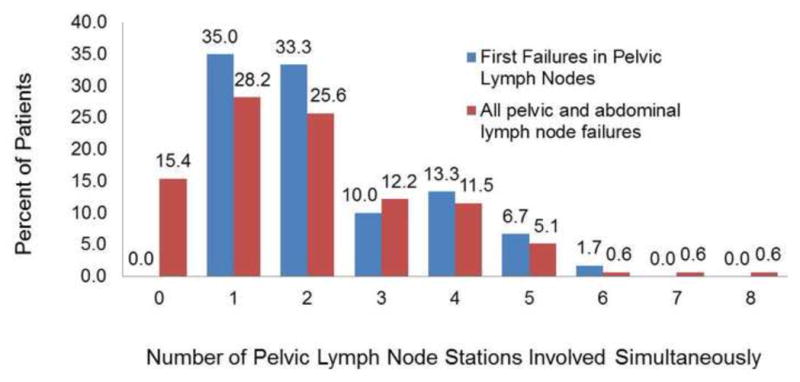
Distribution of the number of pelvic lymph node stations involved simultaneously in patients who had their first failure solely within the pelvic lymph nodes (group 1, n = 60) and all patients (group 4, n = 156) in the cohort.
The locations of pelvic and abdominal LN failures at the time of first failure are listed in Table 2. The left and right common iliac, external iliac, and internal iliac/hypogastric/obturator LNs were all involved to a similar extent (30.0–33.3%) for patients with first recurrence in the pelvis alone (group 1) and for the total cohort (group 4, 25.0–34.6%). In the total cohort of abdominal and pelvic first failures, the para-aortic LNs were most commonly involved (51.3%), followed by the retro-caval and inter-aortocaval LN stations (both 30.8%).
Table 2.
Detailed failure rates and locations
| Nodal station | First failure
|
All pelvic and ABD LN failures a | ||||||
|---|---|---|---|---|---|---|---|---|
| Pelvic LN(s) only | ABD ± pelvic LN(s) | ABD ± pelvic LNs with simultaneous bone metastases | ||||||
|
| ||||||||
| Patients involved (n = 60) (%) | LN stations involved (n = 137) (%) | Patients involved (n = 31) (%) | LN stations involved (n = 127) (%) | Patients involved (n = 65) (%) | LN stations involved (n = 309) (%) | Patients involved (n = 156) (%) | LN stations involved (n = 709) (%) | |
| Diaphragmatic | – | – | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Gastro-esophageal | – | – | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Retrocrural | – | – | 16.1 | 3.9 | 10.8 | 2.3 | 11.5 | 2.5 |
| Left gastric/lesser curvature | – | – | 3.2 | 0.8 | 1.5 | 0.3 | 1.3 | 0.3 |
| Right gastric/greater curvature | – | – | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Left inferior phrenic | – | – | 3.2 | 0.8 | 1.5 | 0.3 | 1.9 | 0.4 |
| Gastroduodenal/pancreaticoduodenal | – | – | 0.0 | 0.0 | 1.5 | 0.3 | 0.6 | 0.1 |
| Celiac axis/common hepatic | – | – | 6.5 | 1.6 | 3.1 | 0.6 | 3.2 | 0.7 |
| Superior mesenteric | – | – | 6.5 | 1.6 | 1.5 | 0.3 | 1.9 | 0.4 |
| Inferior mesenteric | – | – | 0.0 | 0.0 | 30.8 | 6.5 | 19.2 | 4.2 |
| Periportal/hepatoduodenal | – | – | 12.9 | 3.1 | 4.6 | 1.0 | 4.5 | 1.0 |
| Anterior/inferior pancreatic and hepatic | – | – | 0.0 | 0.0 | 1.5 | 0.3 | 0.6 | 0.1 |
| Splenic | – | – | 0.0 | 0.0 | 1.5 | 0.3 | 0.6 | 0.1 |
| Pericolic/colic (right, middle, left) | – | – | 3.2 | 0.8 | 0.0 | 0.0 | 0.6 | 0.1 |
| Mesenteric/juxtaintestinal | – | – | 3.2 | 0.8 | 1.5 | 0.3 | 1.3 | 0.3 |
| Renal hilar | – | – | 32.3 | 7.9 | 21.5 | 4.5 | 21.8 | 4.8 |
| Interaortocaval | – | – | 51.6 | 12.6 | 27.7 | 5.8 | 30.8 | 6.8 |
| Pre-caval | – | – | 29.0 | 7.1 | 24.6 | 5.2 | 19.9 | 4.4 |
| Retro-caval | – | – | 45.2 | 11.0 | 35.4 | 7.4 | 30.8 | 6.8 |
| Lateral caval | – | – | 6.5 | 1.6 | 6.2 | 1.3 | 6.4 | 1.4 |
| Pre-aortic | – | – | 35.5 | 8.7 | 24.6 | 5.2 | 23.1 | 5.1 |
| Retro-aortic | – | – | 32.3 | 7.9 | 24.6 | 5.2 | 21.2 | 4.7 |
| Lateral aortic (para-aortic) | – | – | 80.6 | 19.7 | 60.0 | 12.6 | 51.3 | 11.3 |
| Cecal/ileocolic | – | – | 3.2 | 0.8 | 0.0 | 0.0 | 0.6 | 0.1 |
| Superior rectal | 3.3 | 1.5 | 12.9 | 3.1 | 6.2 | 1.3 | 6.4 | 1.4 |
| Presacral | 10.0 | 4.4 | 6.5 | 1.6 | 18.5 | 3.9 | 14.7 | 3.2 |
| Promontory | 16.7 | 7.3 | 0.0 | 0.0 | 13.8 | 2.9 | 13.5 | 3.0 |
| Right common iliac | 33.3 | 14.6 | 6.5 | 1.6 | 24.6 | 5.2 | 26.9 | 5.9 |
| Left common iliac | 33.3 | 14.6 | 0.0 | 0.0 | 21.5 | 4.5 | 25.0 | 5.5 |
| Right external iliac | 30.0 | 13.1 | 3.2 | 0.8 | 33.8 | 7.1 | 29.5 | 6.5 |
| Left external iliac | 33.3 | 14.6 | 3.2 | 0.8 | 33.8 | 7.1 | 34.6 | 7.6 |
| Right internal Iliac/hypogastric/obturator | 31.7 | 13.9 | 3.2 | 0.8 | 16.9 | 3.6 | 23.7 | 5.2 |
| Left internal Iliac/hypogastric/obturator | 33.3 | 14.6 | 3.2 | 0.8 | 21.5 | 4.5 | 25.6 | 5.6 |
| Perirectal | 3.3 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | 0.3 |
ABD = abdominal; LN = lymph node.
Includes all first failures.
3.3. Pelvic LN failure: first subgroup
To gain a better insight into the patterns of failure that could theoretically be prevented by pelvic radiotherapy, the subgroup of patients who experienced a pelvic LN failure alone (without concurrent abdominal LN failure or bone metastases) was analyzed (n = 60). Among these patients, 59% experienced failure in the external iliac LNs, 59% in the internal iliac/hypogastric/obturator LNs, and 55% in the common iliac LNs (Fig. 2). Isolated failures alone in the external iliac, internal iliac/hypogastric/obturator, and common iliac LN stations were observed in 19%, 14%, and 10% of patients, respectively, while 16% of patients had failure in these three nodal stations simultaneously. Using superior field borders of S1/S2, L5/S1, and L4/L5 would provide target coverage of the identified pelvic LN failures for 33.3%, 41.7%, and 93.4% of patients, respectively (Fig. 3).
Fig. 2.
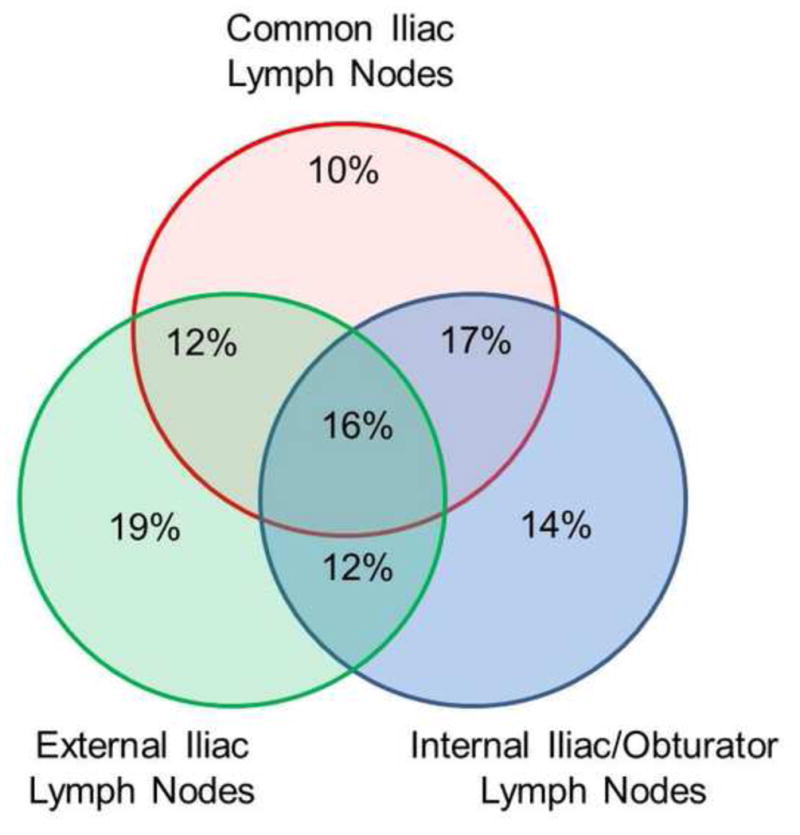
Venn diagram of patients who experienced recurrence solely in the pelvic lymph nodes (group 1) and the distribution of relapse in the common iliac lymph nodes, external iliac lymph nodes, and the internal iliac/obturator/hypogastric lymph nodes.
Fig. 3.
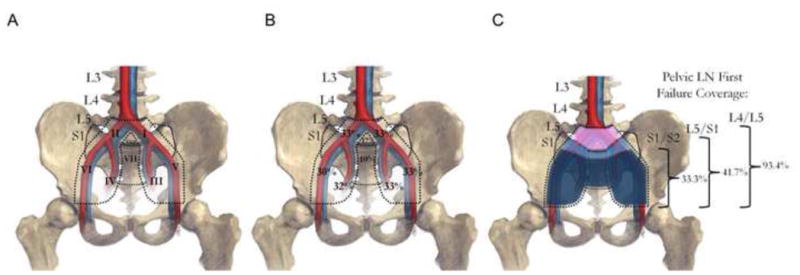
Graphical representation of the distribution of pelvic failures in group 1. (A) Map of pelvic lymph node (LN) stations (I, left common iliac; II, right common iliac; III, left internal iliac/obturator/hypogastric; IV, right internal iliac/obturator/hypogastric; V, left external iliac; VI, right external iliac; VII, presacral; VIII, promontory. (B) Percentage of patients who experienced recurrence in each pelvic lymph node station. (C) Percentage of patients who would have had all pelvic lymph nodes covered if the pelvic radiotherapy field was at S1/2, L5/S1, and L4/5.
To determine predictors of involvement of common iliac and superior promontory LNs that would require extension of the superior field border from L5/S1 to cover the superior border of the common iliac LNs, logistic regression analysis was performed. On multivariate analysis, only ≥T3a disease (OR 5.06, 95% CI 1.19–21.44; p = 0.03) and use of ADT (OR 0.17, 95% CI 0.04–0.67; p = 0.01) independently impacted the presence of common iliac and superior promontory LNs. Importantly, patients who received ADT had higher T stage (p = 0.002), Gleason score (p < 0.001), and baseline PSA (p = 0.002; Supplementary Table 1). Notably, we could not identify any clinicopathologic variables that predicted who would most likely fail first with a pelvic-only recurrence versus an abdominal-only recurrence.
4. Discussion
The importance of elective treatment of pelvic LNs in prostate cancer with either PLND or radiotherapy has been a source of controversy. However, there is continued interest in determining if altering pelvic LN treatment would confer oncologic benefits if performed adequately on the appropriate patient population [16]. Critical factors that may impact the benefit of WPRT in prostate cancer are (1) the baseline risk of pelvic LN involvement and the risk of subsequent LN spread; (2) the extent and adequacy of radiotherapy pelvic LN coverage; (3) concurrent ADT; and (4) the radiotherapy dose to pelvic LNs.
First, to demonstrate a benefit of treating the pelvic LNs, patients must be at sufficient risk of pelvic LN involvement and/or subsequent recurrence. Using modern nomograms, a man with low or favorable intermediate-risk cancer has a likelihood of LN involvement at diagnosis of <5% (Memorial Sloan Kettering Cancer Center online nomogram). Therefore, use of WPRT or PLND is unlikely to have oncologic benefits in this setting. One of the criticisms of the GETUG randomized trial comparing WPRT to prostate-only radiotherapy is that only 45% of the men included in the trial had a risk of LN involvement >15% [2,6].
Second, sufficient coverage of micrometastatic disease is required to demonstrate a benefit of pelvic LN treatment. The GETUG randomized trial implemented routine radiation field coverage to the S1/2 interspace [2,6]. This field design allows coverage of the majority of the internal, external, obturator/hypogastric, and presacral LNs. However, the superior aspect of most of these LN stations would receive ≤50% of the prescribed dose owing to the use of older treatment techniques that would be inadequate for eradicating micrometastatic disease [17]. Similarly, RTOG 9413 and the current RTOG contouring atlas recommend extension of the superior border to the L5/S1 interspace [14,18]. This technique will omit coverage of LNs in the common iliac stations and the superior aspect of the promontory.
Multiple imaging and surgical series have evaluated the patterns of lymphatic drainage and involvement of micrometastatic disease at the time of prostate cancer presentation [8,19]. One approach for determining lymphatic drainage patterns has been to use sentinel LNs as detected by SPECT imaging. Mattei et al [8] used this technique on 34 patients with cT1–2 prostate cancer via intraprostatic injection of 99mTc nanocolloid and confirmed LNs intraoperatively, followed by an extended PLND. They concluded that extended PLND should include the common iliac LNs at least up to the ureteric crossing. However, even with this approach, only 75% of all prostate-draining LNs would be covered. In addition, they noted that 18% of sentinel pelvic LNs were found in the common iliac region. In a larger confirmatory series of 74 patients, Joniau et al [19] used a similar technique and found that 21% (88/421) of pelvic LNs drained to the common iliac region. Multiple other surgical series have confirmed the ability of the extended PLND to increase the detection of LN invasion [20,21].
We estimate that when the superior border is at the L5/S1 interspace, only 41.7% of patients with pelvic LN recurrence would have had full coverage of all sites of recurrence. By contrast, using a superior border to cover the superior promontory and common iliac LNs, 93.4% of pelvic recurrences would have received a full radiation dose. These data in part provide a potential explanation for why randomized trials of WPRT to date have not clearly demonstrated an improvement in outcome. The current open randomized trial assessing the benefit of WPRT, RTOG 0924, now recommends that the routine superior border be raised to L4/5 to include all of the common iliac LNs (NCT01368588). This may be especially relevant for patients with ≥T3a disease, as we demonstrated on multivariate analysis that these patients have a fivefold higher risk of failure above L5/S1 and would warrant the superior border to be extended at least to L4/L5. Ideally, the clinical target volume would encompass all of the common iliac LNs, which may be superior to L4/5 in select patients, and we would recommend using patient anatomy rather than bony landmarks to ensure full coverage.
Finally, use of ADT may impact the benefit of WPRT. The dose of 45 Gy commonly used to treat pelvic LNs may be inadequate for eradicating microscopic disease. Thus, by combining WPRT with ADT there is likely to be a synergistic ability to eradicate tumor cells by capitalizing on the DNA repair inhibitory effects of ADT [22–24]. This is one potential hypothesis as to why the subgroup analysis from RTOG 9413 demonstrated that WPRT plus ADT had improved outcomes over prostate-only radiotherapy [14].
The study limitations warrant further discussion. Most notably, this is a retrospective series and therefore is subject to inherent biases. CT imaging was guided by physician concern after biochemical failure and was not standardized at a strict PSA cutoff, nor were PSA values available at the time of imaging. This could notably bias our estimates of common iliac LN failure that occurred in conjunction with lower pelvic LN failure, in which the common iliac LN failure was a subsequent event. However, we did identify 10% of patients who had isolated common iliac LN involvement, consistent with sentinel LN series [8]. CT, MRI, and PET/CT imaging modalities have inherent limitations regarding the sensitivity and specificity in capturing pathologically involved LNs, and no patients had pathologic confirmation of their nodal disease. However, to the best of our knowledge, a similar large series with pathologic confirmation of all LN failures does not exist. Finally, although we documented LN failures above L5/S1, our data do not attempt to answer the question of whether there is clinical benefit of WPRT, as no patients in our series received pelvic radiotherapy.
5. Conclusions
In summary, we demonstrated that among patients treated with modern dose-escalated radiotherapy without pelvic LN treatment, radiographic failures are frequently found above the L5/S1 interspace. For patients deemed to need WPRT, we recommend extension to include the common iliac LNs. This is consistent with the ongoing RTOG 0924 trial, which will test the clinical benefit of extended pelvic radiotherapy.
Supplementary Material
Table 3.
Multivariate logistic regression
| Odds ratio (95% confidence interval) | p value | |
|---|---|---|
| T stage | ||
| ≤T2a | Reference | |
| T2b/c | 0.59 (0.14–2.53) | 0.48 |
| ≥T3a | 5.06 (1.19–21.44) | 0.03 |
| Neoadjuvant ADT | 0.17 (0.04–0.67) | 0.01 |
ADT = androgen deprivation therapy, analyzed as a dichotomized variable (yes vs no).
Acknowledgments
Funding/Support and role of the sponsor: None.
We would like to thank Sam Spratt for providing the illustration for Figure 3. We also thank the Prostate Cancer Foundation for a 2014 Rebecca and Nathan Milikowsky Young Investigator Award (D.E.S).
Footnotes
Author contributions: Michael J. Zelefsky had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition of data: Spratt, Vargas, Golia Pernicka.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Spratt, Vargas, Golia Pernicka.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Spratt.
Obtaining funding: None.
Administrative, technical, or material support: Pei.
Supervision: Zelefsky.
Other: None.
Financial disclosures: Michael J. Zelefsky certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–55. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–73. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
- 3.Asbell S, Krall J, Pilepich M, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77-06. Int J Radiat Oncol Biol Phys. 1988;15:1307–16. doi: 10.1016/0360-3016(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 4.Aizer AA, James BY, McKeon AM, et al. Whole pelvic radiotherapy versus prostate only radiotherapy in the management of locally advanced or aggressive prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1344–9. doi: 10.1016/j.ijrobp.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 5.Pan C, Kim K, Taylor J, et al. Influence of 3D-CRT pelvic irradiation on outcome in prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:1139–45. doi: 10.1016/s0360-3016(02)02818-3. [DOI] [PubMed] [Google Scholar]
- 6.Morikawa LK, Roach M. Pelvic nodal radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int J Radiat Oncol Biol Phys. 2011;80:6–16. doi: 10.1016/j.ijrobp.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 7.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–65. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Mattei A, Fuechsel FG, Dhar NB, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008;53:118–25. doi: 10.1016/j.eururo.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–9. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–92. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratt DE, Zumsteg ZS, Pei X, et al. Predictors of castration-resistant prostate cancer after dose-escalated external beam radiotherapy. Prostate. 2015;75:175–82. doi: 10.1002/pros.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morón F, Szklaruk J. Learning the nodal stations in the abdomen. Learning. 2014;80:958. doi: 10.1259/bjr/64292252. [DOI] [PubMed] [Google Scholar]
- 13.Zumsteg ZS, Spratt DE, Romesser PB, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol. 2015;194:1624–30. doi: 10.1016/j.juro.2015.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–55. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–7. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa LK, Roach M. Pelvic nodal radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int J Radiat Oncol Biol Phys. 2011;80:6–16. doi: 10.1016/j.ijrobp.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 17.Witte MG, Heemsbergen WD, Bohoslavsky R, et al. Relating dose outside the prostate with freedom from failure in the Dutch trial 68 Gy vs. 78 Gy. Int J Radiat Oncol Biol Phys. 2010;77:131–8. doi: 10.1016/j.ijrobp.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–7. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joniau S, Van den Bergh L, Lerut E, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013;63:450–8. doi: 10.1016/j.eururo.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 20.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–65. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–7. doi: 10.1016/j.eururo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2103;3:1245–53. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt DE, Evans MJ, Davis BJ, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015;75:4688–96. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone–DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


