Abstract
Though functional social support has been shown to serve as a protective factor for HIV viral load suppression in other populations, scant research has examined this relationship among men who have sex with men (MSM) in the United States. We assessed characteristics of social support; effects of social support on HIV viral load; and moderation by social support of the relationship between psychosocial indicators of a synergistic epidemic (syndemic) and HIV viral load. We analyzed longitudinal data from HIV-positive MSM using antiretroviral therapy (ART) who were enrolled in the Multicenter AIDS Cohort Study between 2002—2009 (n=712). First, we conducted reliability assessments of a one-item social support measure. Then, we conducted a series of generalized longitudinal mixed models to assess our research questions. Moderation was assessed using an interaction term. A three-level (low/medium/high) social support variable demonstrated high reliability (ICC=0.72; 95% CI: 0.70, 0.75). Black and Hispanic MSM reported lower social support than their White counterparts (p<.0001). Recent sero-conversion was associated with higher social support (p<.05). Higher numbers of concomitant syndemic indicators (depression, polysubstance use, and condomless anal sex) were associated with lower social support (p<.0001). Medium and high social support levels were associated with greater viral load suppression and lower viral load means (p<.0001). Social support moderated the relationships between syndemic and HIV viral load (p<.05). HIV-positive MSM, particularly those of color, may benefit greatly from interventions that can successfully boost functional social support. Creating strengths-based interventions may also have particularly high impact among HIV-positive MSM with the highest psychosocial burdens.
INTRODUCTION
Globally, social relationships have been shown to have a robust effect on health. Stronger social relationships have been demonstrated to be a robust protective factor against morbidity and all-cause mortality (Holt-Lunstad, Smith, & Layton, 2010; Lett et al., 2007; Murphy et al., 2008). To better understand the effect of social relationships on health, researchers have attempted to refine and distinguish patterns of social relationships into functional and structural realms, wherein functional social relationships can be loosely defined as perceived and received social support, and structural social relationships are comprised of marital status, breadth and density of social networks, social integration and other factors. Both functional and structural social relationships have been shown to have similarly protective effects on health (Holt-Lunstad et al., 2010). Though causality has been difficult to prove and biopsychosocial pathways have not been disentangled, increased functional social support has been shown to positively impact medication adherence, including among people living with HIV/AIDS (PLWHA) who are on antiretroviral therapy (ART) (DiMatteo, 2004; Kelly, Hartman, Graham, Kallen, & Giordano, 2014). Research on a sample of HIV positive African-American and Latino/a MSM and women in Los Angeles demonstrated that HIV status disclosure to higher numbers of social network members was predictive of retention in medical care, although this effect was not seen in MSM alone, and a multidimensional measure of social support was not significantly predictive of care retention (Wohl et al., 2011).
Functional social support has important health-promoting effects for PLWHA, as multiple studies have shown that greater functional social support is associated with lower viral load and greater viral load suppression (Amberbir, Woldemichael, Getachew, Girma, & Deribe, 2008; Burgoyne, 2005; A. Knowlton et al., 2006; A. R. Knowlton et al., 2007; Power et al., 2003; Simoni, Frick, & Huang, 2006). HIV viral suppression reduces the risk of overall mortality, the likelihood of opportunistic infections, and the odds of transmitting HIV to sexual partners (Cohen et al., 2011; Palella Jr et al., 1998). Because increasing the overall proportion of people with undetectable viral loads may, in turn, greatly reduce population-level viral load and HIV incidence, understanding factors like functional social support that are associated with viral suppression is essential to the Treatment as Prevention (TasP) model (Das et al., 2010; Montaner et al., 2010). However, there exists little knowledge about the effects of functional social support among HIV-positive men who have sex with men (MSM) in the United States. Previous investigations into associations between functional social support and viral load have utilized data from samples of injection drug users (A. Knowlton et al., 2006; A. R. Knowlton et al., 2007), heterosexuals (Simoni et al., 2006), men and women combined (Power et al., 2003), and populations outside the U.S. (Amberbir et al., 2008; Burgoyne, 2005). Each of these studies found that greater functional social support is associated with lower viral load (Amberbir et al., 2008; Burgoyne, 2005; A. Knowlton et al., 2006; A. R. Knowlton et al., 2007; Power et al., 2003; Simoni et al., 2006). Because MSM constituted 62% of new HIV diagnoses throughout the United States in 2011, researchers and practitioners alike should understand if functional social support is associated with viral load and suppression in this population (Control & Prevention, 2013).
Though we know little about the relationship between functional social support and viral load among MSM in the U.S., researchers have established robust associations between viral load suppression, ART adherence and often co-occurring psychosocial factors such as depression, substance use, and sexual risk-taking that have been shown to form a synergistic epidemic (syndemic) among MSM (Blashill et al., 2014; M. R. Friedman et al., 2015; Ron Stall, Friedman, & Catania, 2008; Ron Stall et al., 2003). This syndemic cascade among MSM has been theorized and empirically shown to develop as a result of early life adversities, including sexuality-related stigma and marginalization experienced during childhood and adolescence (Herrick et al., 2013; Ron Stall et al., 2003). Compared with heterosexuals, sexual minorities experience significant disparities in social support and peer/family/school connectedness, which can mediate the development of negative psychosocial health outcomes resulting from adversity (Coulter, Herrick, Friedman, & Stall, 2016; Frost, Meyer, & Schwartz, 2016; Saewyc et al., 2009).
Based on these findings, it is likely that HIV positive MSM suffer severe and persistent social support deficits, which are strongly associated with psychosocial outcomes that in turn inflect HIV treatment outcomes. One study found that while adherence partially mediated the relationship between syndemic and viral load, other factors may also be also at play—and one may be resiliencies (M. R. Friedman et al., 2015). For the last several years, researchers have been calling for more resilience research, to empirically assess how diverse protective factors modify the relationship between a psychosocial syndemic and health outcomes (Buttram, 2015; Herrick et al., 2013; Herrick et al., 2011). Functional social support may be one of many factors buffering the association between psychosocial syndemic indicators and viral load.
The purpose of this study was to empirically examine the effects of a one-item measure of functional social support on HIV viral load in a longitudinal cohort that includes a large subset of HIV-positive MSM. First, we examined sociodemographic correlates of functional social support. Second, we investigated independent effects of functional social support on viral load and viral load suppression, controlling for known risk factors (psychosocial syndemic indicators) and ART adherence. Finally, we examined whether social support moderated the relationship between psychosocial syndemic indicators and viral load. We hypothesized that the harmful effects of syndemic indicators on viral load would be modified for those with higher social support versus those with lower social support.
METHODS
Sample
The Multicenter AIDS Cohort Study (MACS), designed as an observational cohort study of the natural and treated history of HIV/AIDS in gay/bisexual men, has enrolled successive cohorts since 1984 in four U.S. cities: Chicago, Pittsburgh, Los Angeles, and Baltimore (KASLOW et al., 1987; Silvestre et al., 2006). MACS participants are invited every six months to undertake comprehensive behavioral and medical questionnaires, blood draws, and physical exams. The current analyses center on biopsychosocial measures collected from MACS participants who were HIV-positive and who reported any sexual activity with men, ART use, and responded to a survey question about functional social support between 2002—2009.
Measures
Sociodemographics
Participants self-reported race/ethnicity and age (date of birth) at enrollment and annual income at most recent visit. Bisexual behavior was addressed via self-reported sexual activity between 2002—2009 (Friedman et al., 2014; Herrick et al., 2013). To avoid potential confounding, recent sero-conversion was also included as a covariate in analyses of viral load suppression. MSM who sero-converted between study visits 38 and 50 were treated as “recent sero-converters” during the first three study visits after sero-conversion (Friedman et al., 2014; M. R. Friedman et al., 2015)
HIV viral load
At each visit, blood from HIV-positive participants was assayed for HIV viral load levels using the COBAS Ultrasensitive Amplicor HIV-1 monitor assay for HIV RNA (Roche Molecular Systems, Branchburg, NJ), with a sensitivity of 50 copies of HIV per RNA/mm3. We analyzed two forms of this variable: (1) continuous viral load, in which we log10-transformed viral load values; and (2) binary viral load suppression, where we used a cut-off point of <200 copies/mm3 to distinguish detectable from undetectable viral load (Department of Health and Human Services, 2015; Health Services Resource Administration, 2015).
ART adherence
We used an ART adherence scale measuring four levels of self-reported adherence since last visit. Participants responded to a single-item question (“On average, how often did you take your medication as prescribed?”) assessing frequency of all ART medication use as prescribed since last visit. Response options included 100% of the time; 95 – 99% of the time; 75 – 94% of the time; and <75% of the time (Carrico et al., 2014; Shoptaw et al., 2012).
Syndemic indicators
The sum (0–3) per subject, by visit, of the number of psychosocial syndemic indicators: depression symptoms (CES-D score>15), polysubstance use (2 or more illegal substances used at least monthly), and any condomless anal sex with casual male partners; treated as a continuous variable. This summed variable has been shown in this sample to be highly predictive of both ART adherence and HIV viral load (M. R. Friedman et al., 2015), with significant synergistic effects on ART adherence when depressive symptoms and polysubstance use are concomitant (M. Friedman et al., 2015). Further details about this measure and its component variables can be found elsewhere (M. R. Friedman et al., 2015).
Functional social support
The following one-item question assessed social support at each visit: “Is there someone you can talk to about things that are important to you – someone you can count on for understanding or support?” Response options included: (1) “No, no one”; (2) “Yes, one person”; (3) 2–3 people; (4) 4–5 people; and (5) 6 or more people. Based on response distributions, we used a tripartite variable that categorized social support as low (0 or 1 people), medium (2–3 people), and high (4 or more people). This measure aligns with the definition for perceptions of social support (“perception of availability of emotional, informational, tangible, or belonging support if needed”) measured in existing scales (Hanson, Elmståhl, Isacsson, & Ranstam, 1997; Holt-Lunstad et al., 2010; I. G. Sarason, Levine, Basham, & Sarason, 1983; Seeman & Berkman, 1988), and shares characteristics with a single-item social support measure that assessed the number of people who could be counted on for tangible assistance, which predicted morbidity (Blake & McKay, 1986).
Statistical analysis
As the one-item social support measure had not to our knowledge been previously tested, we first assessed its reliability within this sample. We used the publicly available SAS macro, %icc9, which estimates intraclass correlation coefficients (ICC) (Hertzmark E. and Spiegelman, 2010). We assessed the overall ICC and the within-subject coefficient of variation for social support, after adjusting for the fixed effect of time (Hankinson et al., 1995). We considered 0.70 as the minimum threshold for adequate measurement quality (Terwee et al., 2007).
To assess our primary research questions, we used SAS PROC GLIMMIX to fit generalized longitudinal mixed models, with a repeated measures statement controlling for within-subject variance over time (13 visits, from 2002—2009), adjusting for sociodemographics, ART adherence, and syndemic indicators. We first explored whether social support was correlated with the effects of time, sociodemographic covariates, and syndemic indicators. Then, we tested whether social support was associated with HIV viral load. Finally, we assessed whether social support moderated the effect of syndemics on HIV viral load by adding an interaction term (i.e., social support × syndemic indicators). We reported parameter estimates and least-squares means estimates for variables of interest. Least-squares means were estimated using observed margins for outcomes (social support and viral load); within-group analyses were conducted by level and further adjusted using the studentized maximum modular approach to minimize error rates associated with heteroscedasticity and subgroup multiplicity. Least-squares means estimates from moderation models were used in graphing regressed moderation slopes. Analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Sociodemographics
Table 1 shows that of the 712 HIV-positive, sexually-active MSM on ART in this sample, 14.9% identified as Hispanic; 59.3% as White; and 24.7% as Black. Participants were most represented in Los Angeles (32.3%), while Baltimore (23.2%), Pittsburgh (21.3%), and Chicago (23.2%) shared similar proportions. A large majority of participants were 40 years of age or older (87.9%) by 2009. Over one-third (36.2%) of participants reported an annual income of less than $20,000. The vast majority of participants (93.8%) reported sexual behavior only with men from 2002—2009; 6.2% reported bisexual behavior.
Table 1.
Sociodemographics of sexually active HIV-positive MSM on ART in the MACS, wave 50 (n=712)
| Sociodemographics | Subcategory | N (%) |
|---|---|---|
| Race/ethnicity | ||
| Hispanic | 106 (14.9%) | |
| White, non-Hispanic | 422 (59.3%) | |
| Black, non-Hispanic | 176 (24.7%) | |
| Other | 8 (1.1%) | |
| MACS site | ||
| Baltimore | 165 (23.2%) | |
| Chicago | 165 (23.2%) | |
| Pittsburgh | 152 (21.3%) | |
| Los Angeles | 230 (32.3%) | |
| Cohort | ||
| 1984 | 313 (44.0%) | |
| 1987 | 72 (10.1%) | |
| 2002 | 327 (45.9%) | |
| Age | ||
| 20–39 | 86 (12.1%) | |
| 40 or older | 626 (87.9%) | |
| Income | ||
| <$10,000–$19,999 | 242 (36.2%) | |
| $20,000 or above | 427 (63.8%) | |
| Sexual behavior (waves 38–50) | ||
| Sex with men only (MSMO) | 668 (93.8%) | |
| Sex with men and women (MSMW) | 44 (6.2%) | |
Reliability of functional social support measure
Nearly all participants (98.3%) completed the social support question more than once, with an average of 9.7 completions over 13 potential visits and a total of 6796 observations (data not shown). Table 2 shows that, adjusting for time, the coefficient of reliability (ICC) was 0.74 (95% CI: 0.72, 0.77), and the estimated coefficient of within-subject variance was 0.17 (95% CI: 0.17, 0.18) for the original social support scale (1–5); observed means ranged from 3.14 to 3.45 by study visit, with standard errors from 0.05 to 0.07. Over study visits 38–50 (2002—2009), 3.2% of subjects reported no social support at a given observation; 21.0% reported 1 social support person; 38.7% reported 2–3 people; 23.7% reported 4–5 people; and 13.2% reported 6 or more people, approximating a normal distribution.
Table 2.
Characteristics of social support scale (original scale=1–5; refined scale=0–2)
| Original Scale (1–5) | Refined Scale (0–2) | |||
|---|---|---|---|---|
| Wave (study visit) | Mean | Standard error | Mean | Standard error |
| 38 | 3.4484 | 0.06946 | 1.2735 | 0.04827 |
| 39 | 3.3504 | 0.06267 | 1.208 | 0.04354 |
| 40 | 3.1971 | 0.05585 | 1.0899 | 0.0388 |
| 41 | 3.1868 | 0.05321 | 1.0974 | 0.03697 |
| 42 | 3.2035 | 0.05167 | 1.0893 | 0.0359 |
| 43 | 3.1421 | 0.05226 | 1.0533 | 0.03631 |
| 44 | 3.2311 | 0.05117 | 1.1046 | 0.03555 |
| 45 | 3.1857 | 0.05062 | 1.0976 | 0.03517 |
| 46 | 3.1533 | 0.05117 | 1.0657 | 0.03555 |
| 47 | 3.1788 | 0.05032 | 1.0824 | 0.03496 |
| 48 | 3.1961 | 0.05136 | 1.0882 | 0.03568 |
| 49 | 3.17 | 0.04906 | 1.0626 | 0.03409 |
| 50 | 3.2039 | 0.04858 | 1.0921 | 0.03375 |
| Social support frequency, by subject | Value | % | Value | % |
| 1 (no one) | 3.2% | 0 (low: 0–1 person) | 24.2% | |
| 2 (one person) | 21.0% | -- | -- | |
| 3 (2–3 people) | 38.7% | 1 (medium: 2–3 people) | 38.7% | |
| 4 (4–5 people) | 23.7% | -- | -- | |
| 5 (6 or more people) | 13.2% | 2 (high: 4 or more) | 36.9% | |
| Reliability assessment | Estimate | 95% CI | Estimate | 95% CI |
| Intra-class coefficient | 0.74 | 0.72, 0.77 | 0.72 | 0.70, 0.75 |
| Within-subject variability | 0.17 | 0.17, 0.18 | 0.38 | 0.36, 0.39 |
Models adjusted for wave, race/ethnicity, annual income below $20,000, ART adherence, bisexual behavior, recent sero-conversion, and syndemics count.
Based on these data, we computed and assessed the reliability of a refined social support scale based on tertiles, reflecting low (0–1 people); medium (2–3 people), and high (4 or more people) functional social support. This refined scale had similar reliability, with an ICC of 0.72 (95% CI: 0.70, 0.75). Though the estimated coefficient of within-subject variance (0.38; 95% CI: 0.36, 0.39) was wider than in the original scale, distributions were similarly centered, ranging from 1.05 to 1.27 (using a scaling of 0–2) by study visit; and standard errors were relatively lower, ranging from 0.03 to 0.05 by visit. We used this refined scale in subsequent analyses for ease of interpretation.
Correlates of functional social support
Table 3 shows that social support did not vary significantly over time (F=1.33; p=0.20). Participants with annual income less than $20,000; Black and Hispanic MSM; bisexually behaving men; and men younger than 40 all reported significantly lower social support than their counterparts (all p-values<.0001). MSM who had sero-converted within the last three study visits prior to observation reported higher social support than MSM who had been living with HIV for longer periods (t=2.34; p<.05). Adherence was not significantly associated with social support (F=1.52, p=0.21). Higher numbers of concomitant psychosocial syndemics were strongly associated with lower social support (F=40.00; p<.0001): compared with those experiencing one syndemic conditions, men experiencing no syndemic conditions reported higher social support (t=4.92; p<.0001); and men experiencing one syndemic condition reported higher social support than men experiencing two syndemic conditions (t=8.2; p<.0001). However, there were no significant social support differences between reporting two syndemic conditions and reporting three syndemic conditions (t=1.56; p=0.53).
Table 3.
Sociodemographic and psychosocial predictors of social support (refined scale) among sexually active HIV-positive MSM in the MACS, n=712.
| Characteristic | Estimate | Standard Error | F | Pr > F | t Value | Pr > |t| |
|---|---|---|---|---|---|---|
| Intercept | 0.7658 | 0.1157 | - | - | 6.62 | <.0001 |
| Wave (omnibus) | - | - | 1.33 | 0.1958 | - | - |
| Recent sero-converter | 0.4007 | 0.1709 | - | - | 2.34 | 0.0191 |
| Non-recent sero-converter (REF) | 0 | . | - | - | . | . |
| ART adherence scale (omnibus) | - | - | 1.52 | 0.2078 | - | - |
| MSMW | −0.2404 | 0.04469 | - | - | −5.38 | <.0001 |
| MSMO (REF) | 0 | . | - | - | . | . |
| Income <$20,000 | −0.3380 | 0.02115 | - | - | −15.98 | <.0001 |
| Income> $20,000 (REF) | 0 | . | - | - | . | . |
| Black | −0.1515 | 0.02407 | - | - | −6.29 | <.0001 |
| Hispanic | −0.5743 | 0.03123 | - | - | −18.39 | <.0001 |
| Other | 0.02368 | 0.09004 | - | - | 0.25 | 0.9999 |
| White (REF) | 0 | . | - | - | . | . |
| Age <40 | −0.3652 | 0.03270 | - | - | −11.17 | <.0001 |
| Age 40 or above (REF) | 0 | . | - | - | . | . |
| Syndemics count (omnibus) | - | - | 40.00 | <.0001 | - | - |
| Syndemics count=0 vs. 1 | 0.1055 | 0.02145 | - | - | 4.92 | <.0001 |
| Syndemics count=1 vs. 2 | 0.2638 | 0.03218 | - | - | 8.20 | <.0001 |
| Syndemics count=2 vs. 3 | 0.1497 | 0.09571 | - | - | 1.56 | 0.5284 |
Models adjusted for wave (time), race/ethnicity, annual income below $20,000, ART adherence, bisexual behavior, recent sero-conversion, and syndemics count. Syndemics count differences compared with next, by-level (e.g. syndemics count=0 vs. syndemics count=1; syndemics count=1 vs. syndemics count=2; syndemics count=2 vs. syndemics count=3).
Associations between social support and HIV viral load
Table 4 shows that, compared to MSM with low social support, MSM with medium or high social support had lower mean viral loads (155.1 copies/mm3 vs. 96.7 copies/mm3 and 103.0 copies/mm3, respectively; p-values<.0001). Mean viral loads did not differ significantly between medium and high social support strata (t=-1.01; p=0.6781; data not shown). Compared to MSM with low social support, MSM with medium or high social support were more likely to have suppressed viral loads (76.4% vs. 85.2% vs. 83.8%, respectively; p-values<.0001) in a similarly adjusted model (Model 3).
Table 4.
Effects of social support on HIV viral load and moderation of effects of syndemics on viral load by social support: estimated means by level of support, with F-values for moderation and class effects.
| Model | Effect | Estimate | Standard Error | F | Pr > F | LSME | t Value | Pr > |t| |
|---|---|---|---|---|---|---|---|---|
| 1. Viral load (copies/mm3) regressed on syndemics count and social support | Intercept | 0.9998 | 0.06944 | - | - | - | 14.40 | <.0001 |
| Syndemics count (omnibus) | - | - | 6.93 | 0.0001 | - | - | - | |
| Low social support | REF | - | - | - | 155.1 copies/mm3 | - | - | |
| Medium social support | −0.09837 | 0.01508 | - | - | 96.7 copies/mm3 | −6.52 | <.0001 | |
| High social support | −0.08466 | 0.01561 | - | - | 103.0 copies/mm3 | −5.42 | <.0001 | |
| 2. Model 1 with interaction term | Intercept | 0.8620 | 0.1664 | - | - | - | 5.18 | <.0001 |
| Syndemics count (omnibus) | - | - | 5.08 | 0.0016 | - | - | - | |
| Low social support | REF | - | - | - | 154.7 copies/mm3 | - | - | |
| Medium social support | −0.09804 | 0.01504 | - | - | 96.6 copies/mm3 | −6.52 | <.0001 | |
| High social support | −0.08436 | 0.01557 | - | - | 102.9 copies/mm3 | −5.42 | <.0001 | |
| Social support × Syndemics count | - | - | 2.47 | 0.0220 | - | - | - | |
| 3. Undetectable viral load regressed on syndemics count and social support | Intercept | 0.6470 | 0.3707 | - | - | - | 1.75 | 0.0810 |
| Syndemics count (omnibus) | - | - | 3.37 | 0.0176 | - | - | - | |
| Low social support | REF | - | - | - | 76.4% | - | - | |
| Medium social support | 0.5730 | 0.09024 | - | - | 85.2% | 6.35 | <.0001 | |
| High social support | 0.4670 | 0.09253 | - | - | 83.8% | 5.05 | <.0001 | |
| 4. Model 3 with interaction term | Intercept | 1.5997 | 0.9250 | - | - | - | −1.73 | 0.0838 |
| Syndemics count (omnibus) | - | - | 1.94 | 0.1213 | - | - | - | |
| Low social support | REF | - | - | - | 76.5% | - | - | |
| Medium social support | 0.5750 | 0.09080 | - | - | 85.2% | 6.33 | <.0001 | |
| High social support | 0.4685 | 0.09300 | - | - | 83.9% | 5.04 | <.0001 | |
| Social support × Syndemics count | - | - | 2.46 | 0.0221 | - | - | - |
Moderation by social support of relationship between syndemic indicators and viral load
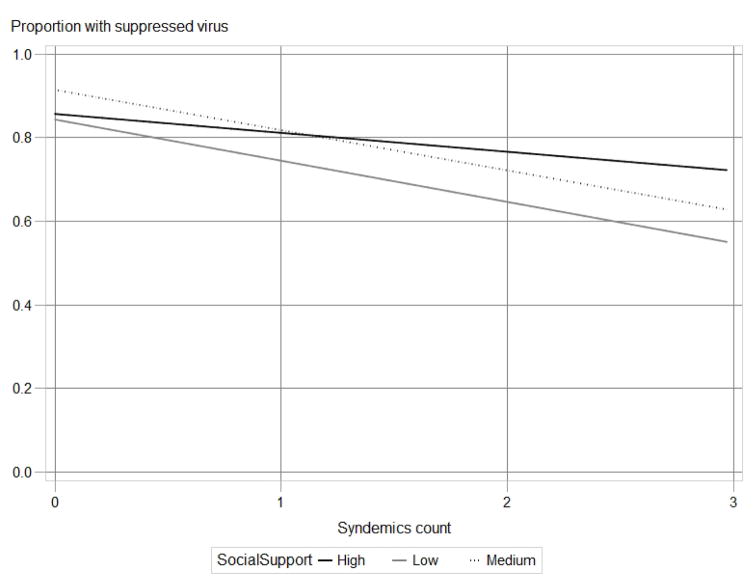
Table 4 shows that syndemic indicators were strongly associated with log10 viral loads (F=6.93; p<.0001) and proportions with suppressed virus (F=3.37; p<.05). Social support had a significant moderating effect (F=2.55; p<.05) on the relationship between syndemic indicators and log10 viral load (Model 2). Social support also had a significant moderating effect (F=2.46; p<.05) on the relationship between syndemic indicators and proportion of participants with suppressed viral load (Model 4). Figure 1 illustrates that, while increasing numbers of concomitant syndemics were negatively associated with proportion of viral load suppression, MSM reporting high social support demonstrated a gentler trajectory as syndemic indicators increased compared to MSM with low reported social support. The proportion of MSM with suppressed virus who reported no syndemic indicators varied little by low, medium, and high social support (81.2% vs. 84.2% vs. 84.5%, respectively), whereas larger differences in viral suppression proportion by low, medium, and high social support (47.5% vs. 53.8% vs. 73.3%) were seen among MSM reporting three syndemic indicators (see Appendix 1). Appendix 1 also shows that the proportion of MSM with suppressed virus who reported one syndemic indicator was highest among those citing medium social support: 73.6% (low social support) vs. 87.5% (medium social support) vs. 84.8% (high social support). This pattern was also demonstrated in MSM reporting two syndemic indicators: 76.0% (low) vs. 82.5% (medium) vs. 73.0% (high).
Figure 1.
Moderation by social support of relationship between syndemics count (0–3) and viral suppression: regression plot using least-squares estimated means.
DISCUSSION
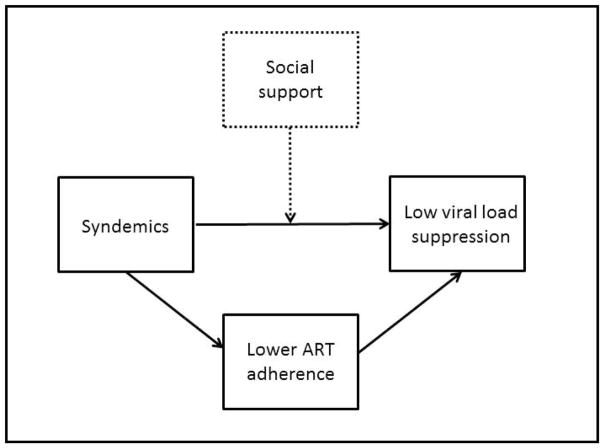
Our findings show that, among sexually active HIV-positive MSM on ART in the Multicenter AIDS Cohort Study, functional social support has a significant protective effect on viral load suppression. These results add to the literature showing functional social support’s protective effect on viral load in other populations (Amberbir et al., 2008; Burgoyne, 2005; A. Knowlton et al., 2006; A. R. Knowlton et al., 2007; Power et al., 2003; Simoni et al., 2006). Previous research has demonstrated that early social adversities inflect the production of syndemics; and that the effects of syndemics do not end with HIV acquisition, but persist to high effect on ART adherence and viral load suppression, whereby ART adherence significantly but incompletely mediates the relationship between syndemics and viral load (Blashill et al., 2014; M. R. Friedman et al., 2015; Herrick et al., 2013). These studies suggest that MSM with concomitant depression, substance use, and sexual risk behaviors are likely at significant risk for poorer HIV-related health outcomes as well as secondary transmission of HIV to sexual partners. Our findings suggest that, by moderating the relationship between syndemic and viral load in model controlling for ART adherence, functional social support has a strong protective effect on viral load suppression among these men with the highest number of concomitant psychosocial conditions and thus holds an important place in the syndemic model as extended to HIV-positive MSM (see Appendix 2).
Social support has been theorized as an important component in the larger framework of resiliency in sexual minority populations (Herrick, Egan, Coulter, Friedman, & Stall, 2014; Herrick, Stall, Goldhammer, Egan, & Mayer, 2014). Perceived and endured stigma related to gay and bisexual identities, MSM behavior, and HIV positive status are strongly related to the mental and physical health of HIV positive MSM; fear of being judged and/or rejected for one’s sexuality and HIV status can lead gay and bisexual men to avoid seeking friendships, relationships, and mental and physical health care, and to non-disclosure of sexuality and HIV status to existing support networks (Valdiserri, 2002; Wolitski, Pals, Kidder, Courtenay-Quirk, & Holtgrave, 2009). Our results provide preliminary evidence of the potency of functional social support in the health of marginalized and stigmatized groups. Our findings add to the evidence that MSM who are Black and Hispanic, bisexually behaving, and/or relatively young may have thinner social support structures, which is theoretically related to the social adversities they disproportionately experience (Ayala, Bingham, Kim, Wheeler, & Millett, 2012; Frost et al., 2016; Saewyc et al., 2009). Findings may be particularly salient for young Black MSM, among whom HIV incidence rates are increasing most sharply in the U.S. (Prejean et al., 2011). Structural interventions for young MSM of color that are designed to provide safe spaces and to strengthen functional and structural social support may be particularly valuable (Friedman, 2014; Garcia et al., 2015; Hull, 2013). While we assessed a theoretical component of functional social support in this study, other resiliencies such as structural social support, community attachment, and social capital may also combine to confer even greater benefit to HIV-positive MSM (Herrick, Egan, et al., 2014; Herrick, Stall, et al., 2014).
This study is subject to several limitations. First, while the MACS is a longstanding prospective cohort study, it should not be considered representative of MSM in the U.S.: participants are disproportionately over the age of 40 and urban, and may be more likely to be in HIV care than other HIV-positive MSM due to the dedicated linkage services the study offers; furthermore, recruitment strategies focused on men who identified as gay or bisexual, and who may thus experience greater sexuality-related social support than MSM who do not identify as such. Second, the social support variable that we analyzed is a one-item measure and conflates the constructs of having people to talk to and to count on, without differentiating the source of support (e.g., friend, family, care provider) or fully discerning the type of support (e.g., informational, emotional, structural) received. In addition, this measure does not assess the quality of support received and how satisfying it is; previous research has indicated that some social support can be negatively perceived (B. R. Sarason, Shearin, Pierce, & Sarason, 1987) and, in certain high-stigma environments, may negatively influence ART medication uptake (Waddell & Messeri, 2006). Although test-retest reliability was high for this one-item measure, construct validity was not assessed. Other validated social support measures, such as the multidimensional scale of perceived social support, may be significantly more robust (Zimet, Dahlem, Zimet, & Farley, 1988). As such, our one-item question may have underestimated the true relationship between social support, viral suppression, syndemic, and effect modification of the syndemic-viral load association. Third, although we controlled for adherence in our models using an established adherence measure (Carrico et al., 2014; Shoptaw et al., 2012), self-reported adherence is subject to recall and response biases, which may explain why we found a relationship between social support and viral load but no relationship between social support and adherence (Lu et al., 2008). Fourth, our measure of syndemic indicators (a count of concomitant depression symptoms, polysubstance use, and condomless anal sex with casual male partners) treats each component with equal weighting, hence ignoring effect size differences within these variables in relation to viral load (Ronald Stall, Coulter, Friedman, & Plankey, 2015; Tsai & Burns, 2015). Though this measure reflects the key syndemic indicators measured longitudinally in this sample, other variables shown to be important to the syndemic model for MSM have not been longitudinally assessed in the MACS, such as sexual compulsivity, sex work, intimate partner violence, and arrest history (Herrick et al., 2013; Kurtz, 2008; Parsons, Grov, & Golub, 2012; Stall R, 2012). While our syndemic measure has been shown to have strong progressive effects on HIV viral load and ART adherence (M. R. Friedman et al., 2015), and significant synergistic effects between component variables (polysubstance use and depressive symptoms) on ART adherence in this sample (M. Friedman et al., 2015), we did not test whether social support moderated synergistic effects in this analysis (Ronald Stall et al., 2015; Tsai & Burns, 2015). Finally, as with all cohort studies, the MACS sample is subject to missing visits as well as missing data within visits; while our models used all observed data, we were only able to fully assess study visits that included complete data. However, these limitations have generally served to heighten a conservative modelling approach, as it is likely that our sampling, measures, and statistical adjustments have served to underestimate the extent of the main and moderating effects conferred by social support on viral load: using more robust measures with a more diverse and representative population of MSM who were less engaged in longstanding HIV research projects would likely find more, not less, variance in both viral load and social support. Despite these limitations, our findings have implications for research and clinical care.
We recommend further psychometric research on the reliability and validity of our one-item measure, to better assess its alignment with validated measures. Future research should explore whether this measure can be feasibly and acceptably used by clinicians to assess the social context of individual patients, which may predict treatment success or failure. One-item surveys, though lacking multi-dimensional nuance, have been used widely in health surveys and have several advantages, including interpretability and low resource burden, and have been utilized in social support assessment (Blake & McKay, 1986; Bowling, 2005). We also recommend the design and testing of small-scale interventions across the HIV care continuum that attempt to increase functional social support experienced by HIV-positive MSM from family, friends, health-care practitioners, and medical social workers. As viral suppression is generally reached only after effective linkage to care, retention in care, ART uptake, and ART adherence are achieved, further studies should examine how social support both predicts outcomes across this continuum and moderates negative syndemic effects on these outcomes among MSM. There is mounting evidence that the emotional and physical benefits of social support are significant, especially to those most burdened by pre-existing psychosocial health conditions.
In the U.S., social isolation is increasing, with Americans reporting fewer confidants over the past 20 years (McPherson, Smith-Lovin, & Brashears, 2006). Men who have sex with men face additional social integration deficits due to stigmatization over their sexual expression, and suffer elevated rates of psychosocial health conditions that are in turn associated with HIV incidence, poorer ART adherence, and lower viral suppression (Blashill et al., 2014; M. R. Friedman et al., 2015; Herrick et al., 2013). Concentrated efforts to increase meaningful social supports in HIV-positive MSM of color, and those presenting with depression, substance use, and sexual risk behavior, may have significant benefits for HIV-related health and wellness and the Treatment as Prevention (TasP) model even when these syndemic indicators are resistant to modulation.
Acknowledgments
Sources of funding: Data collected by the Multicenter AIDS Cohort Study is supported by National Institutes of Health grants UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041.
Appendix 1. Least-squares means estimates of proportion of MSM with suppressed viral loads by syndemics count and social support level, waves 38–50 (n=712)
| Syndemic s count |
Social support level |
Estimat e |
Standar d Error |
t Val ue |
Pr > |t| | Alph a |
Lower | Upper | Mean with suppre ssed viral load |
Standard Error Mean |
Lower Mean (s.e.) |
Upper Mean (s.e.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Low | 1.4601 | 0.1220 | 11.96 | <.0001 | 0.05 | 1.2208 | 1.6993 | 0.8115 | 0.01866 | 0.7722 | 0.8454 |
| 0 | Medium | 1.6760 | 0.08853 | 18.93 | <.0001 | 0.05 | 1.5024 | 1.8496 | 0.8424 | 0.01176 | 0.8179 | 0.8641 |
| 0 | High | 1.6937 | 0.09191 | 18.43 | <.0001 | 0.05 | 1.5135 | 1.8739 | 0.8447 | 0.01206 | 0.8196 | 0.8669 |
| 1 | Low | 1.0254 | 0.1001 | 10.25 | <.0001 | 0.05 | 0.8292 | 1.2215 | 0.7360 | 0.01944 | 0.6962 | 0.7723 |
| 1 | Medium | 1.9453 | 0.09945 | 19.56 | <.0001 | 0.05 | 1.7503 | 2.1402 | 0.8749 | 0.01088 | 0.8520 | 0.8948 |
| 1 | High | 1.7206 | 0.1010 | 17.04 | <.0001 | 0.05 | 1.5226 | 1.9185 | 0.8482 | 0.01300 | 0.8209 | 0.8720 |
| 2 | Low | 1.1531 | 0.1511 | 7.63 | <.0001 | 0.05 | 0.8568 | 1.4494 | 0.7601 | 0.02756 | 0.7020 | 0.8099 |
| 2 | Medium | 1.5468 | 0.1647 | 9.39 | <.0001 | 0.05 | 1.2239 | 1.8698 | 0.8245 | 0.02384 | 0.7727 | 0.8664 |
| 2 | High | 0.9933 | 0.1967 | 5.05 | <.0001 | 0.05 | 0.6077 | 1.3790 | 0.7297 | 0.03880 | 0.6474 | 0.7988 |
| 3 | Low | −0.09988 | 0.4002 | −0.25 | 0.8029 | 0.05 | −0.8844 | 0.6847 | 0.4751 | 0.09980 | 0.2923 | 0.6648 |
| 3 | Medium | 0.1527 | 0.4139 | 0.37 | 0.7122 | 0.05 | −0.6587 | 0.9641 | 0.5381 | 0.1029 | 0.3410 | 0.7239 |
| 3 | High | 1.0121 | 0.8940 | 1.13 | 0.2577 | 0.05 | −0.7406 | 2.7647 | 0.7334 | 0.1748 | 0.3229 | 0.9407 |
Appendix 2
Figure 2.
Heuristic of functional social support and syndemics in HIV-positive MSM.
Footnotes
Conflicts of interest: There are no conflicts of interest to report.
References
- Amberbir A, Woldemichael K, Getachew S, Girma B, Deribe K. Predictors of adherence to antiretroviral therapy among HIV-infected persons: A prospective study in southwest Ethiopia. BMC Public Health. 2008;8(1):265. doi: 10.1186/1471-2458-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala G, Bingham T, Kim J, Wheeler DP, Millett GA. Modeling the impact of social discrimination and financial hardship on the sexual risk of HIV among Latino and Black men who have sex with men. Am J Public Health. 2012;102(S2):S242–S249. doi: 10.2105/AJPH.2011.300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake RL, McKay DA. A single-item measure of social supports as a predictor of morbidity. The Journal of family practice. 1986 [PubMed] [Google Scholar]
- Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, … Safren SA. Psychosocial syndemics are additively associated with worse ART adherence in HIV-Infected individuals. AIDS Behav. 2014:1–6. doi: 10.1007/s10461-014-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. Just one question: If one question works, why ask several? J Epidemiol Community Health. 2005;59(5):342–345. doi: 10.1136/jech.2004.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. Exploring direction of causation between social support and clinical outcome for HIV-positive adults in the context of highly active antiretroviral therapy. AIDS Care. 2005;17(1):111–124. doi: 10.1080/09540120412331305179. [DOI] [PubMed] [Google Scholar]
- Buttram ME. The Social Environmental Elements of Resilience Among Vulnerable African American/Black Men Who Have Sex With Men. Journal of Human Behavior in the Social Environment. 2015;25(8):923–933. doi: 10.1080/10911359.2015.1040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Shoptaw S, Cox C, Stall R, Li X, Ostrow DG, … Plankey MW. Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67(5):508–513. doi: 10.1097/QAI.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Pilotto JH. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Control, C. f. D., & Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012. 2013;17(4) [Google Scholar]
- Coulter RW, Herrick A, Friedman MR, Stall RD. Sexual-Orientation Differences in Positive Youth Development: The Mediational Role of Bullying Victimization. Am J Public Health. 2016;0:e1–e7. doi: 10.2105/AJPH.2015.303005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, Colfax GN. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015 Retrieved from https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/15/virologic-failure-and-suboptimal-immunologic-response.
- DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychology. 2004;23(2):207. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- Friedman M, Coulter R, Silvestre A, Plankey M, Shoptaw S, PJ PS, … Stall R. Someone to count on: the moderating influence of social support on the relationship between syndemics and HIV viral load. Paper presented at the AIDS Impact Conference (oral presentation); Amsterdam, NL. 2015. [Google Scholar]
- Friedman MR. “Is Silk open tonight?”: Lessons learned from Project Silk, an HIV prevention demonstration project for young African American MSM and transgender people. Paper presented at the American Public Health Association; New Orleans, LA. 2014. [Google Scholar]
- Friedman MR, Stall R, Silvestre AJ, Mustanski B, Shoptaw S, Surkan PJ, … Plankey MW. Stuck in the middle: longitudinal HIV-related health disparities among men who have sex with men and women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;66(2):213–220. doi: 10.1097/QAI.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MR, Stall R, Silvestre AJ, Wei C, Shoptaw S, Herrick A, … Plankey MW. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. Aids. 2015;29(9):1087–1096. doi: 10.1097/QAD.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DM, Meyer IH, Schwartz S. Social support networks among diverse sexual minority populations. American Journal of Orthopsychiatry. 2016;86(1):91. doi: 10.1037/ort0000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Parker C, Parker RG, Wilson PA, Philbin MM, Hirsch JS. “You’re Really Gonna Kick Us All Out?” Sustaining Safe Spaces for Community-Based HIV Prevention and Control among Black Men Who Have Sex with Men. PLoS One. 2015;10(10):e0141326. doi: 10.1371/journal.pone.0141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Manson J, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer epidemiology biomarkers & prevention. 1995;4(6):649–654. [PubMed] [Google Scholar]
- Hanson BS, Elmståhl S, Isacsson SO, Ranstam J. Reliability and validity assessments of measures of social networks, social support and control—results from the Malmö Shoulder and Neck Study. Scand J Public Health. 1997;25(4):249–257. doi: 10.1177/140349489702500407. [DOI] [PubMed] [Google Scholar]
- Health Services Resource Administration. HIV/AIDS Bureau Performance Measures. 2015 Retrieved from http://hab.hrsa.gov/deliverhivaidscare/revisedportfoliofaq.pdf.
- Herrick AL, Egan JE, Coulter RW, Friedman MR, Stall R. Raising sexual minority youths’ health levels by incorporating resiliencies into health promotion efforts. Am J Public Health. 2014;104(2):206–210. doi: 10.2105/AJPH.2013.301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL, Lim SH, Plankey MW, Chmiel JS, Guadamuz TT, Kao U, … Stall R. Adversity and syndemic production among men participating in the Multicenter AIDS Cohort Study: a life-course approach. Am J Public Health. 2013;103(1):79–85. doi: 10.2105/AJPH.2012.300810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL, Lim SH, Wei C, Smith H, Guadamuz T, Friedman MS, Stall R. Resilience as an untapped resource in behavioral intervention design for gay men. AIDS Behav. 2011;15(1):25–29. doi: 10.1007/s10461-011-9895-0. [DOI] [PubMed] [Google Scholar]
- Herrick AL, Stall R, Goldhammer H, Egan JE, Mayer KH. Resilience as a research framework and as a cornerstone of prevention research for gay and bisexual men: theory and evidence. AIDS Behav. 2014;18(1):1–9. doi: 10.1007/s10461-012-0384-x. [DOI] [PubMed] [Google Scholar]
- Hertzmark E, Spiegelman D. Intraclass correlation coefficients and their 95 percent confidence intervals. 2010 Retrieved from http://www.hsph.harvard.edu/donna-spiegelman/software/icc9/
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS medicine. 2010;7(7):859. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull SJ. Evaluation of the acceptance journeys campaign: Results from a rolling cross sectional evaluation with 3 comparison groups. Paper presented at the 141st APHA Annual Meeting and Exposition; November 2–November 6, 2013.2013. [Google Scholar]
- KASLOW RA, OSTROW DG, DETELS R, PHAIR JP, POLK BF, RINALDO CR. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: results from the steps study. Journal of the Association of Nurses in AIDS Care. 2014;25(5):405–413. doi: 10.1016/j.jana.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A, Arnsten J, Eldred L, Wilkinson J, Gourevitch M, Shade S, … Team I. Individual, interpersonal, and structural correlates of effective HAART use among urban active injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2006;41(4):486–492. doi: 10.1097/01.qai.0000186392.26334.e3. [DOI] [PubMed] [Google Scholar]
- Knowlton AR, Arnsten JH, Gourevitch MN, Eldred L, Wilkinson JD, Rose CD, … Team IS. Microsocial environmental influences on highly active antiretroviral therapy outcomes among active injection drug users: The role of informal caregiving and household factors. Journal of Acquired Immune Deficiency Syndromes. 2007;46:S110–S119. doi: 10.1097/QAI.0b013e31815767f8. [DOI] [PubMed] [Google Scholar]
- Kurtz SP. Arrest histories of high-risk gay and bisexual men in Miami: Unexpected additional evidence for syndemic theory. J Psychoactive Drugs. 2008;40(4):513–521. doi: 10.1080/02791072.2008.10400657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, Catellier DJ, Carney RM, Berkman LF, … Schneiderman N. Social support and prognosis in patients at increased psychosocial risk recovering from myocardial infarction. Health Psychology. 2007;26(4):418. doi: 10.1037/0278-6133.26.4.418. [DOI] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, Wilson IB. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12(1):86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- McPherson M, Smith-Lovin L, Brashears ME. Social isolation in America: Changes in core discussion networks over two decades. American sociological review. 2006;71(3):353–375. [Google Scholar]
- Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, … Daly P. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. The Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BM, Elliott PC, Le Grande MR, Higgins RO, Ernest CS, Goble AJ, … Worcester MU. Living alone predicts 30-day hospital readmission after coronary artery bypass graft surgery. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15(2):210–215. doi: 10.1097/HJR.0b013e3282f2dc4e. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, … Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Grov C, Golub SA. Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: Further evidence of a syndemic. Journal Information. 2012;102(1) doi: 10.2105/AJPH.2011.300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R, Koopman C, Volk J, Israelski DM, Stone L, Chesney MA, Spiegel D. Social support, substance use, and denial in relationship to antiretroviral treatment adherence among HIV-infected persons. AIDS Patient Care and STDS. 2003;17(5):245–252. doi: 10.1089/108729103321655890. [DOI] [PubMed] [Google Scholar]
- Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, … Lansky A. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saewyc EM, Homma Y, Skay CL, Bearinger LH, Resnick MD, Reis E. Protective factors in the lives of bisexual adolescents in North America. Am J Public Health. 2009;99(1):110–117. doi: 10.2105/AJPH.2007.123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason BR, Shearin EN, Pierce GR, Sarason IG. Interrelations of social support measures: Theoretical and practical implications. J Pers Soc Psychol. 1987;52(4):813. [Google Scholar]
- Sarason IG, Levine HM, Basham RB, Sarason BR. Assessing social support: the social support questionnaire. J Pers Soc Psychol. 1983;44(1):127. [Google Scholar]
- Seeman TE, Berkman LF. Structural characteristics of social networks and their relationship with social support in the elderly: who provides support. Social Science & Medicine. 1988;26(7):737–749. doi: 10.1016/0277-9536(88)90065-2. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, … Plankey MW. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23(8):576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre AJ, Hylton JB, Johnson LM, Houston C, Witt M, Jacobson L, Ostrow D. Recruiting minority men who have sex with men for HIV research: results from a 4-city campaign. Journal Information. 2006;96(6) doi: 10.2105/AJPH.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychology. 2006;25(1):74. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Coulter R, Friedman MR, Plankey MW. Commentary on” Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept” by A. Tsai and B. Burns. Soc Sci Med. 2015;145:129. doi: 10.1016/j.socscimed.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Stall RFM, Buttram ME, Kurtz SP. Party, play and pay: associations between transactional sex and high-risk UAI among substance-using MSM in South Florida. Paper presented at the 19th International AIDS Conference; Washington, D.C. 2012. [Google Scholar]
- Stall R, Friedman M, Catania JA. Interacting epidemics and gay men’s health: A theory of syndemic production among urban gay men. Unequal opportunity: Health disparities affecting gay and bisexual men in the United States. 2008:251–274. [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J, … Catania JA. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health. 2003;93(6) doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, … de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Burns BF. Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept. Social Science & Medicine. 2015;139:26–35. doi: 10.1016/j.socscimed.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiserri RO. HIV/AIDS stigma: an impediment to public health. Am J Public Health. 2002;92(3):341–342. doi: 10.2105/ajph.92.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell EN, Messeri PA. Social support, disclosure, and use of antiretroviral therapy. AIDS Behav. 2006;10(3):263–272. doi: 10.1007/s10461-005-9042-x. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, … Carpio F. Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS Behav. 2011;15(6):1098–1110. doi: 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, Holtgrave DR. The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless and unstably housed persons living with HIV. AIDS Behav. 2009;13(6):1222–1232. doi: 10.1007/s10461-008-9455-4. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]