Abstract
Background
There is growing interest in using diet counselling in the management of patients with irritable bowel syndrome (IBS). Among new emerging diets, vegetarian diets (VD) seem to be experiencing an important popularity, partly because of their alleged health benefits. A recent study performed among a rural Indian population showed that predominant VD could be associated with IBS.
Objective
This cross-sectional study aimed at assessing the association between the VD and IBS, among a large French cohort, the NutriNet-santé study.
Methods
Subjects participating in the NutriNet-Santé cohort study completed a questionnaire based on Rome III criteria (N = 41,682). Anthropometrics, socio-demographical and lifestyle data, including VD, were collected prior to the completion of Rome III questionnaire via self-administered questionnaires. Association between VD and IBS and its subtypes was investigated through multivariate logistic regression.
Results
The included subjects were mainly women (78.0%) and the mean age was 49.8±14.3 years. Among these individuals, 2,264 (5.4%) presented an IBS, and 805 (1.9%) reported a VD. Overall, VD was not associated with IBS or subtypes. A stable VD (i.e. self-declared at least three times) was associated with IBS (aOR 2.60 95%CI [1.37–4.91]), IBS mixed (aOR 2.97 95%CI [1.20–7.36]) and IBS diarrhoea (aOR 2.77 95%CI [1.01–7.59]).
Conclusions
This study suggests that a long term VD could be associated with IBS. Nevertheless, further studies are needed to confirm these results, and investigate the multiple aspects of the vegetarian diet, possibly related to the IBS.
Introduction
Vegetarian diet (VD), that includes the partial or total removal of meat, poultry, fish from the diet, (vegans also exclude dairy products and eggs), is increasingly widespread among the general population [1–4]. The reasons for adopting this dietary profile are attributable to ethical, environmental, and social concerns [1,2,5–10]. Health aspects of such a diet are also more and more emphasized. Indeed, health benefits of the VD, especially on ischemic heart disease and cancer have been widely reported by cross-sectional and prospective cohort studies during the last 50 years [11–14]. Generally speaking, vegetarians tend to be more health conscious, with a lower body mass index (BMI), and in better health when compared with omnivores, giving this type of diet a clear appeal in the population of subjects suffering from chronic diseases [15]. Furthermore, several health crises surrounding meat erupted in recent years (including animal bone meal or mad cow disease), and the world health organisation (WHO) has classified in 2015 red meat and processed meat as Group 2A, that is "probably carcinogenic" to humans [16]. Finally, VD patterns (in comparison to meat-based diets) are more sustainable because they use substantially less natural resources and are less taxing on the environment [17–19]. Adopting a VD may therefore seem a beneficial diet in many ways in the future.
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorder (FGID), defined by abdominal pain and abnormal transit pattern, with the absence of detectable organic illness [20–22]. Prevalence of IBS in the industrialized world is estimated to be approximately 12%, which makes IBS one of the most common gastrointestinal disorder [23]. Among several factors supposed to be involved in the pathogenesis of IBS, diet appears to play a key role [24–29]. Two thirds of IBS patients (64%) report meal-related symptoms to at least one food item [24], and they therefore often express an intense interest in food choice and attempt to identify and remove foods that induce symptoms [30–32]. For example, a cross-sectional study showed that 62% of IBS patients limited or excluded some food items from the diet [24]. Given the lack of scientific evidence supporting specific dietary advice for patients with IBS, they tend to adopt new diets, guided by various way of life (empirical, philosophical, etc), and spread via the media [29,33]. These changes include exclusion diets like VD. Adopting a VD pattern could induce some effects on the digestion process [34–36], and even on digestive diseases: Crowe and colleagues have shown that consuming a VD and a high intake of dietary fibre were both associated with a lower risk of admission to hospital or death from diverticular disease [37]. Moreover, the beneficial effects of a VD on inflammatory bowel diseases (and in particular the prevention of relapses) are increasingly considered [38,39].
A recent cross-sectional study performed among the rural Indian population found that participants with a predominant VD were more at risk for having IBS than those with a non-VD [40]. However, to the best of our knowledge, the association between a VD and IBS has not been studied to date among occidental populations.
This study aimed to assess the association between the vegetarian diet and IBS among a large French sample included in the NutriNet-Santé study.
Methods
Population
The NutriNet-Santé Study is a web-based prospective observational cohort. It aims to investigate the relationships between health and nutrition. The inclusion of subjects aged over 18 years started in France in May 2009 and still ongoing with 158,361 subjects enrolled at the time of the study. At baseline, participants completed self-administered questionnaires about socio-economic, lifestyle, health status, diet, physical activity, and anthropometrics data. During follow-up, additional questionnaires are regularly performed in accordance with the declaration of Helsinki and were approved by the institute Review Board of the French Institute for Health and Medical Research (00000388FWA00005831) and the Commission Nationale de l’Informatique et des Libertés (CNIL 908450 and 909216). All participants provided an electronic informed consent.
Data collection
Irritable bowel syndrome
A questionnaire assessing presence of FGIDs was sent to the whole cohort on 21 June 2013, and the questionnaire was available for completion up to the 6 November 2013, including data on medical digestive history and symptoms using the Rome III criteria. IBS and subtypes of the disease (IBS-Constipation, IBS-C, IBS-Diarrhea, IBS-D, IBS-Mixed, IBS-M and IBS-undefined, IBS-U), were defined according to the Rome III criteria and had to be present at least for the last 6 months [41,42]. Subjects reporting any organic diseases (stomach, esophagus or colorectal cancers, familial adenomatous polyposis coli, Crohn’s disease, coeliac disease, ulcerative colitis) or alarm symptoms (melena, hematemesis, rectal bleeding or significant unintentional weight loss in the past 3 months), were excluded from the present study.
Dietary data
At baseline and every 6 months, participants were requested to complete web based self-administered 24h dietary records. All participants who completed at least three 24h-records before the completion of the questionnaire pertaining to FGIDs were eligible. Each food and beverage consumed was collected according to the three main meal (breakfast, lunch and dinner) and possibilities of snack. Participants had to estimate the portion size for each elements consumed using validated photographs [43]. Dietary intake was estimated using the NutriNet-Santé food composition table, including more than 2,500 different foods and estimating total energy intake. Average energy intake from all dietary questionnaires was took into account in multivariate analysis as a covariate.
Vegetarian diet
Information on VD was collected at baseline and annually through follow-up questionnaires, using the following interrogation: “Currently, do you follow a particular diet? (Medical, pregnancy, vegetarian, vegan, personal or religious conviction)”. In this study we considered self-reported vegetarian diet was considered as a good proxy for vegetarianism. Thus, anyone reporting at least once following a VD was considered vegetarian. We also took into account “stable” vegetarians, i.e. participants who declared at least 3 times they followed a VD (whether at baseline or throughout the follow-up questionnaires) in Nutrinet for analyses. Since they represent a very particular population, we excluded participants who declared they followed a vegan diet (n = 226), but they were considered for sensitivity analyses.
Covariates
At baseline, information on age, gender, BMI (normal/overweight or obese), smoking status (current smoker/former smoker/nonsmoker), marital status (single/living in couple), income level (<1200 € per consumer unit (c.u.)/1200-2300 € per c.u./>2300 € per c.u.) and educational level (no diploma or primary studies/secondary studies or higher educational level) were collected by self-administered questionnaire. Physical activity (PA) level was assessed using International Physical Activity Questionnaire (IPAQ) at baseline, and was divided into three categories according to the mean MET per week [44] as follows: PA was low when mean MET per week was less than 1500, moderate when PA was upper than 1500 and less than 3000, and high above 3000 MET [44].
Statistical analyses
A description of socio-demographical, lifestyle, anthropometrical and medical information was performed according to the gender with t-tests and chi-square tests, according to the type of variable. Comparison of food group consumption and nutrient intake between vegetarians and omnivores was also realized, controlled for gender, age and total energy intake (ANCOVA tests). Interactions according to the IBS status were tested. Multivariate logistic regression models were performed to estimate the association between VD and IBS and subtypes, adjusting for the known or suspected risk factors listed above. Among these factors, those clearly identified in the literature were forced into the models (ie. age, educational level, smoking status, BMI and physical activity), additional factors associated with IBS with p<0.05 in bivariate analyses were included. Results of logistic regression models are presented using adjusted Odds Ratio (aOR) and 95% Confidence Interval (95% CI). To handle missing data of two covariates, multivariate logistic regression models were performed using multiple imputation [45,46]. Imputed values for physical activity (missing data = 5,290, 12.6%) and income level (missing data = 4,446, 10.7%) were estimated conditionally on the following variables: age, gender, marital status and educational level. A second model was performed, with the stable vegetarians as outcome. Finally, we performed sensitivity analyses through multivariate logistic regression models by including vegans in the definition of the outcome. Statistical analyses were conducted using SAS statistical package release 9.4 (SAS institute, Inc., Cary, NC, USA).
Results
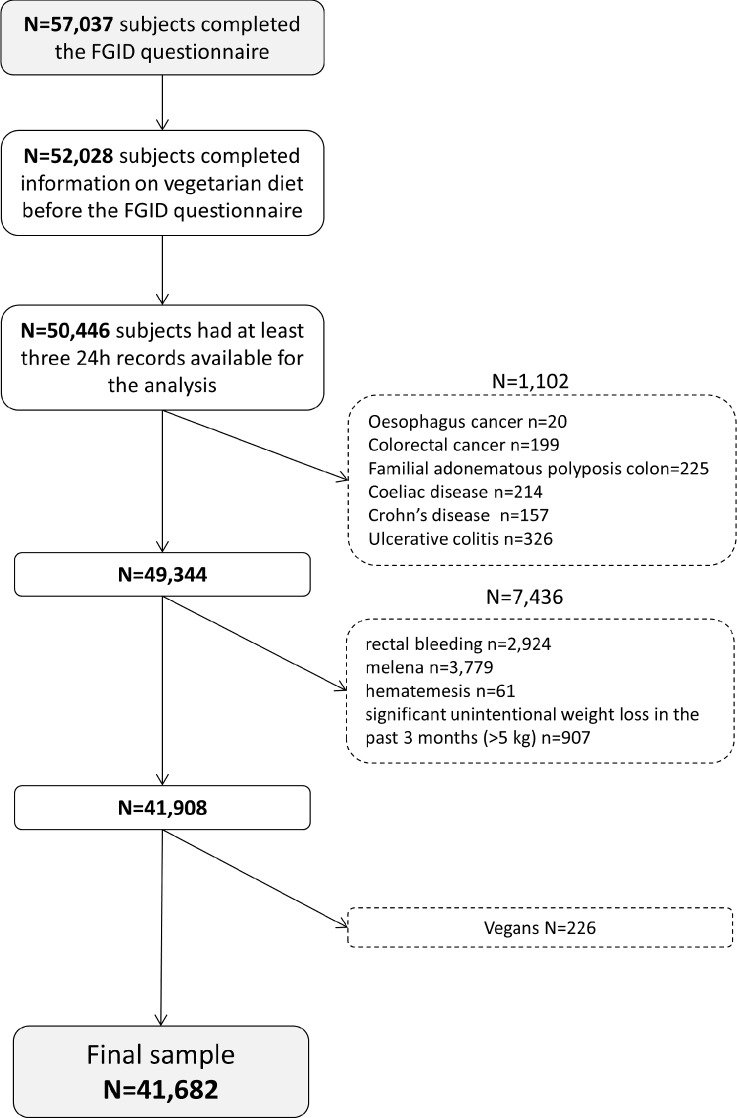
In the NutriNet-Santé Study, 57,037 individuals filled the FGIDs questionnaire. Among them, 52,028 completed information on VD before answering the FGIDs questionnaire. Among them, 50,446 subjects had at least three 24H records available for analysis. The 8,538 participants that reported any organic diseases (stomach, esophagus or colorectal cancers, familial adenomatous polyposis coli, Crohn’s disease, coeliac disease, ulcerative colitis) or alarm symptoms (melena, hematemesis, rectal bleeding or significant unintentional weight loss in the past 3 months) were excluded. Finally, we excluded participants who declared themselves vegans (n = 226) from the analyses (see Fig 1).
Fig 1. Flowchart of the study.
Comparison according to the main characteristics between included subjects (n = 41,682) and those removed (n = 8,784) is shown in S1 Table. Excluded subjects were younger, more often men, current smoker, single with a lower income. The final sample included 41,682 subjects. Subjects included were mainly women (78.0%) and the mean age was 49.8 +/-14.3 years. Overall 2,264 (5.4%) subjects reported an IBS, with a higher prevalence in women compared to men (5.6% vs 4.8%, p = 0.03) (Table 1). Prevalence of IBS subtypes were distributed as follows: 2.0% (n = 847) for IBS-M, 1.7% (n = 727) for IBS-D, 1.2% (n = 478) for IBS-C and 0.5% (n = 212) for IBS-U, with a higher prevalence in women for IBS-c and IBS-u (Table 1). Overall 1.9% (n = 805) subjects declared they followed a VD, mostly women (2.1% vs 1.4%, p<0.001). The proportions of vegetarians in IBS group and control group were similar (respectively 1.9% vs 2.0% with p = 0.84) (Table 2).
Table 1. Description of IBS and subtypes prevalence according to gender (N = 41,682).
| Total | Men n = 9,184 | Women n = 32,498 | p* | |||
|---|---|---|---|---|---|---|
| (22.0%) | (78.0%) | |||||
| N (%) | n | % | n | % | ||
| IBS | <0.01 | |||||
| No | 39,418 (94.6%) | 8,742 | 95.2 | 30,676 | 94.4 | |
| Yes | 2,264 (5.4%) | 442 | 4.8 | 1,822 | 5.6 | |
| IBS Mixed | ||||||
| No | 40,835 (98.0%) | 8,990 | 97.9 | 31,845 | 98.0 | 0.54 |
| Yes | 847 (2.0%) | 194 | 2.1 | 653 | 2 | |
| IBS Diarrhoea | ||||||
| No | 40,955 (98.3%) | 9,010 | 98.1 | 31,945 | 98.3 | 0.21 |
| Yes | 727 (1.7%) | 174 | 1.9 | 553 | 1.7 | |
| IBS Constipation | ||||||
| No | 41,204 (98.8%) | 9,133 | 99.4 | 32,071 | 98.7 | <0.0001 |
| Yes | 478 (1.2%) | 51 | 0.6 | 427 | 1.3 | |
| IBS Undefined | ||||||
| No | 41,470 (99.5%) | 9,161 | 99.7 | 32,309 | 99.4 | <0.0001 |
| Yes | 212 (0.5%) | 23 | 0.3 | 189 | 0.6 | |
* Chi-square tests were performed
Table 2. Comparison of sample characteristics between healthy controls and IBS patients (N = 41,682).
| Controls n = 39,418 (94.6%) |
IBS n = 2,264 (5.4%) |
p* | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
|
Gender |
<0.01 | ||||
| Men | 8,742 | 22.2 | 442 | 19.5 | |
| Women | 30,676 | 77.8 | 1,822 | 80.5 | |
| Age (mean +/-SD) | 49.5 | 14.3 | 56.0 | 11.9 | <0.0001 |
| Marital status | |||||
| Single | 10,531 | 26.7 | 609 | 26.9 | 0.85 |
| Couple | 28,887 | 73.3 | 1,655 | 73.1 | |
| Education level | |||||
| No diploma and primary studies | 1,134 | 2.9 | 78 | 3.4 | <0.01 |
| Secondary studies | 13,011 | 33.0 | 806 | 35.6 | |
| High educational level | 25,273 | 64.1 | 1,380 | 61.0 | |
| Income level | |||||
| <1200 € | 5,460 | 15.5 | 258 | 12.7 | <0.001 |
| 1200–2300 € | 15,404 | 43.4 | 855 | 42.1 | |
| > 2300 € | 14,339 | 40.7 | 920 | 45.2 | |
| Smoking status | |||||
| Non smoker | 20,456 | 51.9 | 1,095 | 48.4 | <0.0001 |
| Former smoker | 13,783 | 35.0 | 924 | 40.8 | |
| Smoker | 5,179 | 13.1 | 245 | 10.8 | |
| Physical activity | |||||
| Low | 7,587 | 22.1 | 432 | 21.4 | 0.57 |
| Moderate | 14,766 | 43.0 | 858 | 42.5 | |
| High | 12,021 | 35.0 | 728 | 36.1 | |
| BMI (kg/cm2) | |||||
| < 25 | 27,610 | 70.0 | 1,532 | 67.7 | 0.05 |
| 25–30 | 8,535 | 21.7 | 525 | 23.2 | |
| ≥ 30 | 3,271 | 8.3 | 207 | 9.1 | |
| Vegetarian diet | 38,658 | 98.1 | 2,219 | 98.0 | |
| 760 | 1.9 | 45 | 2.0 | 0.84 | |
* Chi-square tests were performed in order to compare the proportions of each covariate between IBS and controls
Abbreviations: BMI Body Mass Index; IBS Irritable Bowel Syndrome; SD Standard deviation
Missing data: Income level n = 4,446 (10.7%); Physical activity n = 5,290 (12.6%)
Table 3shows the comparison of the mean food consumption between vegetarians and omnivore subjects, adjusted for age, gender and total energy intake. As expected, compared to omnivorous, vegetarians had significantly lower consumption of meat, poultry, fish and shellfish, processed meat and fish.
Table 3. Comparison of daily intake of food groups (in grams/day) between vegetarians and omnivores (n = 41,682).
| Omnivores | Vegetarians | ||||
|---|---|---|---|---|---|
| n = 40,877 | n = 805 | ||||
| Mean | SE | Mean | SE | p* | |
| Meat, poultry | 97.0 | 0.6 | 37.5 | 3.7 | <0.0001 |
| Porc ham, poultry cuts, processed meat | 43.4 | 0.3 | 17.6 | 2.1 | <0.0001 |
| Fish, shellfish, processed fish and shellfish | 62.9 | 0.5 | 52.5 | 3.4 | <0.01 |
| Eggs | 18.8 | 0.2 | 23.3 | 1.4 | <0.01 |
| Milk, yogurt | 155.8 | 1.6 | 137.3 | 10.2 | 0.07 |
| Cheese, cottage cheese, Petits Suisses | 72.2 | 0.7 | 85.3 | 4.3 | <0.01 |
| Starchy food | 239.3 | 1.0 | 230.3 | 6.8 | 0.18 |
| Wholegrain products | 50.1 | 0.6 | 86.6 | 4.2 | <0.0001 |
| Breakfast cereals | 24.2 | 0.4 | 45.4 | 2.4 | <0.0001 |
| Dry fruits, oleaginous fruits | 9.0 | 0.2 | 16.1 | 1.2 | <0.0001 |
| Fruits | 194.3 | 1.4 | 230.3 | 9.4 | <0.001 |
| Vegetables | 214.2 | 1.1 | 273.1 | 7.5 | <0.0001 |
| Pulses | 20.9 | 0.3 | 37.3 | 2.2 | <0.0001 |
| 100% legumes and fruits juice | 69.6 | 0.9 | 67.7 | 5.9 | 0.75 |
| Condiments, spices | 9.9 | 0.1 | 11.7 | 0.6 | <0.01 |
| Oil | 9.5 | 0.1 | 11.2 | 0.5 | <0.01 |
| Non sugared beverages | 1,094.8 | 5.8 | 1,257.5 | 38.2 | <0.0001 |
| Soft sugary drinks | 56.6 | 1.0 | 74.4 | 6.6 | <0.01 |
| Alcoholic beverages | 127.2 | 1.5 | 103.0 | 9.5 | 0.01 |
| Fat products | 39.7 | 0.2 | 38.0 | 1.4 | 0.22 |
| Fat and sugared products | 177.5 | 1.0 | 169.7 | 6.5 | 0.24 |
| Salty and sweet snack products | 21.6 | 0.2 | 20.6 | 1.5 | 0.53 |
*ANCOVA tests controlled for gender, age and total energy intake
Abbreviations: SE: Standard Error
Vegetarians also had lower consumption of soft sugary drinks and alcoholic beverage, while they had significantly higher consumption of eggs, fruits and vegetables, wholegrain products, pulses, cereals, dry fruits, legumes, oil and non-sugared beverages. Table 4summarizes the mean daily intake in terms of macronutrients in vegetarians and omnivorous subjects controlled for gender, age and total energy intake. The vegetarians reported lower total energy intake, with lower percent energy from fat and proteins, and higher percent energy from carbohydrates. Vegetarians had lower daily intakes of saturated fatty acids (SFA), cholesterol and animal proteins and they tended to reach the recommended level in fibres (>25g/day) more often than omnivorous. Conversely, they had higher intakes poly unsaturated fatty acids (PUFA), Omega 3, Omega 6 and vegetal proteins. Dietary and nutritional intakes were compared between participants who declared themselves vegetarians at least three times in Nutrinet and others (S2 Table and S3 Table). Consistent vegetarians had lower intakes of meat, poultry, and processed meat, and higher intakes of fruits and vegetables. Compared to “simple” vegetarians, consistent vegetarians had also higher intakes of fibres and simple carbohydrates and lower intakes of animal proteins and cholesterol.
Table 4. Comparison of daily intake of macronutrients between omnivorous and vegetarians (N = 41,682).
| Omnivorous n = 10,877 | Vegetarians n = 805 | ||||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | p* | |
| Energy (Kcal) | 2014.8 | 2.3 | 1955.2 | 13.6 | <0.0001 |
| %energy from fat | 37.9 | 0.0 | 37.5 | 0.2 | 0.03 |
| MUFA (g) | 29.3 | 0.0 | 29.6 | 0.2 | 0.24 |
| Omega 3 (g) | 1.4 | 0.0 | 1.5 | 0.0 | <0.0001 |
| Omega 6 (g) | 9.1 | 0.0 | 10.6 | 0.1 | <0.0001 |
| PUFA (g) | 11.1 | 0.0 | 12.8 | 0.1 | <0.0001 |
| SFA (g) | 32.1 | 0.0 | 30.0 | 0.2 | <0.0001 |
| Cholesterol (mg) | 312.8 | 0.6 | 260.0 | 3.2 | <0.0001 |
| %energy from protein | 16.9 | 0.0 | 14.6 | 0.1 | <0.0001 |
| Animal proteins (g) | 54.8 | 0.1 | 37.0 | 0.5 | <0.0001 |
| Vegetal proteins (g) | 24.8 | 0.0 | 31.8 | 0.2 | <0.0001 |
| %energy from carbohydrates | 41.5 | 0.0 | 44.8 | 0.2 | <0.0001 |
| Complex carbohydrates (g) | 105.0 | 0.1 | 112.4 | 0.8 | <0.0001 |
| Simple carbohydrates (g) | 89.0 | 0.1 | 95.8 | 0.8 | <0.0001 |
| Fibers (g) | 19.3 | 0.0 | 24.7 | 0.2 | <0.0001 |
* ANCOVA tests controlled for gender, age and total energy intake except for energy, lipids, proteins and carbohydrates
Abbreviations: Kcal: kilocalories; MUFA: MonoUnsaturated Fatty Acids; PUFA: PolyUnsaturated Fatty Acids; SE: Standard Error; SFA: Saturated Fatty Acids
Consumption of calcium, iron, potassium, magnesium, beta carotene, Vitamins A, B1, B6, B9, C and E were significantly higher in vegetarians compared to omnivores (Table 5). Other micronutrients were significantly higher in omnivores, especially Sodium, and Vitamin D and Vitamin B12.
Table 5. Comparison of daily intake of micronutrients between omnivorous and vegetarians (N = 41,682).
| Omnivores n = 40,877 | Vegetarians n = 805 | ||||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | p* | |
| Calcium (mg) | 911.4 | 1.37 | 965.5 | 7.77 | < .0001 |
| Iron (mg) | 13.3 | 0.02 | 15.2 | 0.12 | < .0001 |
| Potassium (mg) | 2972.4 | 3.48 | 3144.1 | 19.8 | < .0001 |
| Magnesium (mg) | 335.7 | 0.54 | 402.4 | 3.07 | < .0001 |
| Sodium (mg) | 2769.2 | 3.51 | 2510.6 | 19.96 | < .0001 |
| Zinc (mg) | 10.8 | 0.01 | 9.9 | 0.08 | < .0001 |
| Phosphorus (mg) | 1262.6 | 1.42 | 1276.8 | 8.11 | 0.08 |
| Vit A (mg) | 1059.4 | 3.8 | 1140.3 | 21.7 | <0.001 |
| Beta Carotene (μg) | 3361.0 | 13.1 | 4428.2 | 74.3 | < .0001 |
| Vit B1 (mg) | 1.1 | 0.0 | 1.2 | 0.0 | 0.02 |
| Vit B2 (mg) | 1.7 | 0.0 | 1.7 | 0.0 | 0.64 |
| Vit B5 (mg) | 5.3 | 0.0 | 5.2 | 0.0 | 0.11 |
| Vit B6 (mg) | 1.7 | 0.0 | 1.8 | 0.0 | <0.001 |
| Vit B9 (μg) | 320.1 | 0.5 | 380.2 | 3.0 | < .0001 |
| VitB12 (μg) | 5.4 | 0.0 | 3.9 | 0.1 | < .0001 |
| Vit B3 (mg) | 18.9 | 0.0 | 16.8 | 0.2 | < .0001 |
| Vit C (mg) | 112.5 | 0.4 | 123.3 | 2.1 | < .0001 |
| Vit D (μg) | 2.7 | 0.0 | 2.6 | 0.1 | 0.02 |
| Vit E (mg) | 11.2 | 0.0 | 13.3 | 0.1 | < .0001 |
* ANCOVA tests controlled for gender, age and total energy intake
Abbreviations: SE: Standard Error; Vit: Vitamin
No significant association was observed between vegetarians and IBS (Table 6). When studying vegetarians who declared at least three times they followed a VD, significant associations were shown with IBS (aOR 2.60 95%CI: 1.37–4.91), with IBS mixed (aOR 2.97 95%CI: 1.20–7.36), and with IBS-diarrhoea (aOR 2.77 95%CI: 1.01–7.59). Sensitivity analyses, including vegans showed similar results, plus an association between VD and IBS diarrhoea (aOR 1.55 95%CI: 1.02–2.34) (Table 7). Supplementary analyses, with models performed with one outcome splitting our population in three categories (according to the number of self-declarations of VD in Nutrinet, 0, 1 or 2 and at least 3) were performed (S4 Table and S5 Table).
Table 6. Multivariate analyses (logistic regression models) (N = 41 682).
| Vegetarians (n = 805) | Stable vegetarians (n = 106) | ||
|---|---|---|---|
| aOR (95%CI) | aOR (95%CI) | ||
| IBS | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.19 [0.87–1.62] | 2.60 [1.37–4.91] | |
| IBS mixed | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.27 [0.79–2.05] | 2.97 [1.20–7.36] | |
| IBS diarrhoea | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.32 [0.80–2.18] | 2.77 [1.01–7.59] | |
| IBS constipation | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.01 [0.50–2.06] | 2.25 [0.55–9.26] | |
| IBS undefined | Omnivorous | Ref. | Ref. |
| Vegetarians | 0.75 [0.24–2.37] | NA |
Models are adjusted for: Age, educational level, total energy intake, income level, smoking status, BMI, physical activity and gender
Abbreviations: IBS Irritable Bowel Syndrome; NA Not Applicable; OR Odds Ratio; 95%CI Confidence Interval
Table 7. Multivariate analyses including vegans (logistic regression models) (N = 41 908).
| Vegetarians or vegans (n = 1,031) | Stable vegetarians or vegans (n = 134) |
||
|---|---|---|---|
| aOR (95%CI) | aOR (95%CI) | ||
| IBS | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.24 [0.95–1.62] | 2.66 [1.51–4.68] | |
| IBS mixed | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.21 [0.78–1.86] | 2.85 [1.24–6.54] | |
| IBS diarrhoea | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.55 [1.02–2.34] | 3.38 [1.47–7.74] | |
| IBS constipation | Omnivorous | Ref. | Ref. |
| Vegetarians | 1.09 [0.60–2.00] | 1.77 [0.43–7.24] | |
| IBS undefined | Omnivorous | Ref. | Ref. |
| Vegetarians | 0.59 [0.19–1.86] | NA |
Models are adjusted for: Age, educational level, income level, total energy intake, smoking status, BMI, physical activity and gender
Abbreviations: IBS Irritable Bowel Syndrome; NA Not Applicable; OR Odds Ratio; 95%CI Confidence Interval
Discussion
A VD was associated with IBS, IBS-M and IBS-D in consistent vegetarians, i.e. when participants declared at least three times they were vegetarians in the Nutrinet study. To the best of our knowledge, this work is the first to specifically assess the relationship between vegetarianism and IBS in such a large population-based study. Numerous approaches to dietary management of IBS have been investigated [30–32,47–52], including an increase of dietary fibre intakes [53–57], identification and management of lactose intolerance [58], and more recently exclusion of food containing Fermentable Oligo-, Di-, Monosaccharides And Polyols FODMAPs [59]. Given this knowledge, some particular features related to a VD could worsen or improve IBS symptoms. In accordance with previous studies performed on vegetarians, our work highlighted that VD provides relatively large amounts of cereals, pulses, nuts, fruits, vegetables and wholegrain products, strengthening the validity of the vegetarians declaration in our sample [60,61]. Vegetarians have therefore i) higher fibres intake, ii) a greater proportion of energy from carbohydrates and iii) lower intake of lactose. The role of dietary fibres on IBS is complex. Despite years of advising patients to increase their global dietary fibre intakes, recent reviews suggest that the benefit of fibres in IBS appears to be limited to soluble fibres [57,62,63].
An increased proportion of certain types of carbohydrates in diet can also worsen IBS symptoms. In particular sugars (mono and disaccharides) and polyols which are slowly absorbed from the small intestine rather than digested, and can lead to a luminal distension by various mechanisms (water fermentation, rapid fermentation, gas,…) [29,64,65]. In this study, vegetarians had higher intakes of both simple and complex carbohydrates.
Although lactose malabsorption does not appear to be a cause of IBS or to be more prevalent in individuals with IBS than in the general population [31,66–71], lactose is not well digested and absorbed by a majority of adults throughout the world, and individuals with and without IBS may report increased symptoms, similar to those of IBS, following ingestion of lactose-containing foods. Thus, the low lactose intakes of vegetarians could help to improve IBS symptoms. Overall, the vegetarian diet presents both features that might improve and worsen IBS symptoms. This may partly explain the absence of any significant association observed between VD and IBS.
We found a positive association between consistent VD and IBS (aOR = 2.58, 95%CI 1.36–4.87 with p = 0.004), IBS-M (aOR = 2.94, 95%CI 1.19–7.31 with p = 0.02) and IBS-D (aOR = 2.77, 95%CI 1.01–7.59 with p = 0.047). Similar results were shown by including vegans in the outcome, with in addition a significant association with IBS-D with VD (at least once). These results are in line with those of Ghoshal and colleagues [40], where a predominant vegetarian diet was associated with IBS (aOR = 10.77, 95%CI 1.49–77.89) in 2,774 subjects (including 2,654 vegetarians). However, these results should be interpreted carefully. Indeed, the low numbers of consistent vegetarians (n = 106) probably relates to a very particular population, whose dietary behavior and lifestyle can probably not be generalized to the entire vegetarian population [63].
Finally, and in accordance with previous studies, we observed that vegetarians had lower energy [72,73], sodium and SFA intakes, whereas they had higher intakes of PUFA and MUFA. These characteristics have been shown to reduce cardiovascular risk [13,60,74,75].
The identification of IBS was based on the Rome III criteria which was considered the gold standard at the time of inclusion [21]. The prevalence of IBS in our study is in agreement with other studies realized among the French population i.e.: 4% by Bommelaer and colleagues and 4.7% by Dapoigny and colleagues [76,77]. Our prevalence was slightly higher, which could partly be due to the modification of diagnosis criteria for IBS, former criteria tending to have higher detection rates compared to the Rome III [23]. Likewise, the proportion of vegetarians in our sample fits with the estimated proportion of vegetarians in France (about 2%) [1]. Finally, we used a Web-based dietary assessment which was compared with a traditional dietitian’s interview and showed a good agreement with this gold standard [78–80].
However some limitations should be discussed. This is a cross-sectional study, and although we excluded all subjects who declared they were vegetarian after filling-in the FGIDs questionnaire, we were not able to conclude on causality (i.e. determine if vegetarian diet tended to increase IBS symptoms or if participants with an IBS were more likely to adopt such a diet in order to improve their digestive symptoms). Another limitation is that subjects were recruited the general population and were volunteers. They were therefore more likely to be health conscious and have more controlled diets, and probably not representative of the general population. Nevertheless, the representativeness is not necessarily required in analytical studies [81], and the prevalence of IBS in our study was similar to that of the French population, which is not in favor of a selection bias. We observed that vegetarians had some meat and fish intakes in their dietary records. Indeed, we were not able to know precisely what kind of vegetarianism (e.g. ovolactovegetarianism vs. pescovegetarianism, semi-vegetarianism) was followed by self-declared vegetarian participants. Thus, it is possible that these people declared themselves vegetarian and then changed a part of their food habits to include some animal products. Moreover, a report performed by the Human Research Council on current and former vegetarians and vegans in the USA, have shown that almost 85% of vegetarians/vegans abandon their diet [82], mainly because maintaining this type of diet is difficult in the long run. Plus, a recent study performed among the general population in Belgium showed that semi-vegetarian (i.e. flexitarism) represented almost 12% of the surveyed population [9]. This is therefore consistent with our vegetarian population for whom self-reported vegetarianism can vary over time.
Further leads remain to be explored like the modifications of the gut microbiota related to the vegetarianism. Indeed, the composition of the gut microbiota has been shown to be responsive and adaptable to the diet of the host organism [83,84]. And recent works highlighted significant differences between the faecal microbiota of omnivores, vegetarians and vegan [85,86]. Finally, it could be appropriate to focus on (FODMAP’s) content of a usual vegetarian diet.
Conclusion
Overall, vegetarian diet did not appear to be associated with IBS in our study, unless we find positive associations between a stable vegetarian diet and IBS (including IBS mixed and IBS diarrhoea). But more research is needed to assess the association between vegetarian diet and relief of symptoms of IBS patients so clinicians will be able to consider vegetarian diet as one of the treatment options for IBS.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank Anouar Nechba (biostatistician).
We also thank Younes Esseddik, Thi Duong Van, Frédéric Coffinieres, Mac Rakotondrazafy, Régis Gatibelza and Paul Flanzy (computer scientists); and Nathalie Arnault, Véronique Gourlet, Dr. Fabien Szabo, Julien Allegre and Laurent Bourhis (data-manager/biostatisticians) for their technical contribution to the NutriNet-Santé study. We thank all the volunteers of the NutriNet-Santé cohort.
All authors had full access to the data and critically revised the paper for important intellectual content.
Abbreviations
- aOR
adjusted Odds Ratio
- BMI
Body Mass Index
- CU
Consumer Unit
- FGID
Functional GastroIntestinal Disorder
- FODMAPs
Fermentable Oligo-, Di-, Monosaccharides And Polyols
- IBS
Irritable Bowel Syndrome
- IBS-C
IBS Constipation
- IBS-D
IBS diarrhoea
- IBS-M
IBS Mixed
- IBS-U
IBS Undefined
- IPAQ
International Physical Activity Questionnaire
- VD
Vegetarian Diet
- 95% CI
95% Confidence Interval
Data Availability
Data are from the NutriNet cohort. Authors CB, MT, BA, SH and CJ are affiliated to the EREN, which is coordinator of the NutriNet-Santé Study. The NutriNet-Santé study is coordinated by the Nutritional Epidemiology Research Team, Université paris 13, INSERM U1153, INRA U1125, Cnam, CRESS. The data were collected in volunteer participants under the agreement of strict confidentiality of the data. Moreover, instructions from regulatory bodies restrict the capacity of the database to be made publicly available. Any access to the cohort must be part of a more global collaborative research work, with co-authorship on manuscripts. Stability of the data is regularly checked by the EREN team, and any inconsistency investigated thouroughly. Data requests may be sent to support@etude-nutrinet-sante.fr.
Funding Statement
The NutriNet-Santé Study is supported by the French Ministry of Health, the Institut de Veille Sanitaire, the Institut National de la Santé et de la Recherche Médicale, the Institut National de la Recherche Agronomique, the Conservatoire National des Arts et Métiers, the Santé publique France Institute and the Fondation pour la Recherche Médicale and Paris 13 University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Study sponsors had no part in study design, collection, analysis, and interpretation of data and the writing of the article and the decision to submit it for publication.
References
- 1.Leitzmann C. Vegetarian nutrition: past, present, future. Am. J. Clin. Nutr. 2014;ajcn.071365. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen B. To Meat or Not to Meat?: An Analysis of On-line Vegetarian Persuasive Rhetoric. Poroi. 2015;11:1–19. [Google Scholar]
- 3.VEBU. Vegetarische und vegane Lebensmittel auf Erfolgskurs: Umsatzplus von 25.9 Prozent im Jahr 2015. [Internet]. VEBU. 2016 [cited 2016 Dec 5]. Available from: https://vebu.de/presse/pressemitteilungen/2637-2016-03-pm-ifh-umsatzplus-vegane-lebensmittel
- 4.Newport F. In U.S., 5% Consider Themselves Vegetarians [Internet]. Gallup.com. [cited 2016 Dec 5]. Available from: http://www.gallup.com/poll/156215/Consider-Themselves-Vegetarians.aspx
- 5.Craig W, Mangels A. Position of the American Dietetic Association: vegetarian diets. J. Am. Diet. Assoc. 2009;109:1266–82. [DOI] [PubMed] [Google Scholar]
- 6.Scarborough P, Appleby PN, Mizdrak A, Briggs ADM, Travis RC, Bradbury KE, et al. Dietary greenhouse gas emissions of meat-eaters, fish-eaters, vegetarians and vegans in the UK. Clim. Change. 2014;125:179 doi: 10.1007/s10584-014-1169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins IW, Balsam AL, Goldman R. A Survey of Registered Dietitians’ Concern and Actions Regarding Climate Change in the United States. Front. Nutr. 2015;2:21 doi: 10.3389/fnut.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen M, Busch C, Rödiger M, Hamm U. Motives of consumers following a vegan diet and their attitudes towards animal agriculture. Appetite. 2016;105:643–51. doi: 10.1016/j.appet.2016.06.039 [DOI] [PubMed] [Google Scholar]
- 9.Mullee A, Vermeire L, Vanaelst B, Mullie P, Deriemaeker P, Leenaert T, et al. Vegetarianism and meat consumption: A comparison of attitudes and beliefs between vegetarian, semi-vegetarian, and omnivorous subjects in Belgium. Appetite. 2017;114:299–305. doi: 10.1016/j.appet.2017.03.052 [DOI] [PubMed] [Google Scholar]
- 10.Esteve-Saillard M. Market trend in France on the presence of plant proteins in food products. Ocl-Oilseeds Fats Crops Lipids. 2016;23:D403. [Google Scholar]
- 11.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am. J. Clin. Nutr. 1999;70:532S–538S. [DOI] [PubMed] [Google Scholar]
- 12.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? (vol 89, pg 1607S, 2009). Am. J. Clin. Nutr. 2009;90:248–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2016;0. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 2012;15:2287–94. doi: 10.1017/S1368980012000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int. J. Cardiol. 2014;176:680–6. doi: 10.1016/j.ijcard.2014.07.080 [DOI] [PubMed] [Google Scholar]
- 16.WHO | Q&A on the carcinogenicity of the consumption of red meat and processed meat [Internet]. WHO. [cited 2016 Sep 7]. Available from: http://www.who.int/features/qa/cancer-red-meat/en/
- 17.Sabate J, Soret S. Sustainability of plant-based diets: back to the future. Am. J. Clin. Nutr. 2014;100:476S–482S. doi: 10.3945/ajcn.113.071522 [DOI] [PubMed] [Google Scholar]
- 18.Hedenus F, Wirsenius S, Johansson DJA. The importance of reduced meat and dairy consumption for meeting stringent climate change targets. Clim. Change. 2014;124:79–91. [Google Scholar]
- 19.Sabate J, Sranacharoenpong K, Harwatt H, Wien M, Soret S. The environmental cost of protein food choices. Public Health Nutr. 2015;18:2067–73. doi: 10.1017/S1368980014002377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2004;18:613–31. doi: 10.1016/j.bpg.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 21.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional Bowel Disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061 [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction INTRODUCTION. Gastroenterology. 2016;150:1257–61. doi: 10.1053/j.gastro.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 23.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012;10:712–721.e4. [DOI] [PubMed] [Google Scholar]
- 24.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome—etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006;60:667–72. doi: 10.1038/sj.ejcn.1602367 [DOI] [PubMed] [Google Scholar]
- 25.Eswaran S, Muir J, Chey WD. Fiber and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013;108:718–27. doi: 10.1038/ajg.2013.63 [DOI] [PubMed] [Google Scholar]
- 26.Boettcher E, Crowe SE. Dietary Proteins and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013;108:728–36. doi: 10.1038/ajg.2013.97 [DOI] [PubMed] [Google Scholar]
- 27.Farre R, Tack J. Food and Symptom Generation in Functional Gastrointestinal Disorders: Physiological Aspects. Am. J. Gastroenterol. 2013;108:698–706. doi: 10.1038/ajg.2013.24 [DOI] [PubMed] [Google Scholar]
- 28.Shepherd SJ, Lomer MCE, Gibson PR. Short-Chain Carbohydrates and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013;108:707–17. doi: 10.1038/ajg.2013.96 [DOI] [PubMed] [Google Scholar]
- 29.Gibson PR, Varney J, Malakar S, Muir JG. Food components and irritable bowel syndrome. Gastroenterology. 2015;148:1158–1174.e4. doi: 10.1053/j.gastro.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 30.Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol. Clin. North Am. 2011;40:141–62. doi: 10.1016/j.gtc.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 31.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J. Am. Diet. Assoc. 2009;109:1204–14. doi: 10.1016/j.jada.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 32.Morcos A, Dinan T, Quigley EMM. Irritable bowel syndrome: role of food in pathogenesis and management. J. Dig. Dis. 2009;10:237–46. doi: 10.1111/j.1751-2980.2009.00392.x [DOI] [PubMed] [Google Scholar]
- 33.Lacy BE. Making Sense of IBS: A Physician Answers Your Questions about Irritable Bowel Syndrome. JHU Press; 2013. [Google Scholar]
- 34.Nair P, Mayberry JF. Vegetarianism, dietary fibre and gastro-intestinal disease. Dig. Dis. Basel Switz. 1994;12:177–85. [DOI] [PubMed] [Google Scholar]
- 35.Panigrahi MK, Kar SK, Singh SP, Ghoshal UC. Defecation Frequency and Stool Form in a Coastal Eastern Indian Population. J. Neurogastroenterol. Motil. 2013;19:374 doi: 10.5056/jnm.2013.19.3.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BR, Ko YM, Cho MH, Yoon YR, Kye SH, Park YK. Effects of 12-week Vegetarian Diet on the Nutritional Status, Stress Status and Bowel Habits in Middle School Students and Teachers. Clin. Nutr. Res. 2016;5:102–11. doi: 10.7762/cnr.2016.5.2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ. 2011;343:d4131 doi: 10.1136/bmj.d4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riordan AM, Hunter JO, Crampton JR, Neale G, Cowan RE, Davidson AR, et al. Treatment of active Crohn’s disease by exclusion diet: East Anglian Multicentre Controlled Trial. The Lancet. 1993;342:1131–4. [DOI] [PubMed] [Google Scholar]
- 39.Chiba M. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010;16:2484 doi: 10.3748/wjg.v16.i20.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population: Dyspepsia and IBS in a rural Indian community. J. Gastroenterol. Hepatol. [Internet]. 2016. [cited 2016 Sep 15]; Available from: http://doi.wiley.com/10.1111/jgh.13465 [DOI] [PubMed] [Google Scholar]
- 41.Foundation Rome. Guidelines—Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J. Gastrointest. Liver Dis. JGLD. 2006;15:307–12. [PubMed] [Google Scholar]
- 42.Drossman DA. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 43.Hercberg S. Su.Vi.Max: Portions alimentaires; manuel photos pour l’estimation des quantités—(Su.Vi.Max. Photograph Book for the Estimation of portion sizes). [Internet]. Paris: Polytechnica; 2012 [cited 2016 Mar 9]. Available from: http://www.chapitre.com/CHAPITRE/fr/BOOK/su-vi-max/portions-alimentaires-manuel-photos-pour-l-estimation-des-quantites,1212789.aspx
- 44.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity: Med. Sci. Sports Exerc. 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 45.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: a data analyst’s perspective. Multivar. Behav. Res. 1998;33:545–71. [DOI] [PubMed] [Google Scholar]
- 46.Su Y-S, Yajima M, Gelman AE, Hill J. Multiple imputation with diagnostics (mi) in R: opening windows into the black box. J. Stat. Softw. 2011;45:1–31. [Google Scholar]
- 47.Simren M, Mansson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–15. [DOI] [PubMed] [Google Scholar]
- 48.Wald A, Rakel D. Behavioral and complementary approaches for the treatment of irritable bowel syndrome. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2008;23:284–92. [DOI] [PubMed] [Google Scholar]
- 49.Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2009;7:706–708.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review). Int. J. Mol. Med. 2012;29:723–31. doi: 10.3892/ijmm.2012.926 [DOI] [PubMed] [Google Scholar]
- 51.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012;5:1382–90. doi: 10.3892/mmr.2012.843 [DOI] [PubMed] [Google Scholar]
- 52.El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr. J. 2015;14:36 doi: 10.1186/s12937-015-0022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, et al. Systematic review on the management of irritable bowel syndrome in North America. Am. J. Gastroenterol. 2002;97:S7–26. [DOI] [PubMed] [Google Scholar]
- 54.Bijkerk CJ, Muris JWM, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004;19:245–51. [DOI] [PubMed] [Google Scholar]
- 55.Ford AC, Talley NJ, Spiegel BMR, Foxx-Orenstein AE, Schiller L, Quigley EMM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313 doi: 10.1136/bmj.a2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bijkerk CJ, Wit NJ de, Muris JWM, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009;339:b3154 doi: 10.1136/bmj.b3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao SSC, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015;41:1256–70. doi: 10.1111/apt.13167 [DOI] [PubMed] [Google Scholar]
- 58.Irritable bowel syndrome in adults: diagnosis and management | Guidance and guidelines | NICE [Internet]. [cited 2016 Sep 1]. Available from: https://www.nice.org.uk/guidance/cg61
- 59.Dugum M, Barco K, Garg S. Managing irritable bowel syndrome: The low-FODMAP diet. Cleve. Clin. J. Med. 2016;83:655–62. doi: 10.3949/ccjm.83a.14159 [DOI] [PubMed] [Google Scholar]
- 60.Key TJ, Appleby PN, Rosell MS. Health effects of vegetarian and vegan diets. Proc. Nutr. Soc. 2006;65:35–41. [DOI] [PubMed] [Google Scholar]
- 61.Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6:259–69. doi: 10.1079/PHN2002430 [DOI] [PubMed] [Google Scholar]
- 62.Moayyedi P, Quigley EMM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. The Effect of Fiber Supplementation on Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014;109:1367–74. doi: 10.1038/ajg.2014.195 [DOI] [PubMed] [Google Scholar]
- 63.Nagarajan N, Morden A, Bischof D, King EA, Kosztowski M, Wick EC, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015;1. [DOI] [PubMed] [Google Scholar]
- 64.Jane G Muir, Peter R Gibson. Manipulating dietary carbohydrates to treat irritable bowel syndrome. Clin. Insights Irrit. Bowel Syndr. Diagn. Manag. [Internet]. Future Medicine Ltd; 2013 [cited 2016 Sep 20]. p. 81–103. Available from: http://www.futuremedicine.com/doi/abs/10.2217/ebo.13.493
- 65.Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2014;8:819–34. doi: 10.1586/17474124.2014.917956 [DOI] [PubMed] [Google Scholar]
- 66.Hamm LR, Sorrells SC, Harding JP, Northcutt AR, Heath AT, Kapke GF, et al. Original Contributions: Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am. J. Gastroenterol. 1999;94:1279–82. doi: 10.1111/j.1572-0241.1999.01077.x [DOI] [PubMed] [Google Scholar]
- 67.Turnbull GK. Lactose intolerance and irritable bowel syndrome. Nutr. Burbank Los Angel. Cty. Calif. 2000;16:665–6. [DOI] [PubMed] [Google Scholar]
- 68.Parry SD, Barton JR, Welfare MR. Is lactose intolerance implicated in the development of post-infectious irritable bowel syndrome or functional diarrhoea in previously asymptomatic people? Eur. J. Gastroenterol. Hepatol. 2002;14:1225–30. [DOI] [PubMed] [Google Scholar]
- 69.Farup PG (analytic), Monsbakken KW (analytic), Vandvik PO (analytic). Lactose malabsorption in a population with irritable Bowel syndrome: Prevalence and symptoms. A case-control study (English). Scand J Gastroenterol. 2004;39:645–9. doi: 10.1080/00365520410005405 [DOI] [PubMed] [Google Scholar]
- 70.Gupta D, Ghoshal UC, Misra A, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: A case-control study. J. Gastroenterol. Hepatol. 2007;2261 doi: 10.1111/j.1440-1746.2007.04986.x [DOI] [PubMed] [Google Scholar]
- 71.Lomer MCE, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice—myths and realities. Aliment. Pharmacol. Ther. 2008;27:93–103. doi: 10.1111/j.1365-2036.2007.03557.x [DOI] [PubMed] [Google Scholar]
- 72.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 2011;14:275–87. doi: 10.1089/ars.2010.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reddy KS, Katan MB. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. [Internet]. 2004. [cited 2016 Sep 12];7 Available from: http://www.journals.cambridge.org/abstract_S1368980004000199 [DOI] [PubMed] [Google Scholar]
- 75.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885 doi: 10.1136/bmj.39147.604896.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bommelaer G, Dorval E, Denis P, Czernichow P, Frexinos J, Pelc A, et al. Prevalence of irritable bowel syndrome in the French population according to the Rome I criteria. Gastroenterol. Clin. Biol. 2002;26:1118–23. [PubMed] [Google Scholar]
- 77.Dapoigny M, Bellanger J, Bonaz B, des Varannes SB, Bueno L, Coffin B, et al. Irritable bowel syndrome in France: a common, debilitating and costly disorder. Eur. J. Gastroenterol. Hepatol. 2004;16:995–1001. [DOI] [PubMed] [Google Scholar]
- 78.Touvier M, Kesse-Guyot E, Méjean C, Pollet C, Malon A, Castetbon K, et al. Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br. J. Nutr. 2011;105:1055–64. doi: 10.1017/S0007114510004617 [DOI] [PubMed] [Google Scholar]
- 79.Lassale C, Castetbon K, Lapote F, Camilleri GM, Deschamps V, Vernay M, et al. Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. Br. J. Nutr. 2015;113:953–62. doi: 10.1017/S0007114515000057 [DOI] [PubMed] [Google Scholar]
- 80.Lassale C, Castetbon K, Laporte F, Deschamps V, Vernay M, Camilleri GM, et al. Correlations between Fruit, Vegetables, Fish, Vitamins, and Fatty Acids Estimated by Web-Based Nonconsecutive Dietary Records and Respective Biomarkers of Nutritional Status. J. Acad. Nutr. Diet. 2016;116:427–438.e5. doi: 10.1016/j.jand.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 81.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int. J. Epidemiol. 2013;42:1012–4. doi: 10.1093/ije/dys223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Human Research Council. Study of Current & Former Vegetarians & Vegans—initial findings [Internet]. HRC; 2014 Dec. Available from: https://faunalytics.org/wp-content/uploads/2015/06/Faunalytics_Current-Former-Vegetarians_Full-Report.pdf
- 83.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 84.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 85.Ferrocino I, Di Cagno R, De Angelis M, Turroni S, Vannini L, Bancalari E, et al. Fecal Microbiota in Healthy Subjects Following Omnivore, Vegetarian and Vegan Diets: Culturable Populations and rRNA DGGE Profiling. PLoS ONE [Internet]. 2015. [cited 2016 Feb 24];10 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4452701/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72. doi: 10.1136/gutjnl-2014-308209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are from the NutriNet cohort. Authors CB, MT, BA, SH and CJ are affiliated to the EREN, which is coordinator of the NutriNet-Santé Study. The NutriNet-Santé study is coordinated by the Nutritional Epidemiology Research Team, Université paris 13, INSERM U1153, INRA U1125, Cnam, CRESS. The data were collected in volunteer participants under the agreement of strict confidentiality of the data. Moreover, instructions from regulatory bodies restrict the capacity of the database to be made publicly available. Any access to the cohort must be part of a more global collaborative research work, with co-authorship on manuscripts. Stability of the data is regularly checked by the EREN team, and any inconsistency investigated thouroughly. Data requests may be sent to support@etude-nutrinet-sante.fr.