Abstract
Important metabolic changes occur during transition period of late pregnancy and early lactation to meet increasing energy demands of the growing fetus and for milk production. The aim of this investigation is to present an innovative and non-invasive tool using ewe earwax sample analysis to assess the metabolic profile in ewes during late pregnancy and early lactation. In this work, earwax samples were collected from 28 healthy Brazilian Santa Inês ewes divided into 3 sub-groups: 9 non-pregnant ewes, 6 pregnant ewes in the last 30 days of gestation, and 13 lactating ewes ≤ 30 days postpartum. Then, a range of metabolites including volatile organic compounds (VOC), amino acids (AA), and minerals were profiled and quantified in the samples by applying headspace gas chromatography/mass spectrometry, high performance liquid chromatography/tandem mass spectrometry, and inductively coupled plasma-optical emission spectrometry, respectively. As evident in our results, significant changes were observed in the metabolite profile of earwax between the studied groups where a remarkable elevation was detected in the levels of non-esterified fatty acids, alcohols, ketones, and hydroxy urea in the VOC profile of samples obtained from pregnant and lactating ewes. Meanwhile, a significant decrease was detected in the levels of 9 minerals and 14 AA including essential AA (leucine, phenyl alanine, lysine, isoleucine, threonine, valine), conditionally essential AA (arginine, glycine, tyrosine, proline, serine), and a non-essential AA (alanine). Multivariate analysis using robust principal component analysis and hierarchical cluster analysis was successfully applied to discriminate the three study groups using the variations of metabolites in the two stress states (pregnancy and lactation) from the healthy non-stress condition. The innovative developed method was successful in evaluating pre- and post-parturient metabolic changes using earwax and can in the future be applied to recognize markers for diagnosis, prevention, and intervention of pregnancy complications in ewes.
Introduction
Several metabolomic approaches have been conducted for the study of different classes of small-sized metabolites reflecting the key metabolic pathways involved in the transition period between late pregnancy and early lactation in ruminants [1–16]. The majority of these studies utilized traditional biological fluids such as blood, plasma, serum, etc. employing invasive sampling techniques. In this work, we present earwax as an alternative biological matrix for monitoring the changes in the quantitative profiles of a wide range of metabolites during periparturition using targeted and untargeted metabolomics. This could not only help to gain an improved understanding of the metabolic changes occurring during this important period but also in detecting potential derailments which might occur.
Earwax, a waxy substance secreted by ceruminous glands in the ear canal of humans and other mammals, is a mixture of secretions of specialized sweat glands and fatty material from sebaceous glands [17]. Chemically, it is composed of proteins, lipids, glycopeptides, amino acids (AA), short and long chain fatty acids (saturated and unsaturated), aromatic and long chain hydrocarbons, steroids, volatile organic compounds (VOC), and minerals in addition to some environmental pollutants [18].
In humans, it was used as a biological fluid for diagnosis of fungal infections [19] or other pathological conditions as cystic fibrosis, allergic rhinitis, otoschlerosis [20], tumors using certain patterns of sugar, and metabolic diseases [21]. Two metabolic diseases—maple syrup urine disease and alkaptonuria—were identified in earwax before being diagnosed using traditional techniques as blood and urine analysis [22, 23]. Earwax was also used to study lifetime pattern of organic pollutants in a blue whale [24]. In our recent work, earwax was successfully employed in the diagnosis of diabetes in humans as well as discrimination between its types [25]. It has been also applied as an alternative matrix for monitoring of drugs of abuse or drug facilitated crimes [26], exposure to environmental pollutants [27] and in cattle, to detect exposure to fluoroacetate intoxication [28].
Earwax presents a number of advantages in comparison to traditional biological fluids (blood, plasma, serum) as it overcomes the ethical concerns in the invasive sampling techniques involved in sample collection. Thus, it allows acquiring information about an animal without the need for capturing or immobilization which is detrimental and affecting the animal wellbeing. Earwax sampling is also easy and not painful unlike invasive sampling techniques which can pose stress for animals and thus potentially alter the levels of some of the biomarkers detected.
This in addition to merits of earwax over other non-invasive biological matrices (sweat, saliva, hair, urine, feces) including less liability to external contamination as the ear canal is more protected from the external environment, providing sufficient quantities for multiple assays, involving no or minimum pretreatment or time consuming pre-concentration steps, lack of need for vet personnel for sample collection. Moreover, we believe that earwax could serve as an indicator of the cumulative history of physiological changes occurring in the preceding period to the sample collection (up to few weeks) which is the time required for the buildup of the sample [25].
Nevertheless, it presents some limitations as: timely reproduction which makes it not suitable for repeated sampling over short intervals of time due to relatively slow accumulation of earwax (biomarkers are brought into earwax through the blood circulation as it is being secreted and accumulated in the ear), involves complicated instrumentation as gas chromatography/mass spectrometry (GC-MS), liquid chromatography/mass spectrometry (LC-MS) that requires trained personnel, in addition to lack of standardized methods for earwax collection [25, 26]. An overview of the characteristics of earwax in comparison to the other biological matrices is shown in Table 1.
Table 1. Characteristics of earwax in comparison to other biological matrices.
| Characteristics | Earwax | Invasive biological matrices (Blood, plasma, serum) | Other non-invasive biological matrices | ||||
|---|---|---|---|---|---|---|---|
| Saliva | Sweat | Urine | Feces | Hair | |||
| Painful/stressful condition during sample collection | Low | High | Low | Low | Low | Low | Low |
| Liability to external contamination | Low | Low | High | High | High | High | High |
| On-site screening possible | No | No | Yes | No | Yes | No | No |
| Suitability for tracking longterm changes | Yes | No | No | No | No | Yes | Yes |
| Liability to diurnal variations | No | Yes | Yes | Yes | Yes | No | No |
| Possibility of repeated sampling over short intervals | No | Yes | Yes | No | Yes | Yes | No |
| Need for time consuming pre-concentration steps | No | No | Yes | No | Yes | No | No |
| Liability to blood contamination of samples | No | No | Yes | Yes | Yes | Yes | Yes |
| Effect of pH on composition | No | Yes | Yes | Yes | Yes | Yes | No |
| Affected by location of sample collection | No | No | No | Yes | Yes | Yes | Yes (hair position on the body) |
| Need for vet personnel for sample collection | No | Yes | No | No | Yes | No | No |
| Analytical costs | Medium | Medium | Medium | High | Medium | Medium | High |
Materials and methods
Animals, feeding and housing
The trial was conducted in one of the research facilities of the veterinary school of the Federal University of Goiás, Goiânia. A total of 28 healthy Brazilian Santa Inês ewes, aged 2±0.5 years old (average live weight 40 kg), all multiparous were included in this cross-sectional study. Ewes were divided according to their physiological status into 3 subgroups: (1) 9 healthy non-pregnant ewes (HNP); (2) 6 healthy pregnant ewes within the last 30 days of gestation (HP); (3) 13 healthy lactating ewes within 30 days postpartum (HL). All ewes were located in a free stall with straw bedding material with no access to pasture. They were all fed the same basal diet of ground corn grain, soybean meal, sorghum silage, vitamin, mineral supplement, and corn silage. Fresh feed was offered twice a day (0500 and 1600 h). All ewes had free access to water.
Sample collection
For the experiment, earwax samples were collected directly from both ears of the ewes included in the study using plastic curettes. Sample collection was carried out within 30 days before and after parturition for the pregnant (HP) and the lactating (HL) ewes, respectively. Samples were collected once, all at the same day and timing (1100–1200 h), transferred in Eppendorf tubes which were closed, immediately stored in a freezer at -20°C available at the site of collection, and stored there within 7 days until analyzed.
All procedures performed were in strict accordance with the ethical standards of the ethical committee at the Federal University of Goiás where the study was approved and conducted (Protocol number 027/16).
Volatile organic compound analysis
Earwax samples (20 mg) were accurately weighed, transferred into 20-mL GC vials and 0.2 μL of 3-methyl cyclohexanone (Polyscience Corp., USA) was added as internal standard (IS). The vials were then tightly sealed with gas tight polytetrafluoroethylene (PTFE)-lined rubber septum caps and analyzed by headspace gas chromatography/mass spectrometry (HS/GC-MS) using Shimadzu GCMS-QP2010 Ultra system and Shimadzu AOC-5000 headspace analyzer (Shimadzu, Japan). The system was equipped with a 2500 μL gas-tight syringe, a VT32-20 tray for 20-mL standard vials (PAL System, Zwingen, Switzerland) plus an additional preheating module LHS0 Combi PAL for six vials with control of temperature and heating time (PAL System, Zwingen, Switzerland). The preheating module parameters were set at: agitation speed (500 rpm), agitation on time (5 s), agitation off time (5 s), incubation temperature (160°C). For the chromatography, an analytical capillary column NST-100 (25 m × 0.25 mm i.d. × 0.3 μm film thickness) (NST, São Paulo, Brazil) with a polyethylene glycol high-polarity stationary phase was used.
The oven temperature was set to 5 min isothermal heating at 30°C, followed by an increase at 2°C/min until 45°C (held for 5 min) then increased by 2°C/min to 50°C (held for 5 min) then at the same rate to 120°C, then increased at 6°C/min to 200°C (held for 5 min) and finally at 5°C/min to 250°C (held for 10 min).
The headspace sampler parameters were: fill volume (2500 μL), fill speed (100 μL/s), injection volume (2500 μL), injection speed (500 μL/s), syringe temperature (150°C), pre-warm time (10 min), equilibration time (5 min), syringe flushing time (1 min), re-flush time (0 ms), and post-flush time (0 ms).
High purity helium (99.999%—5.0) was used as carrier gas with a constant flow rate of 1.0 mL/min. Injector was operated in the splitless mode at 230°C. Start cut-off time for MS recording was 0 min. The MS was operated in electron ionization (EI) mode at 70 eV. Data acquisition was performed in full scan mode from m/z = 40–500 with a scan time of 0.3 s.
Individual constituents were identified in earwax samples by comparing their mass spectra with reference compounds and/or NIST11s Mass Spectral Library and only compounds with ≥ 80% probability of a match to NIST11s library standards were named. Further authentication was achieved by subsequent manual inspection and retention time matching of selected compounds. Quantification of the investigated VOC was based on peak area ratios versus IS.
Amino acid analysis
Preparation of standard solutions
For calibration samples, stock solutions of the reference standards of 18 AA: arginine (Arg), leucine (Leu), phenyl alanine (Phe Ala), lysine (Lys), tryptophan (Trp), alanine (Ala), glutamine (Glu), valine (Val), threonine (Thr), cysteine (Cys), glycine (Gly), tyrosine (Tyr), proline (Pro), isoleucine (Iso-leu), serine (Ser), aspartic acid (Asp), glutamic acid (Glu acid), and citrulline (Citrull) (Sigma Aldrich, USA) were prepared in 0.1% hydrochloric acid, each at a concentration of 2 mg/mL. Subsequent dilutions were made in 0.1% hydrochloric acid to prepare a combined standard solution (1 μg/mL) of the investigated AA. All calibration stocks and working standards were stored at -20°C until use.
Sample preparation
Earwax samples (10 mg) were accurately weighed, mixed with 800 μL of 0.1% HCl and subsequently vortexed (IKA MS 3 digital, Sigma Aldrich, USA) for 5 min, then centrifuged in Eppendorf Centrifuge (3000 rpm) (Rotana 460R, Hettich, Germany) for 10 min at 4°C. The supernatant was stored at -20°C until analysis.
Chromatography was carried out using an Agilent 1290 series high performance liquid chromatography (HPLC) system equipped with a quaternary pump and 54 vial plate autosampler (Agilent Technologies, Waldbronn, Germany) operating in gradient mode, coupled with thermostated autosampler and fully controlled by Analyst software (Version 1.5.2). The chromatographic run was performed using a Techsphere silica column (250×4.6 mm, 5 μm) (HPLC Technologies, Cheshire, UK) operated at room temperature. Mobile phase was composed of 3 mM ammonium phosphate in HPLC-grade water containing 0.1% formic acid and 0.1% trifluoroacetic acid (TFA) (Sigma Aldrich, USA) as phase A, HPLC-grade water as phase B, and 100% acetonitrile (J.T. Baker, USA) as phase C. The flow rate was maintained at 1.5 mL/min. The injection volume was 1 μL. Separation was accomplished using a linear gradient from 91% to 60% (phase C) over the first 15 min and then returned to the initial condition from 15 to 17 min and then allowed to equilibrate for 3 min while phase A was maintained at 2% throughout the whole run.
MS measurements were done on API 3200 QTRAP triple quadrupole/linear ion trap mass spectrometer (AB SCIEX, Toronto, Canada) equipped with the TurboIonSpray source fully controlled by the Applied Biosystems/MDS Analyst 1.5.2 software. The instrument was operated in the atmospheric pressure chemical ionization (APCI) in positive mode and MS in multiple reaction monitoring mode (MRM). Ion source parameters were: curtain gas (18 psi), ion source gas 1 (40 psi), ion source gas 2 (0 psi), temperature (450°C), collision gas (CAD) (medium), nebulizing current (NC) (4), interface heater (on).
Quantitative data were acquired using Applied Biosystems/MDS Analyst software version 1.5.2.
Validation procedure
Evaluation of method performance including limit of detection (LOD), lower limit of quantitation (LLOQ), linearity, accuracy, precision, carryover, extraction efficiency expressed as recovery (R%), and matrix effect expressed as matrix factor (MF) was performed per ICH guidelines [29].
Linearity and lower limit of quantitation
Quantification was based on external calibration method. Calibration samples were prepared at seven different concentrations (each containing the investigated AA). Concentration ranges were chosen based on sensitivities and levels of AA normally encountered in earwax. The linear least-square regression was used to determine the mean intercepts, mean slope and determination coefficients (R2) of the calibration curves.
The LLOQ is defined as the lowest concentration of analyte on the calibration curve with an acceptable accuracy with ±20% (bias) of the nominal value and precision ≤20% (CV%) from six replicates for a predefined dynamic range.
The LOD is defined as the lowest concentration that gives a reproducible instrument response with S/N ratio ≥3.
Accuracy and precision
Intraday precision and accuracy were determined by analysing five replicates of the calibrators for all amino acids during a single analytical run. Interday precision and accuracy were determined by analysing three replicates of samples at each level through analytical runs made on five different days.
Extraction recovery
The extraction recovery was calculated in terms of R% = C/B x 100, where C is the analyte peak area of blank earwax spiked with a reference standard before the extraction and B is the analyte peak area of earwax spiked with the reference standard after the extraction.
Matrix effect
The matrix effect was calculated in terms of matrix factor (MF), obtained from three concentration levels (low, middle and high calibrators), each in triplicate. The MF is defined as the analyte peak area ratio of blank earwax sample extract spiked with an analyte after extraction to the reference standard in 0.1% HCl containing equivalent amount of the analyte neat sample. If MF is equal to 1, this means that no matrix effect is present; if MF <1, this means that there is ionization suppression, whereas if MF >1, this means that there is ionization enhancement.
Carryover effect
Carryover was investigated by injecting 1 μL of solvent in triplicate immediately after the highest calibration standard and the response was observed at the retention time of the analyte detected. It should not be >20% of the LLOQ response.
Analysis of minerals
A total of 28 minerals and elements were tested including (Ag, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S. Sn, Sr, Ti, U, V, Zn, Se, and I) using an inductively coupled plasma optical emission spectrometer (ICP-OES), model 6300 ICAP Duo (Thermo Fisher Scientific, Massachusetts, USA) with the following accessories: tygon tubes, concentric nebulizer, and cyclonic spray chamber core tube 2 mm.
Samples were used from subjects with the amount of earwax remaining after analysis by HS/GC–MS and high performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS). Samples (10 mg) were weighed and placed into 15-mL PFA-coated pressure vessels, to which 1 mL of 70% v/v ultrapure grade nitric acid (HNO3) (Sigma Aldrich, Brazil), and 1 mL of 30% v/v of ultrapure grade H2O2 (Vetec® Sigma Aldrich, Brazil) were added. The digestion was carried out in a Provecto Analítica model DGT 100 plus microwave oven (Campinas, Brazil) using the following program of 4 steps: (i) 1 min at 320 W power, (ii) 3 min at 500 W power, (iii) 4 min at 800 W power, and finally (iv) 3 min at 0 W. The resulting solution was diluted to 10 mL using Milli-Q® ultrapure water (Merck KGaA, Darmstadt, Germany) in a 10-mL volumetric flask. The diluted samples were stored at 4°C pending analysis.
The wavelengths were chosen taking into account the lines of highest intensity and the lowest number of interference: B (249.773 nm), Ba (455.403 nm), S (234.861 nm), Bi (223.061 nm), Ca (393.366 nm), Co (228.616 nm), Cr (267.716 nm), Cu (324,754nm), Fe (259.940 nm), K (769.896 nm), Li (670.784 nm), Mg (280.270 nm), Mn (257.610 nm), Mo (202.030 nm), Na (589.592 nm), Ni (231.647 nm), P (178.284 nm), Pb (220.353 nm), Sn (189.989 nm), Mr. (421.552 nm), Ti (337.280 nm), V (292.402 nm), Zn (206.200 nm), Se (196.090 nm), and I (178.276 nm).
The readings of all the elements were performed using the axial configuration of the equipment except Ca and Mg, where the radial configuration was used. The instrumental parameters were optimized to achieve greater emission signal corrected to the trace elements. The parameters used were as follows: Rotation (50 RPM pump), gas flow argon aid (0.5 L/min), pressure the nebulizer argon gas (0.16 Mpa) and power source (1250 Watts).
Data pretreatment
Following peak deconvolution and integration, GC/MS data were obtained with initial compound annotation conducted by searching against NIST11 library and/or reference standards. Quantitative data set of the AA obtained by HPLC-MS/MS using external calibration was organized in a Microsoft excel spreadsheet in terms of concentration of each AA in μg/L. The quantitative report of each sample generated from ICP–OES was stored as a separate csv format file by Thermo Scientific™ Qtegra™ Intelligent Scientific Data Solution™ software.
The data matrix was then exported to MATLAB R2014b (The MathWorks Natick, USA) and multivariate analysis was performed using PLS Toolbox 8.1 (Eigenvector Research Inc., Manson, USA).
Statistical analysis
In order to minimize statistical bias resulting from the different magnitudes of the relative compositions of different metabolites, data set derived from GC-MS, HPLC-MS/MS, and ICP-OES was autoscaled prior to multivariate analysis. The intrinsic variations of metabolites in the two stress states (pregnancy and lactation) from the healthy non-stress condition were visualized by robust principal component analysis (RPCA) scores plot and hierarchical cluster analysis (HCA) dendrogram using the furthest neighbor method. Robust instead of classical PCA was employed since outliers can be influential on classical PCA [30]. One-way ANOVA was performed to compare the three groups using a significance level (α) of 0.05.
Results and discussion
Alteration of the earwax volatile organic compound metabolome in periparturient ewes
The important VOC in earwax are alcohols, ketones, non-esterified fatty acids (NEFA), and hydroxy urea.
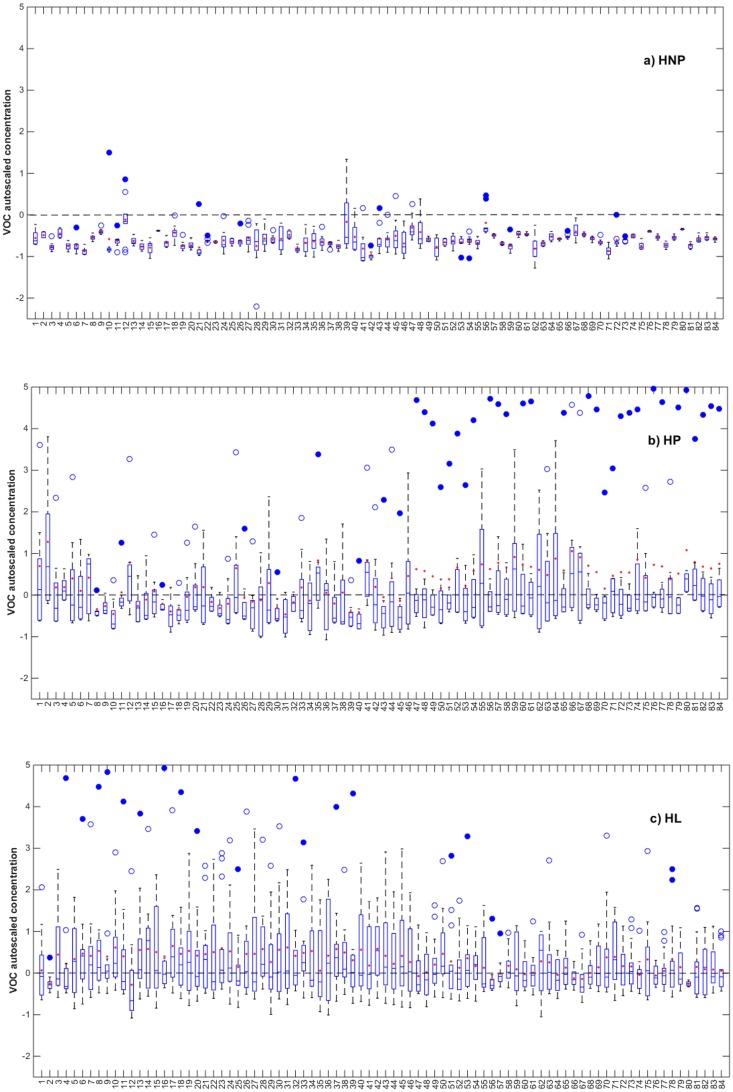
Our results show significant elevation in levels of NEFA with different extents in pregnant and lactating ewes including acetic acid (8); decanoic acid (42); tridecanoic acid (57); tetradecanoic acid (58); hexadecanoic acid (66); octadecanoic acid (80) (P<0.05) (Fig 1a–1c). VOC numbering, CAS no., and retention times (Rt) are demonstrated in Table 2.
Fig 1. Box-plot diagrams showing autoscaled concentrations of 84 volatile organic compounds (VOC) in earwax of Santa Inês sheep: a) Healthy non-pregnant ewes (HNP), b) Healthy pregnant ewes (HP), and c) Healthy lactating ewes (HL).
Unfilled circles (○) represent statistically suspected outliers and filled circles (•) are statistical outliers. VOC numbering is according to Table 2.
Table 2. Volatile organic compounds (VOC) numbering in Fig 1, its CAS No., and retention times (Rt).
| No. | VOC | Rt (min) | CAS No. | No. | VOC | Rt (min) | CAS No. | No. | VOC | Rt (min) | CAS No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hydroxy urea | 1.16 | 127-07-1 | 29 | Phenol, 4-methyl, | 43.04 | 106-44-5 | 57 | Tridecanoic acid | 74.48 | 638-53-9 |
| 2 | Ethanol | 1.32 | 64-17-5 | 30 | Phenol, 4-methoxy, | 43.22 | 150-76-5 | 58 | Tetradecanoic acid | 75.41 | 544-63-8 |
| 3 | Acetone | 1.59 | 67-64-1 | 31 | n-Nonanal | 45.06 | 124-19-6 | 59 | 1-Pentadecanol | 75.92 | 629-76-5 |
| 4 | 1,3-Cyclopentadiene | 1.97 | 542-92-7 | 32 | 2-Oxo-octanoic acid | 50.03 | 328-51-8 | 60 | 1-Hexadecanol | 77.89 | 36653-82-4 |
| 5 | Propanal, 2-methyl | 2.19 | 78-84-2 | 33 | Phenol, 4-ethyl | 50.98 | 123-07-9 | 61 | 1,2-Benzene- dicarboxylic acid, bis (2-methyl- propyl) ester | 78.64 | 84-69-5 |
| 6 | 2-Butanone, 3-methyl | 2.63 | 563-80-4 | 34 | Decanal | 53.74 | 112-31-2 | 62 | Tridecane, 6-methyl- | 78.82 | 13287-21-3 |
| 7 | 2-Butanone | 2.76 | 78-93-3 | 35 | Benzofuran,2,3-dihydro (Coumaran) | 55.27 | 496-16-2 | 63 | 1-Heptadecanol | 79.15 | 1454-85-9 |
| 8 | Acetic acid | 4.24 | 61-19-7 | 36 | (3E)-3-(Amino-methylene) -2H-pyran-2,6(3H)-dione | 55.87 | 20189-42-8 | 64 | 2-Nonadecanone | 79.87 | 629-66-3 |
| 9 | Butanal, 3-methyl, | 4.13 | 590-86-3 | 37 | 2(3H)-Furanone, dihydro-5-octyl-(γ-Dodecalactone) | 57.52 | 2305-05-7 | 65 | 9-Eicosene | 81.46 | 42448-90-8 |
| 10 | Butanal, 2-methyl, | 4.43 | 96-17-3 | 38 | 1H-indole | 60.26 | 120-72-9 | 66 | n-Hexadecanoic acid | 81.64 | 57-10-3 |
| 11 | 2-Propanone, 1-hydroxy, (Acetol) | 4.96 | 116-09-6 | 39 | Tridecane | 61.15 | 629-50-5 | 67 | Hexadecanoic acid, ethyl ester | 82.59 | 628-97-7 |
| 12 | 2,4-Dimethyl furan | 6.57 | 3710-43-8 | 40 | 2-Decen-1-ol | 61.41 | 18409-18-2 | 68 | 1-octadecanol | 84.03 | 112-92-5 |
| 13 | 1H-pyrrole, 1-methyl | 7.92 | 96-54-8 | 41 | 4-Hydroxy-3-methyl acetophenone | 61.65 | 876-02-8 | 69 | Hexadecane-1,2-diol | 84.46 | 6920-24-7 |
| 14 | 1H-pyrrole | 9.02 | 109-97-7 | 42 | Decanoic acid | 64.15 | 334-48-5 | 70 | n-Nonadecanol-1 | 84.98 | 1454-84-8 |
| 15 | Toluene | 9.45 | 108-88-3 | 43 | Tetradecane | 65.86 | 629-59-4 | 71 | 2(3H)-Fuanone, 5-dodecyldihydro- (γ-Palmitolactone) | 85.42 | 730-46-1 |
| 16 | 2-Hexanone | 11,13 | 591-78-6 | 44 | Dodecanal | 66.10 | 112-54-9 | 72 | Cycloeicosane | 86.57 | 296-56-0 |
| 17 | Acetamide | 11.62 | 60-35-5 | 45 | Oxirane, [(dodecyloxy) methyl]- | 68.85 | 2461-18-9 | 73 | 1-Docosene | 86.76 | 1599-67-3 |
| 18 | Methyl pyrazine | 13.53 | 109-08-0 | 46 | 2-Dodecanone | 68.95 | 6175-49-1 | 74 | 1-Eicosanol | 87.15 | 629-96-9 |
| 19 | 1H-pyrrole, 2-methyl | 15.16 | 636-41-9 | 47 | Tridecanal | 69.39 | 10486-19-8 | 75 | Hexadecanoic acid, butyl ester | 87.39 | 111-06-8 |
| 20 | 1H-pyrrole, 3-methyl | 16.03 | 616-43-3 | 48 | 1-Hexadecene | 70.56 | 629-73-2 | 76 | Cyclodocosane, ethyl- | 88,40 | 0-00-0 |
| 21 | 2-Furanmethanol (Furfuryl alcohol) | 17.14 | 98-00-0 | 49 | Dodecane, 2,6,10-trimethyl- | 70.73 | 3891-98-3 | 77 | Cyclotetracosane | 88.81 | 297-03-0 |
| 22 | Pyridine, 3-methyl (Picoline) | 18.68 | 108-99-6 | 50 | 1-Dodecanol | 71.03 | 112-53-8 | 78 | 1-Henicosanol | 89.67 | 15594-90-8 |
| 23 | 2-Heptanone | 20.51 | 110-43-0 | 51 | 2-Tetradecanone | 71.56 | 2345-27-9 | 79 | 2-Hydroxyhexadecyl butanoate | 90.07 | 77899-01-5 |
| 24 | Benzaldehyde | 28.93 | 100-52-7 | 52 | Pentadecanal | 71.77 | 2765-11-9 | 80 | Octadecanoic acid | 91.36 | 57-11-4 |
| 25 | Pyridine, 3,4-dimethyl, (3,4-Lutidine) | 31.92 | 583-58-4 | 53 | 1,4-Methano-naphthalene, 6,7-diethyldecahydro- | 72.05 | 16539-02-9 | 81 | Valeric acid, hexadecyl ester | 91.84 | 125164-54-7 |
| 26 | 2-octanone | 33.64 | 111-13-7 | 54 | 1,5-Cyclooctadiene, 1-t-butyl- | 72.42 | 0-00-0 | 82 | Octadecanoic acid, butyl ester | 92.24 | 123-95-5 |
| 27 | Phenol | 33.87 | 108-95-2 | 55 | 1-Tetradecanol | 73.41 | 112-72-1 | 83 | Eicosanoic acid, 2-ethyl-2-methyl, methyl ester | 92.61 | 55282-04-7 |
| 28 | 2-Cyclohexene-1-one, 3-methyl (Seudenone) | 39.73 | 1193-18-6 | 56 | Hexadecanal | 74.27 | 629-80-1 | 84 | 1-Docosanol (Behenic alcohol) | 94.37 | 661-19-8 |
This was found in accordance with the fact that ruminants use energy from mobilization of body reserves both in late gestation and during lactation. Fat depots are mobilized, via lipolysis of adipose tissue to increase the energy supply. As a result, NEFA are released into the blood circulation and serve as an important source of energy [31].
A significant increase in the levels of some alcohols was also observed in HP and HL principally ethanol (2) (P<0.0005), in addition to furfuryl alcohol (21), 1-dodecanol (50), 1-tetradecanol (55), 1-pentadecanol (59), 1-heptadecanol (63), 1-nonadecanol (70), eicosanol (74), heneicosanol (78), and docosanol (84) with (P<0.05) (Fig 1a–1c). In the literature, pregnant sheep were already used as an experimental model to monitor the effect of alcohol exposure on the maternal metabolism [32]. Thus, the developed method being able to detect alcohols can be applied as a simple method for monitoring alcohol exposure using earwax.
Regarding ketones, all of the ketones detected in earwax samples of HP and HL showed significant increase in their levels in comparison to HNP (P<0.05) with the exception of 2-hexanone (16). These ketones include: acetone (3), 2-butanone, 3-methyl (6), 2-butanone (7), 2-heptanone (23), 2-octanone (26), cyclohexene-1-one 3-methyl, (28), 3E)-3-(Aminomethylene)-2H-pyran-2,6(3H)-dione (36), 4-hydroxy-3-methyl acetophenone (41), 2-dodecanone (46), 2-tetradecanone (51), and 2-nonadecanone (64) (Fig 1a–1c). This also relates the levels of ketones to the negative energy imbalance occurring during pregnancy and lactation. There are also studies confirming the increase in the levels of ketone bodies (acetone, acetoacetate and β-hydroxybutyrate) in other biological fluids such as milk and blood of subclinical ketotic pregnant cows and ketotic dairy cows [33, 34] occurring as a result of negative energy balance.
Hydroxy urea (1), a normal volatile component in earwax of ewes, showed an increase in both HP and HL than in HNP ewes. In the literature, no reports on hydroxyurea were found but there were studies on plasma urea levels [35] showing that it starts increasing in pregnant Barki ewes from the 10th week of pregnancy, reaching the maximum at parturition. Same results were reported by Durak and Altinek [16]. This could be explained that the glomerular filtration and urea clearance in ewes decrease in late pregnancy and lactation [36]. Another explanation of the increase in the level of urea, could be attributed to the negative energy balance as a result of metabolic stress suffered during pregnancy and lactation [37, 38].
In other studies, level of urea was found slightly higher in pregnancy than that of early lactation but it was highest at 55 days lactation [39].
Alteration of the earwax amino acid metabolome in periparturient ewes
The change in AA composition was quantified using ion-pair chromatography coupled with MS/MS detection benefiting of the zwitterionic character of the AA. The positive charged amino group is bounded by the anionic ion pair reagent (TFA). The method was validated and the assay validation parameters results are displayed in Table 3.
Table 3. Results of assay validation parameters of the HPLC-MS/MS method for the analysis of amino acids (AA) in earwax of Santa Inês sheep.
| AA | MRM transitions | Linearity μg/L |
LOD μg/L |
LOQ μg/L |
Precision | R% ±(CV%) | MF ±SD | Carry over (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentrations μg/L |
Intraday (5 replicates) |
Interday (In triplicate for 5 days) |
||||||||||
| Accuracy (%) |
CV (%) |
Accuracy (%) |
CV (%) |
|||||||||
| Arg | 175.3>70.0 | 6.0–100.0 | 1.8 | 6.0 | 6 | 101.1 | 2.63 | 97.8 | 7.00 | 90.2±0.63 | 1.08±0.07 | 0.5 |
| 50 | 106.2 | 4.19 | 83.5 | 7.38 | ||||||||
| 100 | 94.8 | 14.98 | 91.2 | 4.24 | ||||||||
| Leu | 132.2>86.3 | 4.0–100.0 | 1.2 | 4.0 | 4 | 97.5 | 7.96 | 94.8 | 3.40 | 87.2±0.52 | 0.84±0.04 | 1.6 |
| 50 | 108.3 | 8.06 | 115.7 | 5.76 | ||||||||
| 100 | 93.6 | 2.27 | 98.0 | 4.10 | ||||||||
| Phe Ala | 166.4>120.0 | 4.0–100.0 | 1.2 | 4.0 | 4 | 94.2 | 4.06 | 95.9 | 1.74 | 86.3±3.65 | 1.00±0.12 | 1.3 |
| 50 | 88.4 | 6.24 | 107.1 | 15.15 | ||||||||
| 100 | 90.3 | 7.36 | 90.5 | 5.14 | ||||||||
| Lys | 147.3>56.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 95.1 | 17.99 | 110.9 | 12.46 | 89.0±0.52 | 1.05±0.13 | 1.6 |
| 50 | 90.3 | 14.71 | 85.1 | 6.13 | ||||||||
| 100 | 95.8 | 6.03 | 97.1 | 13.63 | ||||||||
| Trp | 205.4>146.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 89.0 | 19.02 | 100.6 | 12.82 | 89.5±2.25 | 0.94±0.07 | 2.0 |
| 50 | 97.2 | 0.58 | 98.5 | 3.15 | ||||||||
| 100 | 87.5 | 0.97 | 87.8 | 2.44 | ||||||||
| Ala | 90.1>44.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 79.3 | 5.80 | 83.6 | 11.34 | 87.6±1.62 | 0.94±0.12 | 1.9 |
| 50 | 99.0 | 6.93 | 101.5 | 9.05 | ||||||||
| 100 | 93.4 | 4.10 | 94.8 | 4.35 | ||||||||
| Glu | 147.2>84.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 98.4 | 6.65 | 104.5 | 7.27 | 90.9±1.21 | 0.94±0.07 | 1.2 |
| 50 | 91.1 | 8.26 | 92.2 | 13.20 | ||||||||
| 100 | 102.0 | 2.99 | 99.8 | 9.86 | ||||||||
| Val | 118.3>72.3 | 4.0–100.0 | 1.2 | 4.0 | 4 | 83.6 | 8.65 | 92.6 | 10.71 | 84.8±1.02 | 1.00±0.15 | 1.3 |
| 50 | 118.0 | 8.39 | 109.9 | 12.39 | ||||||||
| 100 | 101.0 | 1.04 | 101.1 | 8.72 | ||||||||
| Thr | 120.2>74.3 | 4.0–100.0 | 1.2 | 4.0 | 4 | 87.0 | 4.99 | 88.3 | 1.23 | 81.1±0.01 | 0.81±0.05 | 1.8 |
| 50 | 93.1 | 6.45 | 99.7 | 6.65 | ||||||||
| 100 | 98.1 | 0.72 | 94.5 | 3.35 | ||||||||
| Cys | 241.3>152.1 | 1.0–100.0 | 0.3 | 1.0 | 1 | 103.0 | 7.92 | 111.9 | 7.60 | 96.8±0.05 | 1.12±0.03 | 0.5 |
| 50 | 97.7 | 7.55 | 88.4 | 7.24 | ||||||||
| 100 | 88.5 | 5.06 | 105.1 | 15.06 | ||||||||
| Gly | 76.0>30.3 | 1.0–100.0 | 0.3 | 1.0 | 1 | 95.5 | 2.22 | 91.5 | 5.01 | 85.3±3.21 | 0.97±0.16 | 0.7 |
| 50 | 117.7 | 13.18 | 108.2 | 13.77 | ||||||||
| 100 | 89.7 | 5.48 | 99.9 | 12.40 | ||||||||
| Tyr | 182.4>136.3 | 4.0–100 | 1.2 | 4.0 | 4 | 94.7 | 6.89 | 90.0 | 6.95 | 84.1±1.87 | 0.92±0.10 | 3.6 |
| 50 | 94.4 | 12.51 | 108.9 | 12.07 | ||||||||
| 100 | 87.6 | 9.63 | 91.2 | 3.40 | ||||||||
| Pro | 116.2>70.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 77.8 | 4.09 | 85.3 | 10.42 | 89.50±0.30 | 0.83±0.01 | 1.25 |
| 50 | 106.0 | 3.77 | 102.6 | 9.24 | ||||||||
| 100 | 101.6 | 4.97 | 102.0 | 4.10 | ||||||||
| Iso-leu | 132.4>86.2 | 4.0–100.0 | 1.2 | 4.0 | 4 | 86.1 | 10.08 | 86.6 | 2.23 | 89.8±0.10 | 0.84±0.01 | 1.5 |
| 50 | 117.7 | 2.60 | 108.6 | 7.69 | ||||||||
| 100 | 100.5 | 3.01 | 95.7 | 7.60 | ||||||||
| Ser | 106.0>60.0 | 4.0–100.0 | 1.2 | 4.0 | 4 | 89.3 | 7.58 | 88.7 | 3.93 | 78.3±0.25 | 0.95±0.18 | 1.7 |
| 50 | 103.9 | 9.32 | 105.6 | 4.55 | ||||||||
| 100 | 92.8 | 1.49 | 93.4 | 6.11 | ||||||||
| Asp | 134.1>73.9 | 1.0–100.0 | 0.3 | 1.0 | 1 | 86.0 | 15.73 | 89.3 | 7.35 | 81.6±1.06 | 0.95±0.18 | 1.6 |
| 50 | 106.7 | 3.79 | 106.5 | 13.00 | ||||||||
| 100 | 88.1 | 16.19 | 99.3 | 12.30 | ||||||||
| Glu acid | 148.3>84.2 | 1.0–100.0 | 0.3 | 1.0 | 1 | 83.7 | 0.69 | 90.7 | 6.84 | 90.2±0.71 | 0.80±0.06 | 1.3 |
| 50 | 92.4 | 7.22 | 97.8 | 13.06 | ||||||||
| 100 | 95.2 | 5.58 | 100.2 | 15.57 | ||||||||
| Citrull | 176.3>70.0 | 1.0–100.0 | 0.3 | 1.0 | 1 | 110.9 | 11.39 | 107.4 | 3.64 | 89.8±0.26 | 1.07±0.02 | 7.6 |
| 50 | 93.5 | 4.71 | 93.8 | 9.22 | ||||||||
| 100 | 95.6 | 4.89 | 95.1 | 9.34 | ||||||||
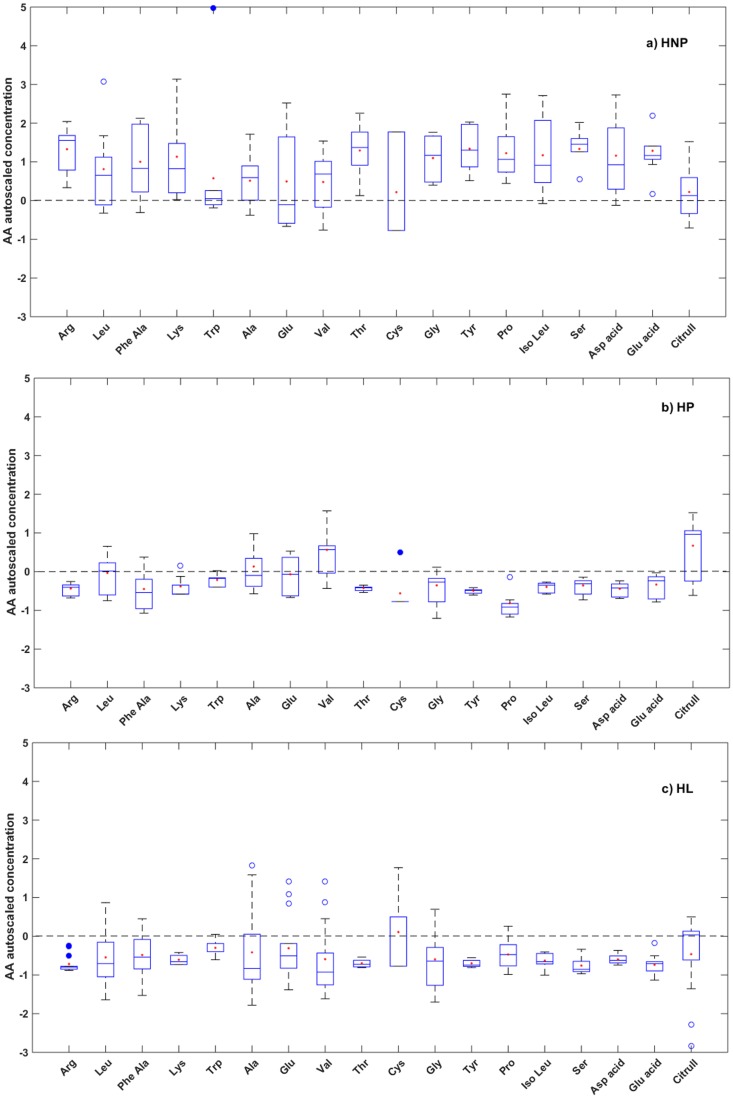
According to our results, there are 18 AA in earwax, of which 14 show significant variations in response to physiological changes during periparturition as Arg, Lys, Thr, Gly, Tyr, Pro, Iso Leu, Ser, Asp acid, Glu acid (P<0.0001), and to a lesser extent Phe Ala (P<0.0005), Leu (P<0.005), Val (P<0.01), and Citrull (P<0.05) (Fig 2a–2c). In the overall, HNP AA levels (Fig 2a) are higher than AA levels for both HP (Fig 2b) and HL (Fig 2c). The results indicate variations in essential AA (Leu, Phe Ala, Lys, Iso Leu, Thr, Val) in accordance with results reported [40], some conditionally essential AA (Arg, Gly, Tyr, Pro, Ser), and minor variation in Ala, a non-essential AA principally in HL group. For conditionally essential AA, their synthesis can be limited under special physiological/pathophysiological conditions, such as catabolic distress such as in pregnancy and lactation [41].
Fig 2. Box-plot diagrams showing autoscaled concentrations of 18 amino acid metabolites (AA) in earwax of Santa Inês sheep: a) Healthy non-pregnant ewes (HNP), b) Healthy pregnant ewes (HP), and c) Healthy lactating ewes (HL).
Unfilled circles (○) represent statistically suspected outliers and filled circles (•) are statistical outliers.
This can be explained that during pregnancy, AA are actively transported across the placenta to be utilized by the fetus for protein synthesis and tissue growth. They are also oxidized as an energy source particularly in underfed ewes where the supply of glucose to the fetus is limited [42], especially that glucose and AA are known to be the main energetic substrates for fetal development and colostrum/milk production [43].
It should be also considered that during pregnancy, even if the energy intake is adequate for sufficient total protein production, the supply of certain essential AA may not be enough in relation to the needs of the fetus [44]. Moreover, there were reports on the reduction in the serum AA concentration due to increased maternal blood volume during gestation [45] which, in turn, is caused by increase in serum estrogen levels [46].
As for lactation, some of the AA available from feed are used for gluconeogenesis when energy is not adequate as well as for energy production [47].
Alteration of the earwax minerals in periparturient ewes
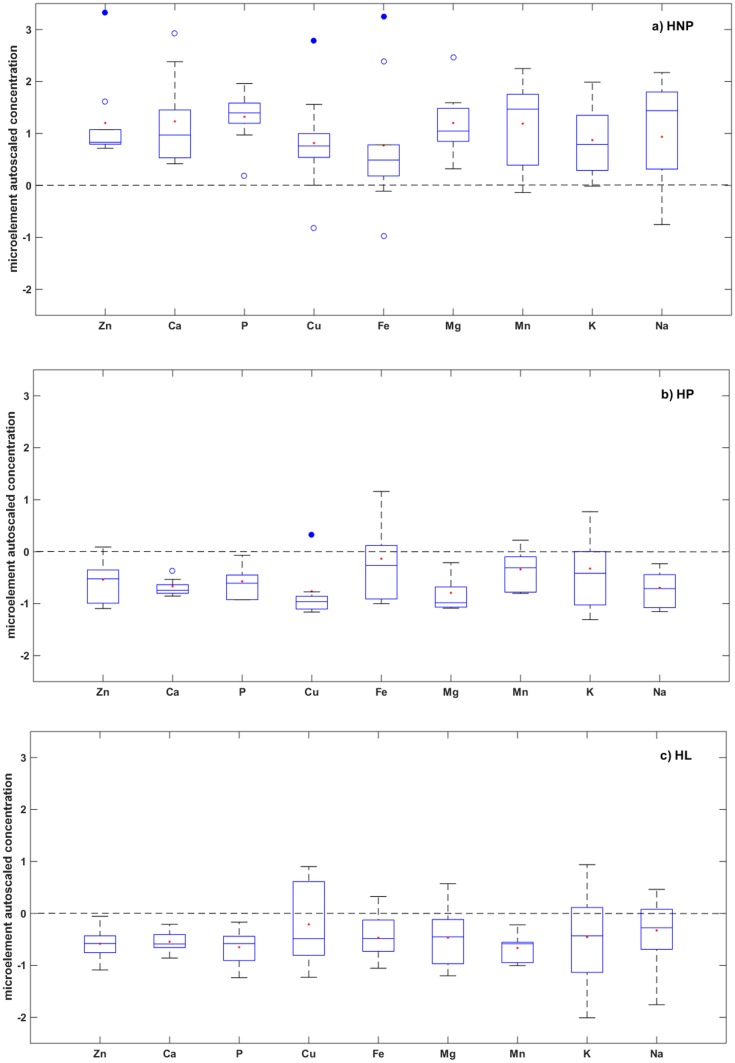
From the 28 elements tested in earwax samples, only 9 were successfully detected and quantified namely (Zn, Ca, P, Cu, Fe, Mg, Mn, Na, and K). They all showed a decline in their concentration in periparturient periods (P<0.005) (Fig 3a–3c).
Fig 3. Box-plot diagrams showing autoscaled concentrations of 9 trace elements in earwax of Santa Inês sheep: a) Healthy non-pregnant ewes (HNP), b) Healthy pregnant ewes (HP), and c) Healthy lactating ewes (HL).
Unfilled circles (○) represent statistically suspected outliers and filled circles (•) are statistical outliers.
Regarding Ca levels in earwax, they significantly decrease in pre- and postparturient samples (P<0.0001) matching results on Ca levels in plasma and serum presented in earlier studies [48], where it was reported that Ca levels markedly decrease during late pregnancy, reaching minimum levels at parturition, then continued to decrease for the first few weeks postpartum, ultimately returning to the level before pregnancy [45]. This could be attributed to increased demand for Ca for mineralization of fetal skeleton.
Same results were reported on blood Ca concentrations in ruminants with clinical and subclinical ketosis or pregnancy toxemia due to mineral deficiencies that typically result from ketosis due to disturbances in feed consumption [49].
Meanwhile, results obtained on Na, K, and Mg concentrations showed that they all decreased significantly in HP and HL in agreement with results previously reported on the same minerals in blood, where erythrocytic Ca, Mg, Na, and K concentrations were found to decrease during the last three weeks of pregnancy, day of parturition, and remained low 2 weeks postpartum as a result of loss of these ions through the colostrum [45].
In other reports, serum Na levels were low before and at parturition but started to increase after parturition being highest at weeks 3 and 4 postpartum however, returning to levels at parturition by week 5. By the contrary, Dakka and Abd El All [49] showed that serum Na level increased during late pregnancy in sheep.
With regard to P, it was found to decrease significantly in both groups HP and HL (P<0.0001). In the literature, there were studies confirming no significant differences in plasma P concentrations between different physiological stages [50] while others showed significant decrease of plasma P levels during pregnancy in ewes [51].
This, to an extent, showed similarity of our results obtained using earwax and other biological fluids such as serum and plasma.
Multivariate analysis
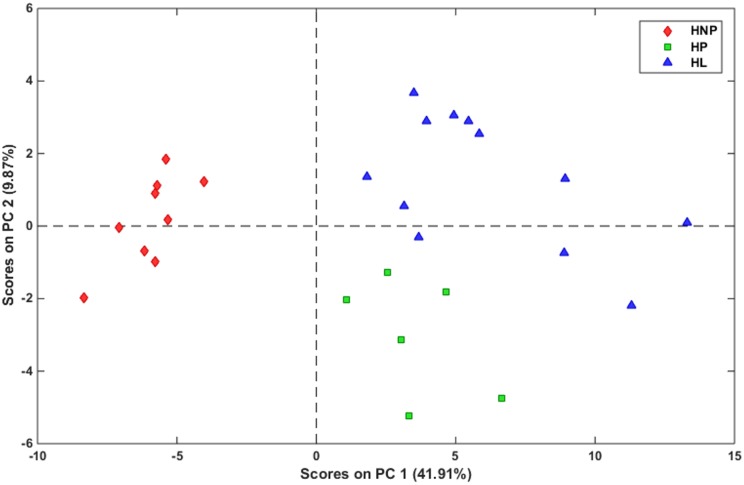
To relate physiological changes associated with pregnancy and lactation with changes in the metabolome, RPCA of marker compounds (metabolites and micronutrients) was performed on the analytical data obtained from HS-GC/MS, HPLC-MS/MS, and ICP-OES of all samples. Fig 4 shows the score plot of the first two principal components (PCs) that explains 51.78% of the total data variance. This plot indicates a clear distinction between the three groups: HNP, HP, and HL. As observed, the HNP samples (left side of Fig 4) could be discriminated from the other two groups (HP and HL) using PC1 alone, as they are concentrated on the negative side of PC1. Meanwhile, PC2 was able to discriminate to a great extent between the HP and HL located on negative and positive sides of PC2, respectively with just few exceptions where 3 HL samples were grouped together with HP group. As seen, HL ewes showed greater heterogeneity than the other two groups studied due to the variation in the metabolite composition among the days during lactation in agreement with the results in the literature indicating significant variations in the metabolite compositions in plasma, serum, and other biological fluids among different days during lactation period in ewes [52]. The sample heterogeneity found in HL group justifies using RPCA instead of the classical PCA. This separation was further confirmed using HCA (Fig 5).
Fig 4. RPCA score plot of 28 earwax samples from Santa Inês sheep using 111 metabolite signals analyzed by HS-GC/MS, HPLC-MS/MS, and ICP-OES: a) Healthy non-pregnant ewes (HNP) (♦), b) Healthy pregnant ewes (HP) (■), and c) Healthy lactating ewes (HL) (▲).
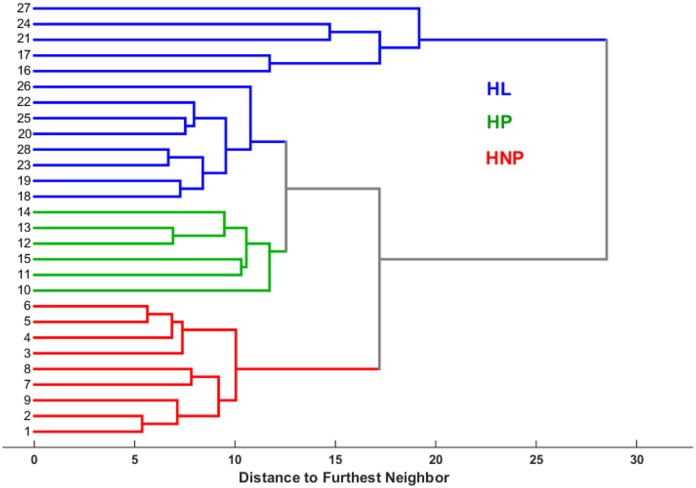
Fig 5. Dendrogram of hierarchial cluster analysis (HCA) of 28 earwax samples from Santa Inês sheep using 111 metabolite signals analyzed by HS-GC/MS, HPLC-MS/MS, and ICP-OES: a) Healthy non-pregnant ewes (HNP) (red), b) Healthy pregnant ewes (HP) (green), and c) Healthy lactating ewes (HL) (blue).
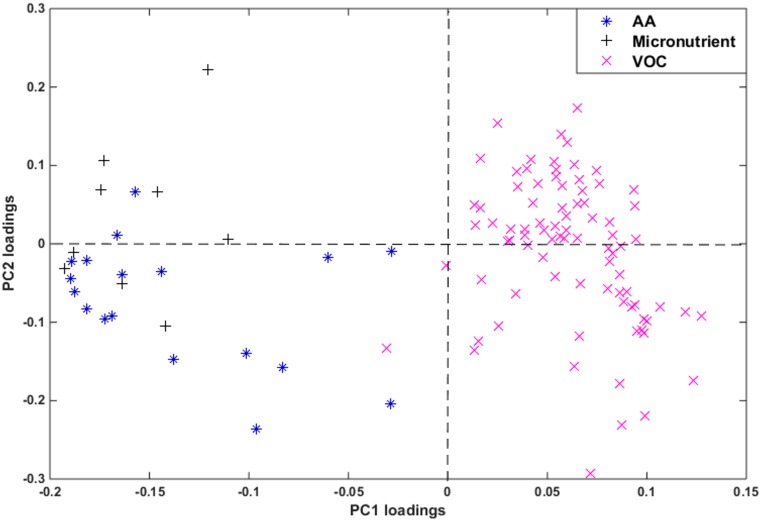
As seen in the dendrogram of Fig 5, clear distinction was obtained between the three groups using HCA. Fig 6 shows the RPCA loadings plot. Two major groups of metabolites emerged from this analysis, corresponding to compounds that either accumulate or decline in response to these physiological changes. The AA (* symbols) and micronutrients (+ symbols) were found to influence most HNP ewes while the VOC (× symbols) influence most the other two groups (HP and HL). This can be confirmed after comparing Figs 4 and 6.
Fig 6. RPCA loadings plot of 111 metabolite signals analyzed by HS-GC/MS, HPLC-MS/MS, and ICP-OES: Amino acids (AA, *), minerals (+), and volatile organic compounds (VOC, ×).
It should be put into consideration that even though RPCA and HCA methods were successfully applied in the current work, these results are only indications of grouping patterns considering the limited number of samples analyzed.
Based on our results, we believe that that metabolomics using earwax as a biological matrix reflects the metabolic changes associated with periparturition. The present approach involved monitoring a wide range of metabolites (fatty acids, alcohols, ketones, amino acids and minerals) using mass spectrometry-based metabolomics (MS-based metabolomics) and ICP-OES. This could be advantageous as the biological complexity of the agricultural animals unavoidably requires a system biology approach that can study the complex interactions in the biological systems using a method of integration and not reduction [53].
Generally, MS-based metabolomics has this power being able to simultaneously analyse several hundreds of metabolites in a biological sample, which are the functional end products of the biological processes [54]. Thus, it allows to differentiate individual phenotypes better than with conventional clinical end points or with small set of metabolites and consequently exploring the physiological status in a more global way [55]. It also presents high sensitivity, high throughput, better identification power for unknown molecules in samples (i.e. by using mass spectral libraries), greater simplicity in handling complex samples by eliminating or minimizing the sample work up, preseparation and chemical manipulation being coupled to separation techniques such as LC and GC [56]. In the same time, it does not suffer the problems related to cross reactivity where high compound specificity is achieved by combined separation and spectral identification which makes it highly sensitive even for minor changes [57]. Moreover, there is no need to develop different methods for different metabolites as in many commercial assays [57]. That is why the applications of MS-based metabolomic studies both targeted and untargeted in the field of animal production and nutrition research are rapidly growing [58].
The earwax is also a promising alternative biological matrix being not only non-invasive but also overcoming several disadvantages faced in other biological fluids such as easy collection, no diurnal variations (results not shown), less liability to external contamination, minimum or no sample pre-concentration or pretreatment, no need for vet personnel for sample collection, and ability for relatively long term monitoring (e.g. few weeks) required for sample build-up, where the biomarkers reach the earwax through the blood circulation as it is being secreted and accumulated in the ear [27]. On the other hand, the study of some important parameters that may influence the application of earwax as a diagnostic biological matrix in animals such as effect of grazing, diet, quantity and quality of earwax in the same breed, etc.) should be addressed in future studies by the authors.
In conclusion, we believe that combining advantages of metabolomics with non-invasive sampling using earwax could hold a great promise in pregnancy and lactation research keeping in mind the technical and economic challenges.
Based on our results, we believe that that metabolomics using earwax as a biological matrix reflects the metabolic changes associated with periparturition. The present approach involved monitoring a wide range of metabolites (fatty acids, alcohols, ketones, amino acids and minerals) using mass spectrometry-based metabolomics and ICP-OES. This could be advantageous as the biological complexity of the agricultural animals unavoidably requires a system biology approach that can study the complex interactions in the biological systems using a method of integration and not reduction [53].
Generally, MS-based metabolomics has this power being able to simultaneously analyse several hundreds of metabolites in a biological sample, which are the functional end products of the biological processes [54]. Thus, it allows to differentiate individual phenotypes better than with conventional clinical end points or with small set of metabolites and consequently exploring the physiological status in a more global way [55]. It also presents high sensitivity, high throughput, better identification power for unknown molecules in samples (i.e. by using libraries), greater simplicity in handling complex samples by eliminating or minimizing the sample work up, preseparation and chemical manipulation being coupled to separation techniques such as LC and GC [56]. In the same time, it does not suffer the problems related to cross reactivity where high compound specificity is achieved by combined separation and spectral identification which makes it highly sensitive even for minor changes [57]. Moreover, there is no need to develop different methods for different metabolites as in many commercial assays [57]. That is why the applications of MS-based metabolomic studies both targeted and untargeted in the field of animal production and nutrition research are rapidly growing [58].
The earwax is also a promising alternative biological matrix being not only non-invasive but also overcoming several disadvantages faced in other biological fluids such as easy collection, no diurnal variations (results not shown), less liability to external contamination, no need for vet personnel for sample collection, minimum or no sample pre-concentration or treatment, and ability for relatively longterm monitoring (e.g. few weeks) required for sample build-up.
On the other hand, the study of some important parameters that may influence the application of earwax as a diagnostic biological matrix in animals such as effect of grazing, diet, quantity and quality of earwax in the same breed, etc.) should be addressed in future studies by the authors.
In conclusion, we believe that combining advantages of metabolomics with non-invasive sampling using earwax could hold a great promise in pregnancy and lactation research keeping in mind the technical and economic challenges.
Conclusion
Identification of changes of metabolism could aid in the determination of abnormal metabolic states and prediction of some metabolic disorders such as pregnancy toxemia and fatty liver which could be advantageous to producers. In this work, metabolic profiles obtained using earwax analysis as a non-invasive potential alternative biological matrix, were successfully used to predict pre- and post-parturient metabolic changes which, in the future could possibly be applied for diagnosis of metabolic diseases and assessment of nutritional status of sheep.
Acknowledgments
We wish to acknowledge Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the research fund (grant number 1516965) provided within the postdoc program (PNPD) for the first author, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research productivity grant (grant number 312280) to Nelson Antoniosi and FUNAPE for management of financial resources.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Quantitative data of metabolites for implementing the multivariate models applied in this work are currently fully available in Figshare at https://doi.org/10.6084/m9.figshare.5302141.v1.
Funding Statement
We wish to acknowledge Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the research fund (grant number 1516965) provided within the postdoc program (PNPD) for the first author, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research productivity grant (grant number 312280) to Nelson Antoniosi and FUNAPE for management of financial resources. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Özpinar A, Firat A. Metabolic profile of pre-pregnancy, pregnancy and early lactation in multiple lambing sakiz ewes. 2. Changes in plasma progesterone, estradiol-17B and cholesterol levels. Ann Nutr Metab. 2003; 47(3–4): 139–43. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Kumar R, Sharma KB. Studies on the metabolic profile of ewes during gestation and possible relation with neonatal mortality. Indian J Small Rumin. 2007. July 23; 13(2): 192–5. [Google Scholar]
- 3.Ceylan E, Tanritanir P, Dede D. Changes in some macro-minerals and biochemical parameters in female healthy Siirt Hair goats before and after parturition. J Anim Vet Adv. 2009; 8(3): 530–3. [Google Scholar]
- 4.Greenfield RB, Gurgoze SY, Zonturlu AK, Ozuertlu N, Icen H. Investigation of some biochemical parameters and mineral substance during pregnancy and postpartum period in Awassi ewes. Kafkas Üniversitesi Veteriner Fakültesi dergisi 2009. January; 15(6): 957–63. [Google Scholar]
- 5.Piccione G, Caola G, Giannetto C, Pennisi P. Selected biochemical serum parameters in ewes during pregnancy, post- parturition, lactation and dry period. Anim Sci Pap Rep. 2009. January; 27(4): 321–30. [Google Scholar]
- 6.Waziri MA, Ribadu AY, Sivachelvan N. Changes in the serum proteins, hematological and some serum biochemical profile in the gestation period in the Sahel goats. Veterinarski arhiv. 2010; 80(2): 215–24. [Google Scholar]
- 7.Antunovic Z, Novoselec J, Sauerwein H, Speranda M, Vegara M, Pavić V. Blood metabolic profile and some of hormones concentration in ewes during different physiological status. Bulgarian J Agric Sci. 2011a. October; 17(5): 687–91. [Google Scholar]
- 8.Antunovic Z, Novoselec J, Speranda M, Vegara M, Pavić V, Mioč B, & Djidara M, et al. Changes in biochemical and hematological parameters and metabolic hormones in Tsigai ewes blood in the first third of lactation. Archiv Tierzucht 2011b. October; 54(5): 535–45. [Google Scholar]
- 9.Khatun A, Wani GM, Bhat JIA, Choudhury AR., Khan MZ. Biochemical indices in sheep during different stages of pregnancy. Asian J Anim Vet Adv. 2011. July 10; 6(2): 175–81. [Google Scholar]
- 10.Maeda Y, Ohtsuka H, Oikawa M. Effect of the periparturient period on blood free amino acid concentration in dairy cows/healthy cows. J. Vet Med Anim Health. 2012. December; 4(9): 124–9. [Google Scholar]
- 11.Araz OB. Changes in Some Haemato-Biochemical and Electrolytes Parameters in Female Meriz Goats during Pregnancy and After Parturition. Animal Sci. 2013; 2(1): 11–4. [Google Scholar]
- 12.Soliman EP. Effect of physiological status on some hematological and biochemical parameters of Ossimi sheep. EASG journal 2014; 9(2): 33–42. [Google Scholar]
- 13.Castagnino D, Härter CJ, Rivera AR, Dorneles de Lima L, Silva HG, Biagioli B, et al. Changes in maternal body composition and metabolism of dairy goats during pregnancy. R Bras Zootec. 2015; 44(3): 92–105. [Google Scholar]
- 14.Schlumbohm C, Harmeyer J. Hyperketonemia impairs glucose metabolism in pregnant and non pregnant ewes. J Dairy Sci. 2004. February; 87(2): 350–8. doi: 10.3168/jds.S0022-0302(04)73174-4 [DOI] [PubMed] [Google Scholar]
- 15.Harmeyer J, Schlumbohm C. Pregnancy impairs ketone body disposal in late gestating ewes: Implications for onset of pregnancy toxaemia. Res Vet Sci. 2006. October; 81(2): 254–64. doi: 10.1016/j.rvsc.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Durak MH, Altinek E. Effect of energy deficiency during late pregnancy in Chios ewes on free fatty acids, β-hydroxy butyrate and urea metabolites. Turk J Vet Anim Sci. 2006; 30: 497–502. [Google Scholar]
- 17.Alvord LS, Farmer BL. Anatomy and orientation of the human external ear. J Am Acad Audiol. 1997. December; 8(6): 383–90. [PubMed] [Google Scholar]
- 18.Bortz JT, Wertz PW, Downing DT. Composition of cerumen lipids. J Am Acad Dermatol. 1990. November; 23(5): 845–849. [DOI] [PubMed] [Google Scholar]
- 19.Yassin A, Mostafa MA, Moawad MK. Cerumen and its microchemical analysis. J Laryngol Otol. 1966. September; 80(9): 933–8. [DOI] [PubMed] [Google Scholar]
- 20.Brand-Auraban A, Kopita L, Shwachman H. Chemical analysis of some inorganic elements in cerumen from patients with cystic fibrosis. J Invest Dermatol. 1972. January; 58(1): 14–5. [DOI] [PubMed] [Google Scholar]
- 21.Burkhart CN, Burkhart CG, Williams S, Andrews PC, Adappa V, Arbogast J. In Pursuit of Ceruminolytic Agents: A study of earwax composition. Am J Otolaryngol. 2000. March; 21(2): 157–60. [DOI] [PubMed] [Google Scholar]
- 22.Monell.org [Internet]. Philadelphia: Monell Chemical Senses Center; 2014. [cited 2015 Apr 15]. http://www.monell.org/news/newsreleases/ earwax _odors.
- 23.Kataoka H, Saito K, Kato H, Masuda K. Non-invasive analysis of volatile markers in human emanations for healthy and early disease diagnosis. Bioanalysis 2013. June; 5(11): 1443–59. doi: 10.4155/bio.13.85 [DOI] [PubMed] [Google Scholar]
- 24.Trumble SJ, Robinson EM, Berman-Kowalewski M, Potter CW, Usenko S. Proc Natl Acad Sci USA 2013. October 15; 110(42): 16922–6. doi: 10.1073/pnas.1311418110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shokry E, de Oliveira AE, Avelino MA, de Deus MM, Filho NR. Earwax: a neglected body secretion or a step ahead in clinical diagnosis. J Proteomics 2017. April 21; 159: 92–101. doi: 10.1016/j.jprot.2017.03.005 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Shokry E, Marques JG, Ragazzo P, Pereira NZ, Filho NRA. Earwax as an alternative specimen for forensic analysis. Forensic Toxicol 2017; 35(2): 348–58. doi: 10.1007/s11419-017-0363-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokry E, de Oliveira AE, Avelino MA, de Deus MM, Pereira NZ, Filho NR. Earwax: an innovative tool for assessment of tobacco use or exposure. A pilot study in young adults. Forensic Toxicol 2017; 35(2): 389–98. doi: 10.1007/s11419-017-0370-0 [Google Scholar]
- 28.Shokry E, dos Santos FC, DA da Cunha PHJ, Fioravanti MCS, Noronha ADF, Pereira NZ, Filho NR. Earwax: A clue to discover fluoroacetate intoxication in cattle. Toxicon 2017; 137: 54–57. https://doi.org/10.1016/j.toxicon.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Guidelines on bioanalytical method validation. Committee for Medicinal Products for Human Use (CHMP) [Internet]. European Medicines Agency [Cited 2016 Mar 15]. http://www.ema.europa.eu/docs/en_GB /document_library/ Scientific_guideline/2011/08/WC500109686.pdf.
- 30.Varmuza K, Filzmoser P. Introduction to multivariate statistical analysis in chemometrics. London: CRC Press; 2009. [Google Scholar]
- 31.Chilliard Y, Ferlay A, Faulconnier Y, Bonnet M, Rouel J, Bocquier F. Adipose tissue metabolism and its role in adaptions to undernutrition in ruminants. Proc Nutr Soc. 2000. February; 59(1): 127–34. [DOI] [PubMed] [Google Scholar]
- 32.Balaraman S, Raine Lunde E, Sawant O, Cudd TA, Washburn SE, Miranda RC. Alcohol Clin Exp Res 2014; 38(5): 1390–400. doi: 10.1111/acer.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enjalbert F, Nicot MC, Bayourthe C, Moncoulon R. Ketone bodies in milk and blood of dairy cows: Relationship between concentrations and utilization for detection of subclinical ketosis. J Dairy Sci 2001; 84(3): 583–9. doi: 10.3168/jds.S0022-0302(01)74511-0 [DOI] [PubMed] [Google Scholar]
- 34.Detection of ketosis in dairy cattle by determining infrared milk ketone bodies in milk [Internet]. France: Conseil Élevege France. Donner du sens à la mesure. [Cited 2016 January 20]. http://www.icar.org/wp-content/uploads/2015 /09/Daviere.pdf.
- 35.El-Sherif MM, Assad F. Changes in some blood constituents of Barki ewes during pregnancy and lactation under semiarid conditions. Small Rum Res 2001; 40: 269–77. [DOI] [PubMed] [Google Scholar]
- 36.Shetaewi MM, Daghash HA. Effect of pregnancy and lactation on some biochemical components in the blood of Egyptian coarse-wool ewes. J Am Vet Med Assoc 1994; 30, 64–73. [Google Scholar]
- 37.Rίos C, Marίn MP, Catafau M, Wittwer F. Relationship between blood metabolites (β-hydroxybutirate, NEFA, cholesterol and urea) and nutritional balance in three dairy goat herds under confinement. Arch Med Vet. 2006; 38(1): 19–23. [Google Scholar]
- 38.De Oliveira RPM, Maduro AHP, Lima ES, De Oliveira FP. Perfil metabólico de ovelhas Santa Inês em diferentes fases de gestação criadas em sistema semi intensivo no estado do amazonas. Ci Anim Bras 2014; 15(1): 81–6. [Google Scholar]
- 39.Rodruigez MN, Tebot I, Le Bas A, Nievas C, Leng L., Cirio A. Renal function and urea handling in pregnant and lactating Corriedale ewes. Can J Anim Sci 1996; 76: 469–72. [Google Scholar]
- 40.Regnault TRH, Friedman JE, Wilkening RB, Anthony RV, Hay WW Jr. Fetoplacental transport and utilization of amino acids in IUGR—A Review. Placenta. 2005; 26 (19 Suppl. A): S52–S62. [DOI] [PubMed] [Google Scholar]
- 41.Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C.: Institute of Medicine of the National Academies; 2006. January. Report No. 0-309-10091-7. Contract No. 4500096095. Sponsored by the National Academies of Science and Health Canada. [Google Scholar]
- 42.Faichney GJ. Amino acid utilization by the foetal lamb. Proc Aust Nutr Soc.1981; 6: 48–53. [Google Scholar]
- 43.Banchero GE, Clariget P, Bencini R, Lindsay DR, Milton JTB, Martin GB. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod Nutr Dev. 2006; 46: 447–60. doi: 10.1051/rnd:2006024 [DOI] [PubMed] [Google Scholar]
- 44.Robinson JJ, Sinclair KD, McEvoy TG. Nutritional effects on fetal growth. Anim Sci. 1999; 68: 315–31. [Google Scholar]
- 45.Azab ME, Abdel-Maksoud HA. Changes in some hematological and biochemical parameters during prepartum and postpartum periods in female Baladi goats. Small Rum Res. 1999; 34: 77–85. [Google Scholar]
- 46.Bell C. Control of uterine blood flow in pregnancy. Obstet Gynecol Surv.1975; 30: 359–60. [Google Scholar]
- 47.O´Doherty JV, Crosby TF. The effect of diet in late pregnancy on colostrum production and immunoglobulin absorption in sheep. Anim Sci 1997; 64(1): 87–96. [Google Scholar]
- 48.Eşki F, Taşal İ, Karsli MA, Şendağ S, Uslu BA, Wagner H, et al. Concentrations of NEFA, β-HBA, triglycerides, and certain blood metabolites in healthy colored Angora goats during the peripartum period. Turk J Vet Anim Sci 2015; 39: 401–5. [Google Scholar]
- 49.Dakka AA, Abdel All TS. Studies on mineral picture in the blood sera of Egyptian sheep. Assiut Vet Med J. 1992; 28(55): 242–9. [Google Scholar]
- 50.Kamel FM. Studies on some minerals and trace elements deficiency diseases of goats in Giza Governarates. [dissertation] Cairo: Cairo University; 1988.
- 51.Sansom BF, Bunch KJ, Dew SM. Changes in plasma calcium, magnesium, phosphorus and hydroxylproline concentrations in ewes from 12 weeks before till 3 weeks after lambing. Br Vet J. 1982; 138: 393–401. [DOI] [PubMed] [Google Scholar]
- 52.Firat A, Ozpinar A. The study of changes in some blood parameters (glucose, urea, bilirubin AST) during and after pregnancy in association with nutritional conditions and litter size in ewes. Turk J Vet Anim Sci. 1996; 20: 387–93. [Google Scholar]
- 53.Loor JJ. Transcriptomics of muscle and fat development in growing cattle In: Crovetto GM, editor. Energy and protein metabolism and nutrition. Milan: Wageningen Academic Publishers; 2010. p. 59–68. [Google Scholar]
- 54.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, et al. Mass spectrometry-based metabolomics for future progress with particular focus on nutrition research. Metabolomics. 2009; 5: 435–458. doi: 10.1007/s11306-009-0168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assfalg M, Bertini I, Colangiuli D, Luchinat C, Schafer H, Schutz B, et al. Evidence of different metabolic phenotypes in humans. Proc Natl Acad Sci USA. 2008; 105: 1420–4. doi: 10.1073/pnas.0705685105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mesquita ES, D´Alexandri FL, Scolari SC, Membrive CMB, Papa PC, Cardoso D, et al. P´robing the uterine microenvironment through systems biology approaches. Anim Reprod. 2012. Oct-Dec; 9(4): 713–22. [Google Scholar]
- 57.Murtagh R, Behringer V, Deschner T. LC-MS as a method for non-invasive measurement of steroid hormones and their metabolites in urine and faeces of animals. Wien Tierarztl Monatsschr 2013; 100: 247–254. [Google Scholar]
- 58.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007; 26: 51–78. doi: 10.1002/mas.20108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Quantitative data of metabolites for implementing the multivariate models applied in this work are currently fully available in Figshare at https://doi.org/10.6084/m9.figshare.5302141.v1.