Abstract
Arsenic contamination of drinking water is a serious threat to the health of hundreds of millions of people worldwide. In the United States ~3 million individuals drink well water that contains arsenic levels above the Environmental Protection Agency (EPA) maximum contaminant level (MCL) of 10 μg/L. Several technologies are available to remove arsenic from well water including anion exchange, adsorptive media and reverse osmosis. In addition, bottled water is an alternative to drinking well water contaminated with arsenic. However, there are several drawbacks associated with these approaches including relatively high cost and, in the case of bottled water, the generation of plastic waste. In this study, we tested the ability of five tabletop water pitcher filters to remove arsenic from drinking water. We report that only one tabletop water pitcher filter tested, ZeroWater®, reduced the arsenic concentration, both As3+ and As5+, from 1,000 μg/L to < 3 μg/L, well below the MCL. Moreover, the amount of total dissolved solids or competing ions did not affect the ability of the ZeroWater® filter to remove arsenic below the MCL. Thus, the ZeroWater® pitcher filter is a cost effective and short-term solution to remove arsenic from drinking water and its use reduces plastic waste associated with bottled water.
Keywords: Arsenic, water filter, filtration, water quality, drinking water
1. Introduction
Arsenic contamination of drinking water is a serious threat to the health of hundreds of millions of people worldwide (Carlin et al. 2016, Flanagan et al. 2015a, Smith et al. 2016, Zheng and Ayotte 2015). In the United States (U.S.), for example, forty-three million people use private wells and the United States Geological Survey estimates that ~3 million people in the U.S. drink private well water that contains arsenic levels above the World Health Organization (WHO) standard and U.S. EPA MCL of 10 μg/L, which was established in 2001 (Zheng and Ayotte 2015). However, arsenic levels in private wells are unregulated. It is up to the homeowner to test to determine if there is arsenic in the water and to take appropriate action to reduce the arsenic concentration (Carlin et al. 2016, Spayd et al. 2015, Zheng and Ayotte 2015).
A major emphasis of the Dartmouth Superfund Research Program (http://www.dartmouth.edu/~toxmetal/ and http://www.dartmouth.edu/~arsenicandyou/), as well as Superfund Research Programs at Columbia University (http://superfund.ciesin.columbia.edu/), University of Arizona at Tucson (https://superfund.arizona.edu/https://superfund.arizona.edu/), University of California at Berkley (http://superfund.berkeley.edu/), University of California at San Diego (http://superfund.ucsd.edu/), University of Washington (http://deohs.washington.edu/srp/) and the University of North Carolina at Chapel Hill (http://sph.unc.edu/superfund-pages/srp/), as well as private, government and state agencies (for example, New Hampshire Department of environmental Services (http://des.nh.gov/organization/divisions/water/dwgb/capacity/arsenic.htm), is to encourage individuals who drink water from private wells to test their well water for arsenic every three years. If well water arsenic is above 10 μg/L it is recommended that the consumer change to bottled water immediately, contact the local or state health department, and install either a whole house water treatment system (i.e., point of entry, POE) or a point of use (POU) filtration system, which treats the water at a single tap, to reduce the arsenic concentration to as close to zero as possible ([http://www.dartmouth.edu/~arsenicandyou/index.html](Flanagan et al. 2015a, Spayd et al. 2015, Stanton et al. 2015). The choice of a treatment system for arsenic depends on several features of water chemistry including, but not limited to, other contaminants, pH, manganese and iron concentrations, and the arsenic oxidation state and concentration (Sarkar and Paul 2016). One relatively inexpensive approach to eliminate exposure to arsenic in well water is to use bottled water, which has been estimated to cost several hundred dollars per year (Flanagan et al. 2015a, Smith et al. 2016). Other approaches to remove arsenic from well water include POU treatment systems that can cost from $300 USD up to several thousand dollars to install plus yearly maintenance costs (~$100/yr.), and POE treatment systems that cost $2,000 to $3,000 USD to install plus yearly maintenance costs (~$200 to $300 USD/yr.)(Flanagan et al. 2015b, Smith et al. 2016). In addition to cost, which is known to be a barrier to remediation, there are other limitations to the use of bottled water and reverse osmosis systems, including the generation of plastic waste and backwash waste water disposal, respectively (Flanagan et al. 2015b, Smith et al. 2016, Spayd et al. 2015).
In this study, we tested the effectiveness of five inexpensive (~$20 to $35 USD for the filtration unit and ~$10 to $15 USD for replacement filters) and readily available tabletop water pitcher filters to remove arsenic from drinking water. The impetus for this study was to identify a robust, low cost and easy to use system to reduce arsenic in drinking water obtained from private wells. In a recent review of arsenic and environmental health it was noted that a key research need is to improve remediation strategies (Carlin et al. 2016). We report that only one tabletop water pitcher filter tested, ZeroWater®, reduced arsenic, both As3+ and As5+ in spiked municipal water, from 100 μg/L to below 1 μg/L. Moreover, the ZeroWater® water pitcher filter also reduced the arsenic concentration from 1,000 μg/L to 2.6 μg/L, a value below the U.S. EPA MCL of 10 μg/L. In addition, the ZeroWater® filter also reduced arsenic in well water samples obtained in New Hampshire from 42 μg/L to below detection. The amount of total dissolved solids did not affect the ability of the ZeroWater® water pitcher to remove arsenic below the MCL.
2. Materials and methods
2.1 Tabletop water pitcher filters
Five commercially available tabletop water pitcher filtration units, including two of the most popular brands in the U.S., Pur® (model# PPT700W) and Brita® (model# OB36/OB03) were purchased from local merchants. In addition, tabletop water pitcher filtration units by ZeroWater® (model# ZD-013-D), Great Value® (Wal-Mart-model# QP6-OS) and HDX® (Home Depot-model# QP8-07) were also tested. For each brand three different lots of filters were tested.
2.2 Arsenic solutions
To make influent solutions containing arsenic, As+5 and As+3 stock (1,000 mg/L) were purchased from Inorganic Ventures, Christiansburg, VA. Appropriate amounts of each stock solution were added to tap water (Hanover, NH public water supply, soft water) to make solutions with a final total arsenic concentration of 10 μg/L (5 μg/L As+3 and 5 μg/L As+5), 100 μg/L (50 μg/L As+3 and 50 μg/L As+5), and 1,000 μg/L (500 μg/L As+3 and 500 μg/L As+5). As+3 and As+5 were added to the influent water since both arsenic species can be present in well water: the relative concentration of each depends primarily on the pH and O2 content (Sorg et al. 2014). Arsenic concentrations of 10 μg/L and as high as 100 μg/L are not uncommon in well water in the U.S. (Spayd et al. 2015, Zheng and Ayotte 2015). Arsenic concentrations of 1,000 μg/L in well water are less common, but are observed occasionally in the U.S. as well as world-wide. A second set of influent solutions was made in moderately hard water (see below) with a final total arsenic concentration of 10 μg/L (5 μg/L As+3 and 5 μg/L As+5) and 100 μg/L (50 μg/L As+3 and 50 μg/L As+5). The salt composition of the soft water solution was (Na+, 11.1 ppm; Mg++, 1.28 ppm; K+, 1.7 ppm; Ca++, 8.46 ppm; and hardness as CaCO3 was 26.41 mg/L)(CE 2000). The salt composition of the moderately hard water solution was (Na+, 16.3 ppm; Mg++, 4.9 ppm; K+, 1.7 ppm; Ca++, 16.6 ppm; and hardness as CaCO3 was 61.4 mg/L)(CE 2000). A third set of arsenic solutions was made in distilled water and contained either 100 μg/L of As+3 or 100 μg/L of As+5. Also, water samples were obtained from two wells in New Hampshire known to contain arsenic (~42 μg/L): one well was in Concord, NH and the other well was in Kensington, NH. These well water samples, although representative of samples obtained in New Hampshire (https://nh.water.usgs.gov/project/nawqa/data_gw.htm), differ significantly from other aquifers in the US and other countries that are characterized by higher levels of silica and sulfate. The concentration of As+3 and As+5, as well as Si, P, S, and Fe, in all influent solutions was measured by ICP-MS.
2.3 ICP-MS
Arsenic concentration in the influent (i.e., raw unfiltered water) and the filter effluent (i.e., filtered water) was measured by ICP-MS (Agilent 7900 and 8800) following U.S. EPA 200.8 but using He as a collision gas. The instrument was calibrated using National Institute of Standards and Technology (NIST) traceable standards and an initial and continuing calibration verification was performed every 10 samples. Detection limit for arsenic was 0.05 μg/L.
2.4 Filtration tests
The first set of experiments was performed on the five filters described above. Briefly, ten liters of influent soft water containing arsenic was added to each filter, in 1 L increments in the following order: control (no arsenic added), 10 μg/L, 100 μg/L and 1,000 μg/L. This was repeated with three lots of each filter brand, except for HDX®, which did not have lot numbers, instead three different filters were purchased from three different Home Depot locations. The second set of experiments was limited to the ZeroWater® filter because it was the only filter to reduce the arsenic concentration in all influent samples tested to a value below 10 μg/L. Since the ZeroWater® performance data sheet suggests that the filter be replaced after 15 gallons (~57 L) studies were also conducted to test the ability of the filter (three different lots) to reduce the arsenic concentration in 100 L of water, in 1 L increments, containing either 10 μg/L or 100 μg/L arsenic in soft and hard water. The third set of experiments was conducted to test the ability of the ZeroWater® filter to remove either 100 μg/L of As+3 or 100 μg/L of As+5 from distilled water. The fourth set of experiments was conducted to test the ability of the ZeroWater® filter to remove naturally occurring arsenic from water obtained from two wells in NH.
2.5 Data analysis and statistics
Graphpad Prism version 6.0 for Macintosh (Graphpad, San Diego, CA) was used to perform a statistical analysis of the data. Means were compared using a t-test or ANOVA followed by Tukey’s test, as appropriate. P<0.05 was considered significant, and all data are expressed as the mean ± SEM.
3. Results
3.1 Comparison of five tabletop pitcher filtration units
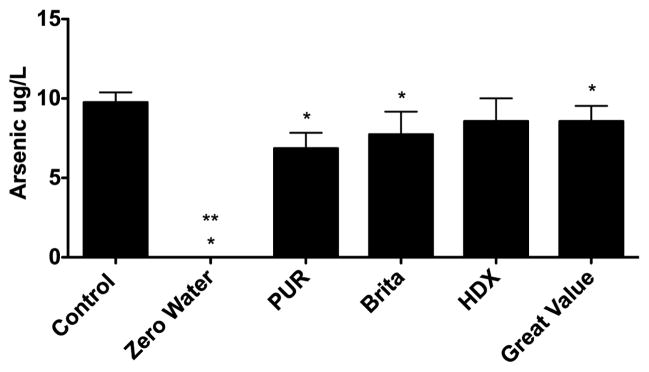
Figure 1 presents the results of studies conducted to examine the ability of five tabletop water pitcher filtration units to reduce the arsenic concentration in the influent containing 10 μg/L. ZeroWater®, Pur®, Brita® and Great Value® reduced the arsenic concentration below 10 μg/L: however, HDX® did not reduce the arsenic concentration below 10 μg/L. Only the ZeroWater® filter reduced the arsenic concentration to less than 1 μg/L.
Figure 1.
The ability of five tabletop water pitcher filters, listed by the brand name, to remove arsenic (10 μg/L) from soft water. Control represents ICP-MS measurement of the arsenic solution before it was added to the filters. Three lots of each filter were tested, 1 L/filter. *P<0.05 versus 10 μg/L. **P<0.001, indicates that the ZeroWater® filter reduced the arsenic concentration in the filtrate to less than 0.05 μg/L. Data presented as the mean ± SEM. Effluent arsenic concentrations were: ZeroWater® (below detection, 100% removed by filtration), PUR® (6.9 ± 0.6 μg/L, 31% removed by filtration), Brita® (7.7 ± 0.8 μg/L, 23% removed by filtration), HDX® (8.6 ± 0.8 μg/L, 14% removed by filtration), and Great Value® (8.6 ± 0.6 μg/L, 14% removed by filtration).
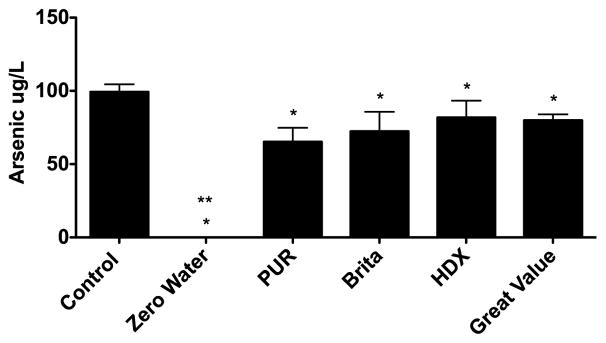
Next, studies were conducted to test the ability of the filters to reduce the arsenic concentration in the influent containing 100 μg/L. All filters tested reduced the arsenic concentration below 100 μg/L: however, only the ZeroWater® filter reduced the arsenic concentration to less than 1 μg/L (Figure 2).
Figure 2.
The ability of five tabletop water pitcher filters, listed by the brand name, to remove arsenic (100 μg/L) from soft water. Control represents ICP-MS measurements of the arsenic solution before it was added to the filters. Three lots of each filter were tested, 1 L/filter. *P<0.05 versus 100 μg/L **P<0.001, indicates that the ZeroWater® filter reduced the arsenic concentration in the filtrate to less than 0.05 μg/L. Data presented as the mean ± SEM. Effluent arsenic concentrations are: ZeroWater® (below detection, 100% removed by filtration), PUR® (65.2 ± 5.5 μg/L, 34.8% removed by filtration), Brita® (72.4 ± 7.7 μg/L, 27.6% removed by filtration), HDX® (81.8 ± 6.6 μg/L, 18.2% removed by filtration), and Great Value® (79.9 ± 2.4 μg/L, 20.1% removed by filtration).
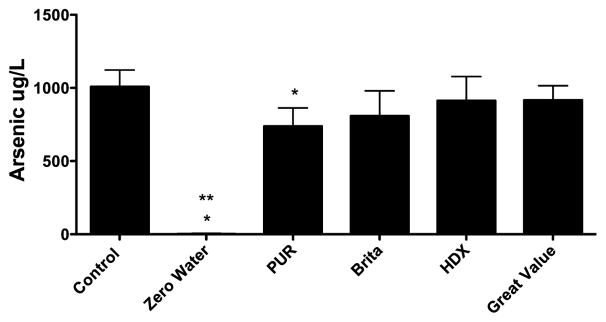
Finally, studies were conducted to test the ability of the filters to reduce the arsenic concentration in the influent containing 1,000 μg/L. Only ZeroWater® and Pur® filters reduced the arsenic concentration below 1,000 μg/L (Figure 3). Moreover, only the ZeroWater® filter reduced the arsenic concentration from 1,000 μg/L to less than 10 μg/L (Figure 3).
Figure 3.
The ability of five tabletop water pitcher filters, listed by the brand name, to remove arsenic (1,000 μg/L) from soft water. Control represents ICP-MS measurements of the arsenic solution before it was added to the filters. Three lots of each filter were tested, 1 L/filter. *P<0.05 versus 1,000 μg/L **P<0.001, indicates that the ZeroWater® filter reduced the arsenic concentration in the filtrate to less than 10 μg/L (mean 2.63 μg/L ± 2.06 μg/L). Data presented as the mean ± SEM. Effluent arsenic concentrations were: ZeroWater® (2.7 ± 2.0 μg/L, 99.7% removed by filtration), PUR® (737.7 ± 72.4 μg/L, 26.2% removed by filtration), Brita® (808.2 ± 99.2 μg/L, 19.2% removed by filtration), HDX® (913.5 ± 95.0 μg/L, 8.7% removed by filtration), and Great Value® (916.8 ± 56.8 μg/L, 8.3% removed by filtration).
3.2 ZeroWater® Filter: Robustness and effect of water hardness
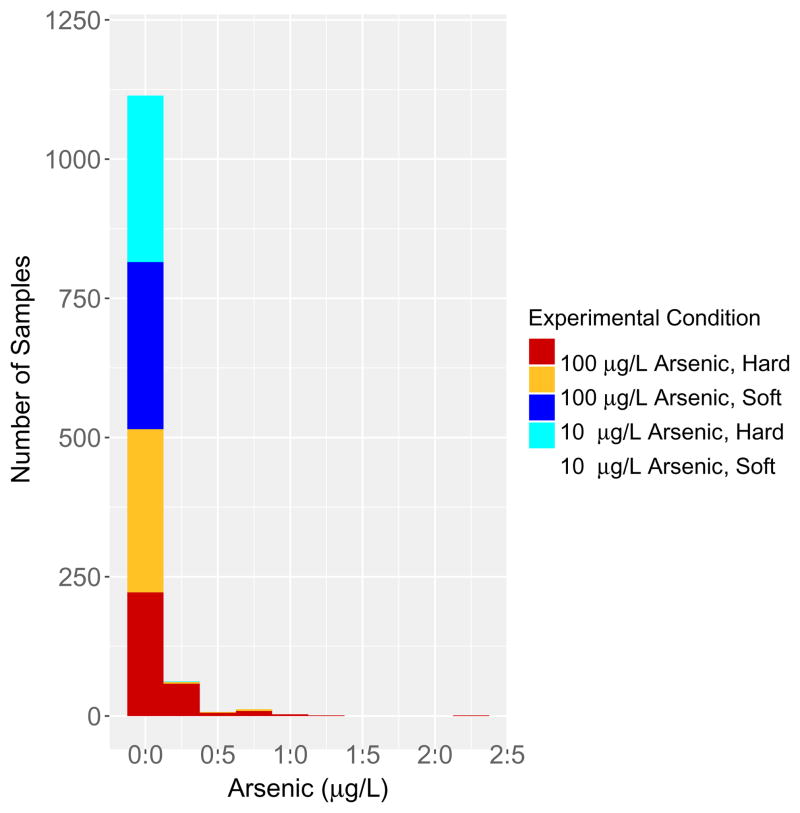
The goals of the next series of studies were to: (1) examine the ability of the ZeroWater® filter to reduce the arsenic concentration in the effluent of 100 L of water in 1 L increments and (2) to examine the effect of water hardness on the ability of the ZeroWater® filter to reduce the arsenic concentration in 100 L of water in 1 L increments. First, studies were conducted with 10 μg/L arsenic in the soft and moderately hard water solutions. The ZeroWater® filter reduced the arsenic concentration in the 10 μg/L arsenic influent to values between 0 and <0.125 μg/L in 300 of 300 arsenic containing solutions made in the soft water (Figure 4) and in 299 of 300 arsenic containing solutions made in the hard water (Figure 4). Thus, the ZeroWater® filter reduced the arsenic concentration in 599 of the 600 liters of water tested from 10 μg/L to levels below 0.125 μg/L.
Figure 4.
The ability of the ZeroWater® pitcher filter to remove arsenic from 100 liters of water (measured in 1 L aliquots) containing 10 μg/L or 100 μg/L arsenic dissolved in either soft water or moderately hard water. Three lots of filters were tested. ICP-MS was used to measure arsenic in a total of 1,200 samples. Data are plotted in a stacked bar chart. The Y-axis represents the number of samples plotted as a function of the arsenic concentration measured in the filtered water (X axis). Data in each bin is centered on the value (e.g., the first stacked bar is centered on 0, indicating that the arsenic concentration in those samples was between the limit of detection of 0.05 μg/L and <0.125 μg/L. The second bar from the left is centered on 0.25 μg/L, indicating that the arsenic concentration in those samples is >0.125 μg/L and <0.375 μg/L. An increase in the hardness of the water reduced the ability of the ZeroWater® filter to remove arsenic from the 100 μg/L solution according to a Fisher’s exact test (p < 2.2e-16).
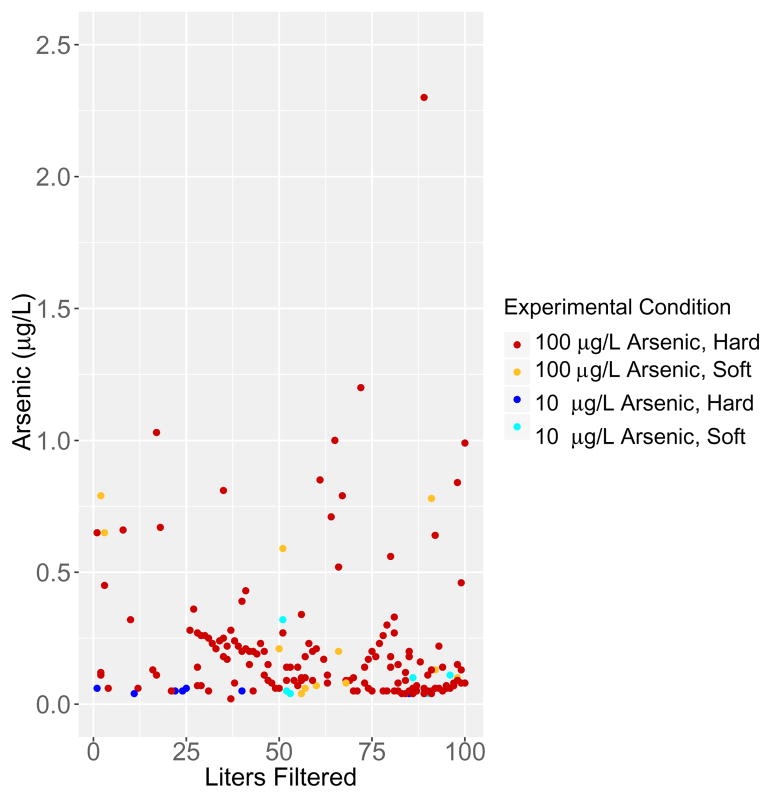
Next, studies were conducted to examine the ability of the ZeroWater® filter to remove arsenic from the 100 μg/L soft and hard water solutions. The ZeroWater® filter reduced the arsenic concentration in the 100 μg/L solution made in soft water to values between 0 and <0.125 μg/L in 299 of 300 samples (Figure 4). The ZeroWater® filter reduced the arsenic concentration in the 100 μg/L solution made in the hard water to values between 0 and <0.125 μg/L in 222 of the 300 samples (Figure 4). The concentration of arsenic measured in the 78 samples in which the arsenic concentration was >0.125 μg/L are shown in Figure 4. Seventy-five of the 78 samples in which the arsenic concentration was >0.125 μg/L had arsenic concentrations less than 1.0 μg/L (Figure 4). Figure 5 plots the individual data points representing 1,200 effluent samples as a function of the number of liters filtered. Only three of the 1,200 effluent samples had a measureable arsenic level between 2.125 μg/L and 2.375 μg/L, well below the EPA MCL of 10 μg/L.
Figure 5.
The arsenic concentration in the 1,200 filtered samples is presented as individual data points as a function of the liters filtered (measured in 1 L aliquots). 1,197 of the 1,200 samples tested were <1 μg/L.
3.3 ZeroWater® Filter: Arsenic removal from distilled water
Because chlorine and some of the salts in the water obtained from the public water supply used to make the arsenic solutions described above may influence the efficacy of the ZeroWater® filter, the next set of experiments was conducted to examine the ability of the ZeroWater® filter to remove either 100 μg/L of As+3 or 100 μg/L of As+5 from distilled water. The ZeroWater® filter reduced both As+3 and As+5 from 100 μg/L to levels below the detection limit of 0.05 μg/L (n=3 for the As+3 and the As+5 solutions).
3.4 ZeroWater® Filter: Arsenic removal from well water
Many of the ions in well water, for example iron, manganese, phosphate, silica and sulfate may influence the efficacy of the ZeroWater® filter. Therefore, the last set of experiments was conducted to examine the ability of the ZeroWater® filter to remove arsenic from water collected from private wells in New Hampshire. In well water sample #1 the arsenic was 42 μg/L (100% As+5), and in well water sample #2 the arsenic was 42 μg/L (97% As+5, 3% As+3) (Table 1). The ZeroWater® filter reduced the arsenic to levels below the detection limit of 0.05 μg/L in all 50 liters of water from each well that was passed through the filter (Table 1).
Table 1.
“The concentrations of the major ions in these two well water samples are representative of the main aquifers supplying potable groundwater to the New Hampshire population (https://nh.water.usgs.gov/project/nawqa/data_gw.htm)”
| As | Si | S | P | Fe | Mn | |
|---|---|---|---|---|---|---|
| Well #1 | 42 μg/L | 11,262 μg/L | 7,436 μg/L | 16.4 μg/L | 551.5 μg/L | 8.9 μg/L |
| Well #2 | 42 μg/L | 7,700 μg/L | 23,318 μg/L | 3.2 μg/L | 4.89 μg/L | 159.0 μg/L |
4. Discussion
The goal of this study was to test the ability of a set of readily available tabletop water pitcher filters to reduce the arsenic concentration in drinking water. Our goal was to identify tabletop pitcher filters that could be recommended by our Superfund Research Program and our stakeholders to reduce the arsenic concentration to levels below the WHO standard and U.S. EPA MCL of 10 μg/L. We report that only one tabletop water pitcher filter tested, ZeroWater®, reduced the arsenic concentration from 100 μg/L and 10 μg/L to below 1 μg/L. Moreover, the ZeroWater® water pitcher filter also reduced the arsenic concentration from 1,000 μg/L to 2.6 μg/L, a value well below the EPA MCL of 10 μg/L. In addition, the amount and composition of the total dissolved solids did not have a meaningful impact on the ability of the ZeroWater® filter to remove either As3+ or As5+. Thus, the ZeroWater® pitcher filter is an effective way to remove arsenic from private well drinking water and reduces plastic waste associated with bottled water. Moreover, ZeroWater® filters can be recycled. Hence, the ZeroWater® pitcher filter can be a short-term solution to reduce exposure to arsenic in drinking water until a POU or a POE system is installed or a relatively low cost option for individuals and families that may not have the resources to purchase more expensive POU or POE options.
Our results are in general agreement with data on the ZeroWater® web site (http://www.zerowater.com/, as of 2/22/2017) where they report that the ZeroWater® filter removes 99% of As+3 and As+5, whereas the Brita filter removes 11% and 2%, respectively. We confirm the ability of the ZeroWater® filter to reduce the arsenic concentration by 99%, and observed that the Brita filter reduced the arsenic concentration by 22.6% and 28.6% when the influent arsenic concentration is 10 μg/L and 100 μg/L, respectively. Although many water pitcher filters are certified under the Water Quality Association (WQA) Gold Seal Product Certification Program and have been tested and certified by the WQA using NSF/ANSI (American National Standards Institute) standards to reduce many metals, including lead, volatile organic compounds, and several pesticides, a search of web sites and peer reviewed publications (PubMed search, February 22, 2017) revealed that none are certified by the WQA or NSF to remove arsenic. Thus, we recommend independent NSF/ANSI testing and certification for arsenic by all manufacturers of tabletop water pitcher filters under a variety of conditions to identify those that remove arsenic.
Several limitations of the present study should be noted. Only five tabletop water pitcher filters were tested, and in limited conditions (i.e., soft and moderately hard water, distilled water, equal concentrations of As+3 and As+5, As+3 alone, As+5 alone, and water from two private wells in New Hampshire). The effectiveness of filtration systems in removing arsenic depends on a variety of factors including the pH, oxygen content, chlorine content, species of arsenic, and the concentration of Si, P, S Mn and Fe. Thus, it is possible that testing of these five filters under different conditions may produce different results and that studies on additional filters may identify other tabletop water pitcher filters that remove arsenic from drinking water. In addition, the well water results reflect the ability of the ZeroWater® filter to remove arsenic from water sampled from aquifers in New Hampshire, and not necessarily from other aquifers in the US or other countries that are characterized by higher levels of silica and sulfate. Finally, the adequacy of a pitcher filtering method to meet all cooking and drinking need of a family, especially a large family, and how a pitcher method compares to POU or POE entry systems to reduce arsenic needs to be evaluated.
Tabletop water pitcher filters have several advantages compared to bottled water as a short-term solution to avoid consuming water contaminated by arsenic. First, the use of tabletop filters does not generate as much plastic waste as bottled water, and some companies, including ZeroWater®, recycle the filter cartridges, thereby reducing waste from spent filters. Second, there is concern that water in plastic bottles may contain perfluorooctanoic acid (PFOA), polybrominated diphenyl ethers (PDBE), phalates and bisphenol A (BPA) and antimony (http://www.who.int/water_sanitation_health/dwq/chemicals/antimony.pdf), all of which have been linked to adverse health effects. Third, since ZeroWater® pitchers cost ~$35 USD and the company suggests replacing filter units after filtering 15 gallons1 (~$15 USD/filter) the cost per gallon (~$1 USD/gallon) is similar to the cost of a gallon of bottled water (~$1USD/gallon). The reduced waste associated with the ZeroWater® filter compared to bottled water may reduce barriers to compliance, and thereby reduce the exposure to arsenic in well water (Flanagan et al. 2015b, Smith et al. 2016, Spayd et al. 2015). Similar to bottled water the use of table top water pitcher filters to remove arsenic from drinking and cooking water is an effective, short term solution than can be utilized until a POU or POE filtration system is installed.
There are several drawbacks from the use of tabletop water pitcher filters to remove arsenic, and any contaminant, from drinking water. These include the relatively slow filtration process, the need to refill the pitcher on a regular basis, and the need for regular monitoring and replacement of filters. However, several companies provide email notifications to replace filters and some filters come with the ability to monitor filter efficacy for removal of dissolved solids. Another potential drawback with the use of tabletop water pitcher filters as a long-term solution to remove arsenic from drinking water is that like bottled water, which reduces urinary arsenic by 21%, tabletop filters may not be as effective as POE or POU systems, which reduce urinary arsenic by ~60% (Flanagan et al. 2015b, Josyula et al. 2006, Smith et al. 2016, Spayd et al. 2015). Additional studies are required to determine the utility of tabletop water pitcher filters to reduce arsenic exposure compared to POE and POU systems.
We also suggest filtering water provided by public water supplies for three major reasons. First, although the Safe Drinking Water Act established in 1974 authorized the U.S. EPA to set health-based guidelines for contaminants in drinking water and regulates the presence of 103 contaminants for water that leaves the treatment plant, water can be contaminated at any point on its way to the tap (Lothrop et al. 2015). The recent water crisis in Flint, Michigan USA is an excellent example in which lead leached from the supply pipes and contaminated the water supply and led to significant exposure to those who drank the lead contaminated water, most notably children who are the most at risk for lead poisoning (Bellinger 2016). Second, despite the Safe Drinking Water Act for public water supplies in the U.S., in 2011 there were 7,170 exceedances of the EPA standards affecting ~1,800,000 people (Lothrop et al. 2015). Third, a recent study on 3rd to 5th grade children has shown that even low levels of arsenic below the EPA MCL (≥ 5 μg/L compared to < 5 μg/L) was associated with reduced IQ as well as decreased perceptible reasoning, working memory, and verbal comprehension (Wasserman et al. 2014). Accordingly, the only way to know what is in both public water supplies at the tap and well water is to test the water, and, if warranted, drink bottled water or water filtered using an effective tabletop water pitcher filter that removes the contaminant of interest and/or remediate using one of the approaches available including anion exchange, adsorptive media or reverse osmosis systems (Smith et al. 2016).
5. Conclusions
In this study, we have tested the ability of five relatively inexpensive and readily available tabletop water pitcher filters to remove arsenic from water. We report that one tabletop water pitcher filter tested, ZeroWater®, reduced the arsenic concentration from 10 μg/L and 100 μg/L to below 0.05 μg/L It also reduced the arsenic concentration from well water samples obtained in NH containing 42 μg/L of arsenic to below 0.05 μg/L, and from water containing 1,000 μg/L of arsenic to 2.6 μg/L, a value below the EPA MCL level of 10 μg/L. If properly used and maintained, the ZeroWater® water pitcher filter is a cost effective, short term solution to remove arsenic from drinking water and it eliminates plastic waste associated with bottled water. In addition, the ZeroWater® pitcher filter is an option for individuals and families that may not have the resources to purchase more expensive POU and POE options.
Highlights.
Arsenic contamination of drinking water is a serious threat to public health.
We identify a filter that reduces arsenic from 1,000 μg/L to below the MCL.
The filter is a short-term solution to remove arsenic from well water.
The filter is a cost-effective way to remove arsenic from well water.
Acknowledgments
Funding Source
The NIEHS P42-ES007373 funded this study. The NIEHS was not involved in the collection, analysis or interpretation of data, in writing the report or in the decision to submit the article for publication.
We thank Celia Chen, Kathrin Lawlor, Laurie Rardin, and Shannon Rogers for reading the manuscript and providing valuable comments. We also thank Joseph Ayotte (United States Geological Survey) for assisting with identification and collection of the well water samples. The NIEHS (grant number P42-ES007373) funded this study.
ABBREVIATIONS
- U.S. EPA
United States Environmental Protection Agency
- POU
point of use
- POE
point of entry
- ICP-MS
inductively coupled plasma mass spectrometry
- SEM
standard error of the mean
- PFOA
perfluorooctanoic acid
- PDBE
polybrominated diphenyl ethers
- BPA
bisphenol A
- NIST
National Institute of Standards and Technology
- WQA
Water Quality Association
- ANSI
American National Standards Institute
- USD
United States Dollars
Footnotes
We tested the ability of the ZeroWater® filter to remove arsenic from 100 L (26.4 gallons) of water, a volume that exceeds the manufactures suggestion that filters be replaced after filtering 15 gallons. It is not our intention or recommendation that the filter be used to remove arsenic from more than 15 gallons of water.
CONFLICT OF INTEREST
The authors declare no competing financial interest.
Author Contributions
All authors contributed to the experimental design and analysis of data and the manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellinger DC. Lead Contamination in Flint--An Abject Failure to Protect Public Health. N Engl J Med. 2016;374(12):1101–1103. doi: 10.1056/NEJMp1601013. [DOI] [PubMed] [Google Scholar]
- Carlin DJ, Naujokas MF, Bradham KD, Cowden J, Heacock M, Henry HF, Lee JS, Thomas DJ, Thompson C, Tokar EJ, Waalkes MP, Birnbaum LS, Suk WA. Arsenic and Environmental Health: State of the Science and Future Research Opportunities. Environ Health Perspect. 2016;124(7):890–899. doi: 10.1289/ehp.1510209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEB . Water Quality: An Introduction. Spring Science + Business Media; New York, NY: 2000. [Google Scholar]
- Flanagan SV, Marvinney RG, Johnston RA, Yang Q, Zheng Y. Dissemination of well water arsenic results to homeowners in Central Maine: influences on mitigation behavior and continued risks for exposure. Sci Total Environ. 2015a;505:1282–1290. doi: 10.1016/j.scitotenv.2014.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SV, Marvinney RG, Zheng Y. Influences on domestic well water testing behavior in a Central Maine area with frequent groundwater arsenic occurrence. Sci Total Environ. 2015b;505:1274–1281. doi: 10.1016/j.scitotenv.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josyula AB, McClellen H, Hysong TA, Kurzius-Spencer M, Poplin GS, Sturup S, Burgess JL. Reduction in urinary arsenic with bottled-water intervention. J Health Popul Nutr. 2006;24(3):298–304. [PMC free article] [PubMed] [Google Scholar]
- Lothrop N, Wilkinson ST, Verhougstraete M, Sugeng A, Loh MM, Klimecki W, Beamer PI. Home Water Treatment Habits and Effectiveness in a Rural Arizona Community. Water (Basel) 2015;7(3):1217–1231. doi: 10.3390/w7031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Paul B. The global menace of arsenic and its conventional remediation - A critical review. Chemosphere. 2016;158:37–49. doi: 10.1016/j.chemosphere.2016.05.043. [DOI] [PubMed] [Google Scholar]
- Smith AE, Lincoln RA, Paulu C, Simones TL, Caldwell KL, Jones RL, Backer LC. Assessing arsenic exposure in households using bottled water or point-of-use treatment systems to mitigate well water contamination. Sci Total Environ. 2016;544:701–710. doi: 10.1016/j.scitotenv.2015.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg TJ, Chen AS, Wang L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res. 2014;48:156–169. doi: 10.1016/j.watres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Spayd SE, Robson MG, Buckley BT. Whole-house arsenic water treatment provided more effective arsenic exposure reduction than point-of-use water treatment at New Jersey homes with arsenic in well water. Sci Total Environ. 2015;505:1361–1369. doi: 10.1016/j.scitotenv.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton BA, Caldwell K, Congdon CB, Disney J, Donahue M, Ferguson E, Flemings E, Golden M, Guerinot ML, Highman J, James K, Kim C, Lantz RC, Marvinney RG, Mayer G, Miller D, Navas-Acien A, Nordstrom DK, Postema S, Rardin L, Rosen B, SenGupta A, Shaw J, Stanton E, Susca P. MDI Biological Laboratory Arsenic Summit: Approaches to Limiting Human Exposure to Arsenic. Curr Environ Health Rep. 2015;2(3):329–337. doi: 10.1007/s40572-015-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Loiacono NJ, Kline J, Factor-Litvak P, van Geen A, Mey JL, Levy D, Abramson R, Schwartz A, Graziano JH. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Health. 2014;13(1):23. doi: 10.1186/1476-069X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ayotte JD. At the crossroads: Hazard assessment and reduction of health risks from arsenic in private well waters of the northeastern United States and Atlantic Canada. Sci Total Environ. 2015;505:1237–1247. doi: 10.1016/j.scitotenv.2014.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]