Abstract
Intrathecal baclofen (ITB) has been known to reduce spasticity which did not respond to oral medications and botulinum toxin treatment. However, few results have been reported comparing the effects of ITB therapy in patients with cerebral palsy (CP) and acquired brain injury. This study aimed to investigate beneficial and adverse effects of ITB bolus injection and pump therapy in patients with CP and to compare outcomes to patients with acquired brain injury such as traumatic brain injury and hypoxic brain injury. ITB test trials were performed in 37 patients (19 CP and 18 acquired brain injury). Based on ambulatory function, CP patients were divided into 2 groups: 11 patients with nonambulatory CP and 8 patients with ambulatory CP. Change of spasticity was evaluated using the Modified Ashworth Scale. Additional positive or negative effects were also evaluated after ITB bolus injection. In patients who received ITB pump implantation, outcomes of spasticity, subjective satisfaction and adverse events were evaluated until 12 months post-treatment. After ITB bolus injection, 32 patients (86.5%) (CP 84.2% versus acquired brain injury 88.9%) showed a positive response of reducing spasticity. However, 8 patients with CP had negative adverse effects. Particularly, 3 ambulatory CP patients showed standing impairment and 1 ambulatory CP patient showed impaired gait pattern such as foot drop because of excessive reduction of lower extremity muscle tone. Ambulatory CP patients received ITB pump implantation less than patients with acquired brain injury after ITB test trials (P = .003 by a chi-squared test). After the pump implantation, spasticity was significantly reduced within 1 month and the effect maintained for 12 months. Seventeen patients or their caregivers (73.9%) were very satisfied, whereas 5 patients (21.7%) suffered from adverse events showed no subjective satisfaction. In conclusion, ITB therapy was effective in reducing spasticity in patients with CP and acquired brain injury. Before ITB pump implantation, it seems necessary to perform the ITB bolus injection to verify beneficial effects and adverse effects especially in ambulatory CP.
Keywords: acquired brain injury, cerebral palsy, intrathecal baclofen, spasticity
1. Introduction
Spasticity or spastic hypertonia accompanied with various neurological diseases such as cerebral palsy (CP),[1] spinal cord injury,[2] and acquired brain injury such as traumatic brain injury[3] has been known to limit the quality of life, function, and patient care.[4] The first way of adjusting spasticity is to take oral antispasticity medications; however, it is often avoided due to systemic side effects when used for high-dose oral medication. Second, focal spasticity can be adjusted by injecting botulinum toxin, phenol, alcohol, and and so on; however, the antispastic effects are not permanent. Third, if the above treatments are ineffective, orthopedic musculoskeletal surgery, selective posterior rhizotomy, intrathecal baclofen (ITB) pump implantation, and potentially deep brain stimulation can be tried.[4–7]
The baclofen is a GABA receptor agonist that is most frequently used for the treatment for spasticity. However, the use has been limited due to systemic side effects such as drowsiness, confusion, and headache.[8] The ITB therapy can directly and effectively control spasticity by selectively acting as a GABA receptor agonist at the phase of the spinal cord with less systemic side effects.[9,10] Moreover, while orthopedic musculoskeletal surgery and selective posterior rhizotomy are irreversible surgeries, ITB therapy is reversible and continuously controls spasticity.[11] It has been reported that ITB therapy significantly reduced spasticity which did not respond to oral medications and botulinum toxin treatment.[9,10] However, few results have been reported comparing the immediate effects or long-term effects of ITB therapy in patients with CP and acquired brain injury.
Among the common cerebral disorders with severe spasticity, it is difficult to estimate whether the outcomes of ITB was related to underlying disease.[12] Therefore, the objectives of this research were to investigate beneficial and adverse effects of ITB bolus injection and ITB pump therapy in adults with CP and to compare the outcomes to patients with acquired brain injury such as traumatic brain injury and hypoxic brain injury. Additionally, the outcomes of ITB bolus injection were evaluated in patients with ambulatory CP and nonambulatory CP in this study.
2. Methods
2.1. Subjects
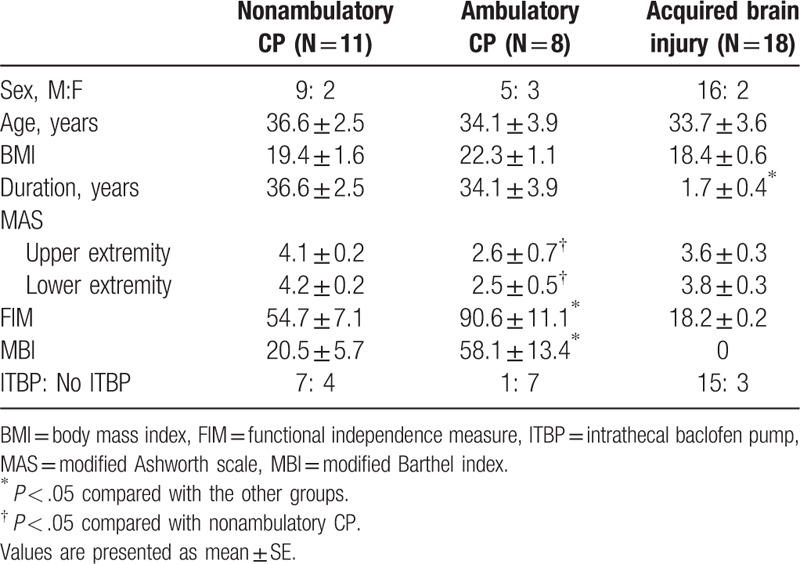
Total 37 patients (30 males and 7 females) with cerebral origin were recruited in our study. The subjects consisted of 19 CP and 18 acquired brain injury (13 traumatic brain injury and 5 hypoxic brain injury) (Table 1). All subjects showed spasticity showing more than grade 2 in the Modified Ashworth Scale (MAS), which did not effectively respond to oral antispasticity medications. Average age of subjects was 34.7 ± 2.1 years old. Average duration of disease was 19.1 ± 3.1 years. Based on ambulatory function, CP patients were divided into 2 groups; 11 patients with nonambulatory CP and 8 patients with ambulatory CP. Among ambulatory CP patients, 4 CP patients could walk outdoors independently, and 4 CP patients could walk using a gait aid such as walker. The other 29 patients were wheelchair bounded. The procedure of this study was approved by the Institutional Review Board (4-2016-0004).
Table 1.
Baseline characteristics in patients with CP and acquired brain injury.
2.2. Intrathecal baclofen test trial and pump implantation
Effects of ITB therapy have been verified through the test trials of the ITB bolus injection before ITB pump implantation to decide the surgery. The ITB bolus injection was provided through lumbar puncture up to 3 days. If spasticity reduced more than 1 grade in the MAS during the test trial at the most severe limb, it was regarded as having a definitive positive response. ITB bolus injection was usually given 50 μg at the first day. If there was no response to the first trial, the testing dose was increased to 75 μg at the second day. In this way, 100 μg was given at the third day if the second day dose was ineffective.
For each patient, vital signs and the response of reducing spasticity measured using the MAS have been closely monitored 2, 4, 6, and 8 hours after the ITB bolus injection. Spasticity was measured in all 4 limbs using the MAS; that is, shoulder adductors, elbow flexors, and wrist flexors in the upper extremities and hip adductors, knee flexors, and ankle plantar flexors in the lower extremities. The MAS grades of right extremity and left extremity were averaged and the MAS grades in upper extremity and lower extremity were compared among groups. For convenience of statistical analysis, as a 5-point scoring scale, MAS grade 1+ was matched to point 2; grades 2, 3, and 4 to points 3, 4, and 5, respectively.[13,14] The MAS grade was selected for analysis when the spasticity reduced most during the 3 days of the test trials. Beneficial effects such as reducing dystonic movement, excessive sweating, chronic pain and improving sitting posture, and adverse effects such as headache, drowsiness, and generalized hypotonia due to excessive reduction of muscle tone have been also observed.
2.3. Outcome measurement after ITB pump implantation
The ITB pump implantation was operated for the patients who showed definitive positive response to the test trials, and no adverse events. The surgery was conducted after a written consent and all procedures were operated by experienced neurosurgeons. After the pump implantation, individual patient was monitored using the MAS at 3-month intervals to maintain appropriate dose to control spasticity efficaciously without adverse effects. The primary outcome after ITB pump implantation on spasticity using MAS were evaluated as described above at initial, 1 month, 3 months, 6 months, and 12 months after ITB pump implantation. Moreover, functional level measured using the modified Barthel index (MBI) and functional independence measure (FIM) were evaluated at initial,1 month, 3 months, 6 months, and 12 months after the pump implantation.
Spasticity and functional levels such as MBI and FIM were evaluated by physical therapist and occupational therapist specialized in the field of physical and rehabilitation medicine. Drug side effects, pump mechanical complications, catheter malfunction, and baclofen withdrawal symptoms were carefully monitored by physiatrist and neurosurgeon who had performed the operation. In addition, the changes of sitting posture and other beneficial or adverse effects described above were observed. The dosage for individual administration after the ITB pump implantation was carefully adjusted within the range of 10% based on the MAS spasticity grade and other beneficial or adverse effects.
Subjective satisfaction of the patients or caregivers was also measured from scores of 0 to 10. The point 10 indicated the highest satisfaction and the point 0 indicated dissatisfaction which showed that if the score was high, the satisfaction tended to be high as well. If patients were not able to express their subjective satisfaction clearly due to cognitive impairment, the satisfaction of caregivers was measured.
2.4. Statistical analysis
Baseline characteristics were compared among nonambulatory CP, ambulatory CP, and acquired brain injury using one-way ANOVA, and between the ITB pump group and no ITB pump group using the independent t-test. After the ITB test trials, the MAS grade was compared between pre-trial and post-trial outcomes in patients with CP and acquired brain injury using the paired t-test. The MAS grade was also compared using the paired t-test to investigate the immediate effects after the ITB bolus injection in the ITB pump group and no ITB pump group. Descriptive statistics using a chi-squared test were used to compare the number of nonambulatory CP, ambulatory CP, and acquired brain injury who received ITB pump implantation after ITB test trials. In addition, the MAS grade was analyzed to compare the within-group changes of spasticity using repeated measures ANOVA after the ITB pump implantation. A P < .05 was considered statistically significant.
3. Results
3.1. Comparison of baseline characteristics
The ITB test trials were conducted for total 37 patients (11 nonambulatory CP, 8 ambulatory CP, and 18 acquired brain injury). When baseline characteristics were compared among nonambulatory CP, ambulatory CP, and acquired brain injury, and between the ITB group and no ITB group, there was no statistical difference in demographic data of gender, age, and body mass index among groups. However, patients with acquired brain injury showed shorter disease duration compared with CP patients (P < .001 by one-way ANOVA). Among CP patients, ambulatory CP showed lower MAS grade in upper extremity (P = .034) and in lower extremity (P = .019), and higher functional levels measured using MBI and FIM (P < .001 respectively) (Table 1).
After ITB test trials, 23 patients (62.2%) (7 nonambulatory CP, 1 ambulatory CP and 15 acquired brain injury) proceeded to receive the ITB pump implantation. Patients with CP, particularly ambulatory CP, received ITB pump implantation less than patients with acquired brain injury (P = .003 by a chi-squared test) (Table 1).
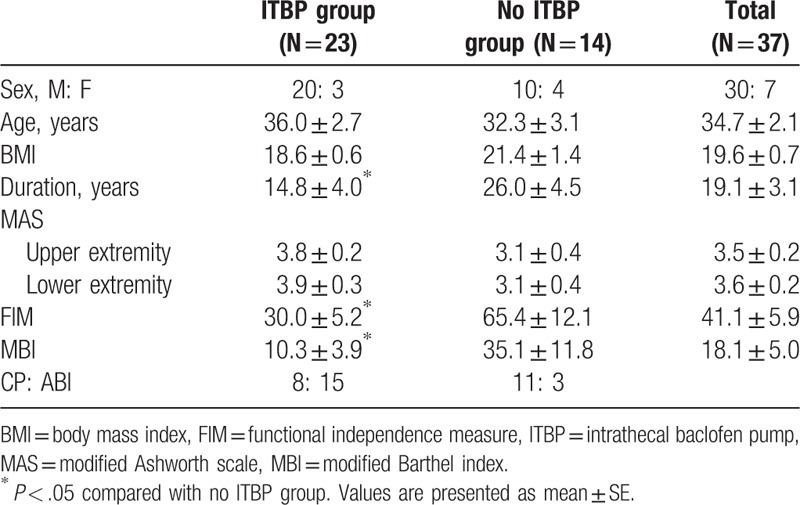
On the other hand, when baseline characteristics were compared between the ITB pump group and patients who did not receive the pump implantation (no ITB pump group), there was no statistical difference in gender, age, body mass index, and MAS spasticity grade. However, the ITB pump group showed shorter disease duration (P = .027) and lower functional levels measured using MBI (P = .016) and FIM (P = .003) (Table 2).
Table 2.
Baseline characteristics in the ITBP Group and No ITBP Group.
3.2. Effects of intrathecal baclofen test trials
All patients showed beneficial effects such as positive response of reducing spasticity or other positive effects. Thirty-two patients (86.5%) (CP 84.2% versus acquired brain injury 88.9%) showed a definitive positive response of reducing spasticity after the ITB test trials. Seventeen patients (45.9%) (CP 68.4% versus acquired brain injury 22.2%) showed additional beneficial effects such as decreased dystonic movement, improved sitting posture and sleep pattern, or decreased excessive sweating and chronic pain. Among them, 8 patients (21.6%) showed decreased dystonic movement. Other positive effects were decreased chronic pain in 6 patients (16.2%), improved sitting posture and decreased excessive sweating in 4 patients (10.8%) respectively, and improved sleep pattern in 3 patients (8.1%) (Table 3).
Table 3.
Responses of subjects tested with intrathecal baclofen bolus injection.
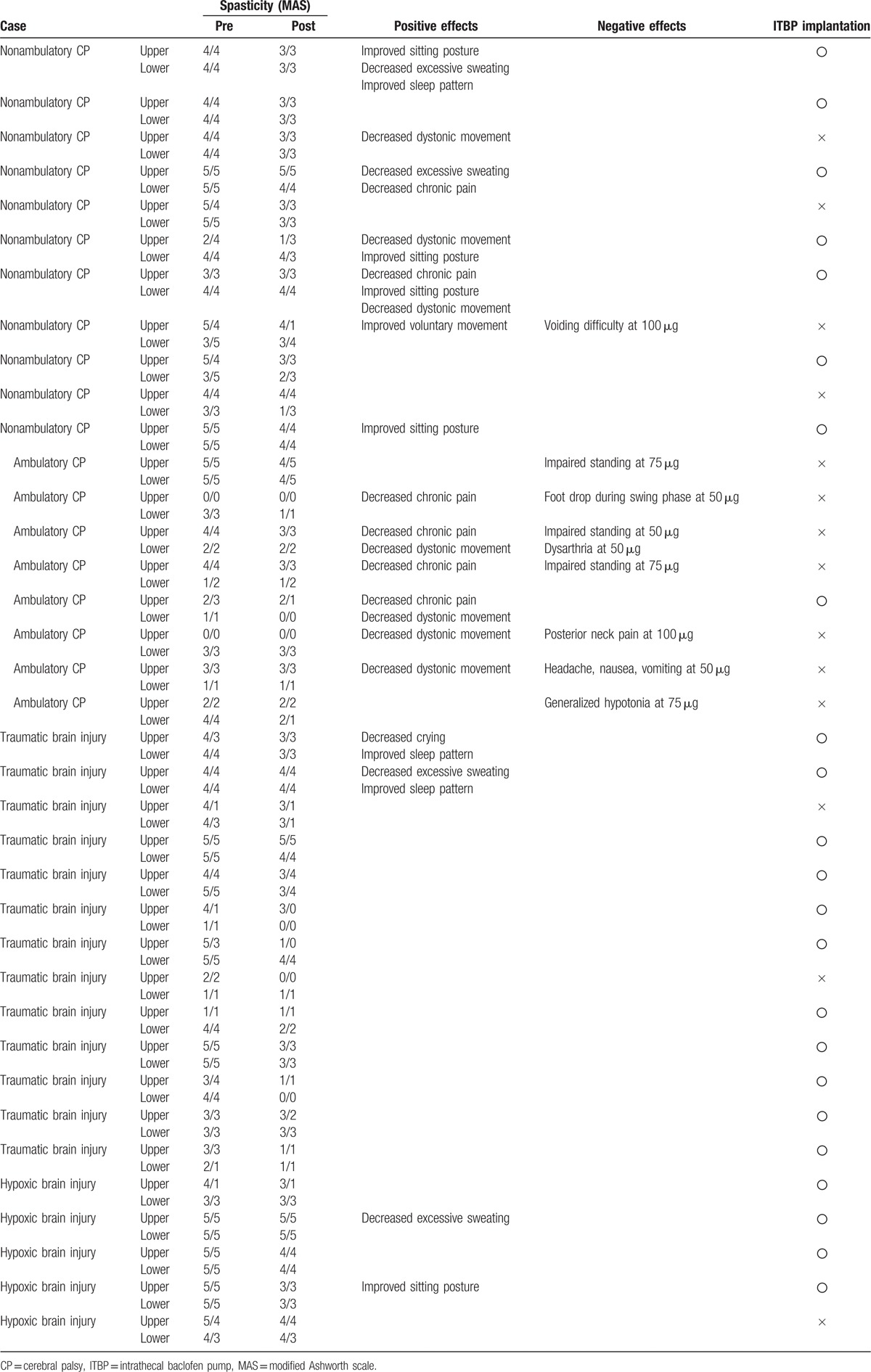
Of 14 patients who did not receive the ITB pump implantation after the test trials, 12 patients showed improved spasticity. However, 8 patients with CP had negative adverse effects. Particularly, 3 ambulatory CP patients showed standing impairment at 50–75 μg and 1 ambulatory CP patient showed impaired a gait pattern such as foot drop during the swing phase at 50 μg because of excessive reduction of lower extremity muscle tone. In addition, voiding difficulty at 100 μg, dysarthria at 50 μg, posterior neck pain at 100 μg, headache, nausea, and vomiting at 50 μg, and generalized hypotonia at 50 μg were shown, respectively (Table 3). Five patients who showed a reduction of spasticity without negative adverse effects gave up receiving the pump implantation due to cost effectiveness and financial difficulty.
3.3. Spasticity reduction in CP and ABI after intrathecal baclofen bolus injection
Spasticity reduced both groups of CP and acquired brain injury after ITP bolus injection. In all subjects, the ITB bolus injection significantly decreased the MAS grade from 3.5 ± 0.2 to 2.7 ± 0.2 in upper extremity and from 3.6 ± 0.2 to 2.7 ± 0.2 in lower extremity (P < .001 respectively by paired t-tests).
In patients with acquired brain injury, the ITB bolus injection significantly decreased the MAS grade from 3.6 ± 0.3 to 2.6 ± 0.4 in upper extremity (P < .001) (Fig. 1A) and from 3.8 ± 0.3 to 2.7 ± 0.3 in lower extremity (P = .001) (Fig. 1B). In nonambulatory CP, the MAS grade also significantly decreased after the ITB bolus injection from 4.1 ± 0.2 to 3.2 ± 0.2 in upper extremity (P = .001) (Fig. 1A), and from 4.2 ± 0.2 to 3.2 ± 0.2 in lower extremity (P < .001) (Fig. 1B). However, in ambulatory CP, the MAS grade significantly decrease after the ITB bolus injection in upper extremity (P = .041) (Fig. 1A), but not in lower extremity (P = .129) (Fig. 1B).
Figure 1.
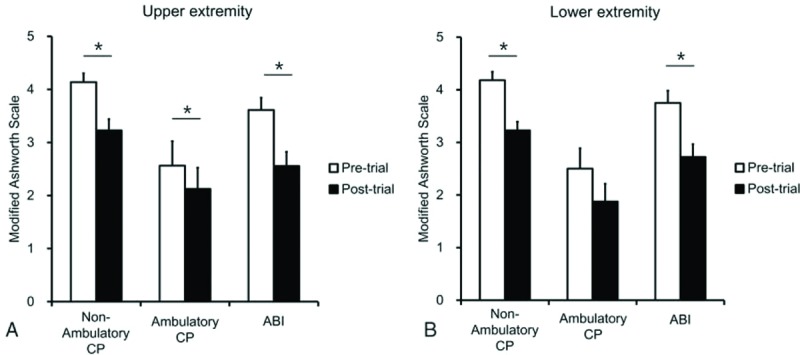
Effects of intrathecal baclofen bolus injection in CP and acquired brain injury. In patients with acquired brain injury, the ITB bolus injection significantly decreased the MAS grade in upper extremity (A) and lower extremity (B). In nonambulatory CP, the MAS grade also significantly decreased after the ITB bolus injection in upper extremity (A) and lower extremity (B). However, in ambulatory CP, the MAS grade significantly decreased after the ITB bolus injection in upper extremity (A), but not in lower extremity (B). ABI = acquired brain injury, CP = cerebral palsy, ITB = intrathecal baclofen, MAS = modified Ashworth scale. ∗P < .05, pre-ITB test trial versus post-ITB test trial.
3.4. Spasticity reduction in ITB pump and no ITB pump groups after intrathecal baclofen bolus injection
In the ITB pump group, the MAS grade significantly decreased from 3.8 ± 0.2 to 2.8 ± 0.3 in upper extremity (Fig. 2A) and from 3.9 ± 0.3 to 2.9 ± 0.3 in lower extremity (Fig. 2B) (P < .001 respectively by paired t-tests). No ITB pump group also showed the ITB bolus injection significantly decreased the MAS grade from 3.1 ± 0.4 to 2.4 ± 0.4 in upper extremity (P = .003) (Fig. 2A) and from 3.1 ± 0.4 to 2.3 ± 0.3 in lower extremity (P = .006) (Fig. 2B).
Figure 2.
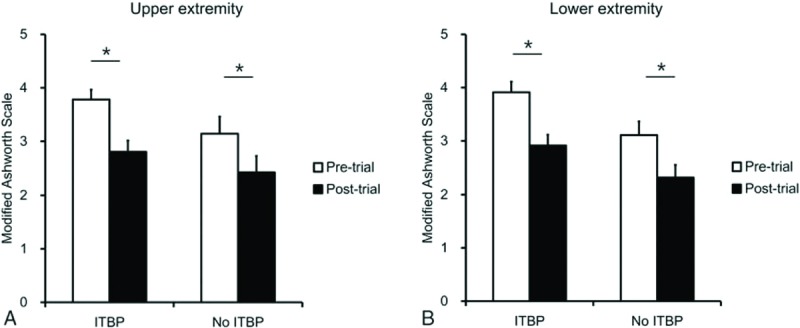
Effects of intrathecal baclofen bolus injection in ITBP and no ITBP groups. In the ITB pump group, the MAS grade significantly decreased in upper extremity (A) and lower extremity (B). No ITB pump group also showed the ITB bolus injection significantly decreased the MAS grade in upper extremity (A) and lower extremity (B). CP = cerebral palsy, ITBP = intrathecal baclofen pump, MAS = modified Ashworth scale. ∗P < .05, pre-ITB test trial versus post-ITB test trial.
When spasticity reduction was also compared between ITB pump and no ITB pump groups, the MAS grade did not show a difference between 2 groups, suggesting the reason of no ITB pump group not proceeding with the pump implantation not because of little spasticity reduction after the ITB bolus injection, but because of the negative adverse effects.
3.5. Effects of intrathecal baclofen pump implantation
In all subjects, the ITB pump therapy significantly reduced the MAS grade from 3.7 ± 0.2 to 2.2 ± 0.2 in upper extremity and from 3.9 ± 0.3 to 2.2 ± 0.2 in lower extremity (P < .001 respectively by repeated ANOVA) (Fig. 3A). Looking at Fig. 3 and Table 4, the beneficial effect was shown significantly within 1 month after the implantation and maintained for a 12-month follow-up period. In patients with CP, the ITB pump therapy significantly decreased the MAS grade from 3.9 ± 0.3 to 2.6 ± 0.4 in upper extremity (P = .046) and lower extremity (P = .001) (Fig. 3B). In patients with acquired brain injury, the ITB pump therapy also significantly decreased the MAS grade from 3.6 ± 0.3 to 2.0 ± 0.3 in upper extremity and from 4.0 ± 0.3 to 2.2 ± 0.3 in lower extremity (P < .001 respectively) (Fig. 3C).
Figure 3.
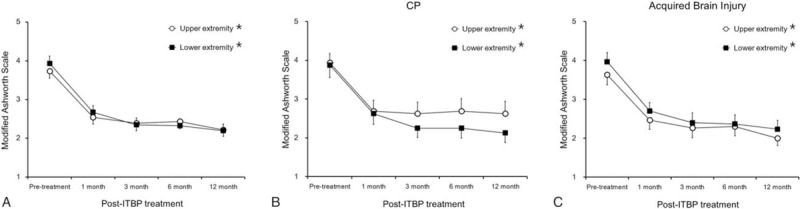
Effects of intrathecal baclofen pump implantation. In all subjects, the ITB pump therapy significantly reduced the MAS grade in upper extremity and lower extremity (A). The beneficial effect was shown significantly within 1 month after the implantation and maintained for a 12 month follow-up period. In patients with CP, the ITB pump therapy significantly decreased the MAS grade in upper extremity and lower extremity (B). In patients with acquired brain injury, the ITB pump therapy also significantly decreased the MAS grade in upper extremity and lower extremity (C). CP = cerebral palsy, ITBP = intrathecal baclofen pump, MAS = modified Ashworth scale. ∗P < .05 by repeated measures ANOVA.
Table 4.
Responses of subjects with intrathecal baclofen pump implantation.
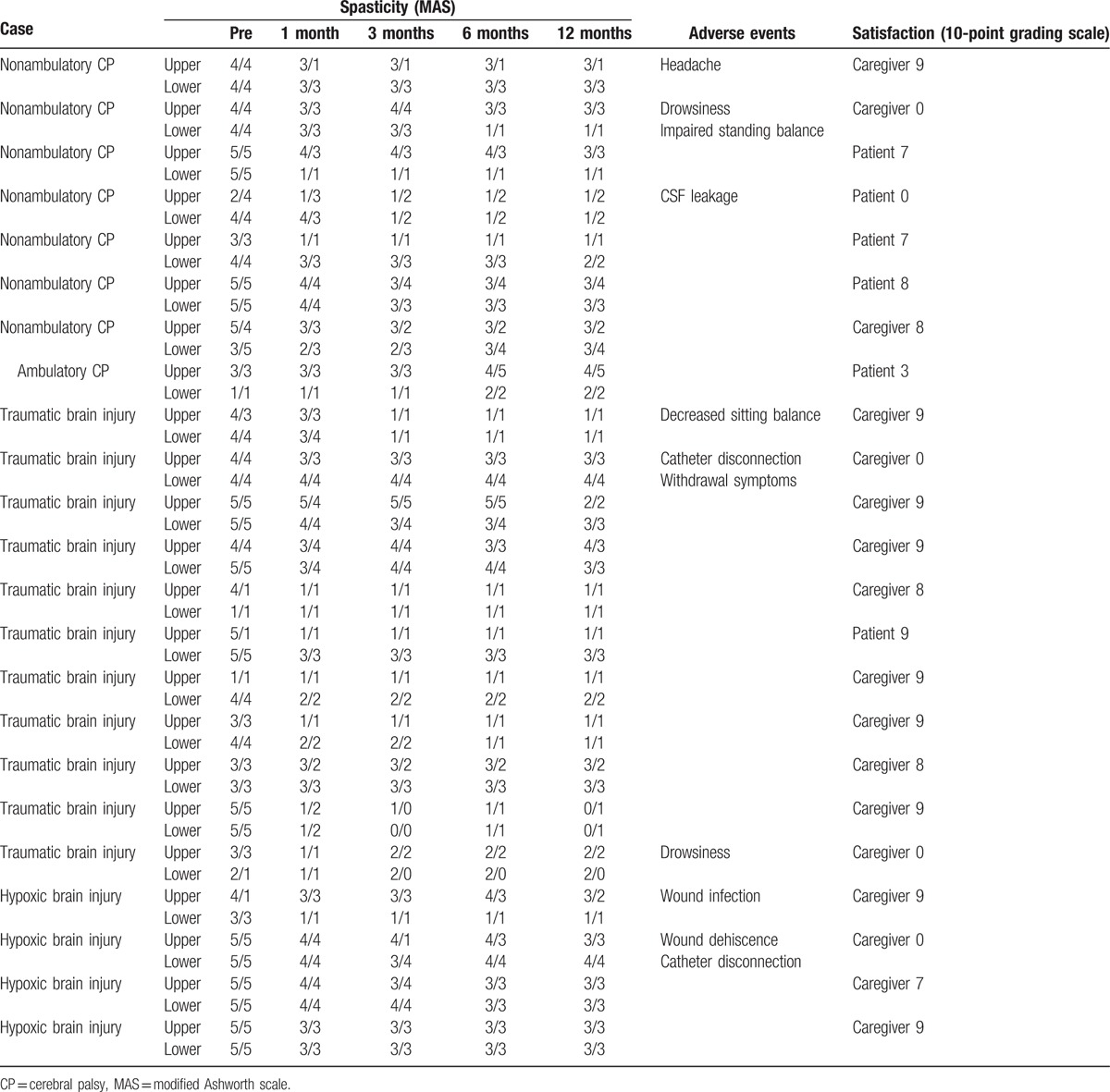
Seventeen patients (73.9%) among 23 patients who received the ITB pump implantation were very satisfied, showing subjective satisfaction score was 8.4 ± 0.2 either by the patients or caregivers. One patient (4.3%) was somewhat satisfied showing subjective satisfaction score was 3. However, 5 patients (21.7%) who were suffered from negative adverse effects after the pump implantation expressed no satisfaction. Therefore, overall satisfaction score was 6.3 ± 0.8 out of maximum score 10.
When the complications after the ITB pump implantation were evaluated, 8 patients (34.8%) experienced adverse events. Among them, 4 patients (17.4%) showed reversible adverse events such as headache, drowsiness, and decreased sitting or standing balance. Additional 4 patients (17.4%) showed catheter or wound problems such as catheter disconnection and CSF leakage, or wound infection, which required revision surgeries.
On the other hand, when the functional outcomes were evaluated in patients who had received the ITB pump implantation, the MBI and FIM scores did not significantly increase after the pump therapy (from 9.8 ± 3.9 to 10.4 ± 3.5 in MBI, from 33.6 ± 5.4 to 34.3 ± 4.8 in FIM). It suggests that ITB pump therapy could not reverse the functional impairment in patients with severe disability.
4. Discussion
Spasticity causes musculoskeletal deformities and abnormal posture, which also causes chronic pain and impairs sleep pattern and activities of daily living.[15] In patients with severe spasticity, oral antispasticity medications might be insufficient to effectively manage these symptoms. Botulinum toxin injection can also be inefficacious to handle generalized spasticity. On the other hand, it has been verified that ITB treatment decreases severe generalized spasticity.[15,16] In this study, most patients showed a definitive response of spasticity reduction or other beneficial effects such as improved sitting posture and sleep pattern, and decreased excessive sweating and chronic pain after the ITB bolus injection. Then, the patients who received the ITB pump implantation showed a significant reduction of generalized spasticity, which was consistent with the previous results.[15,16] Additionally, patients who did not receive the pump implantation after ITB test trials continued to take oral antispasticity medications whereas patients who received the pump implantation usually reduced the dose of oral antispasticity medications.
Likewise, the ITB treatment has prominent effects in decreasing spasticity; however, in safety issues, it has been known that it could cause side effects such as headache, dizziness, drowsiness, respiratory depression, seizure, and loss of consciousness.[17–21] Moreover, catheter-related complications may occur, leading to increased morbidity and mortality.[22–24] In addition, spasticity reduction did not always induce functional improvement; instead, it caused excessive reduction of lower extremity muscle tone, resulting in instability of walking and standing.[17] Thus, it is very important to verify functional changes of patients by the ITB test trials before conducting the ITB pump implantation.[25,26] In other words, ITB treatment should be selectively conducted for the cases that are expected to reduce spasticity without functional impairments. In the CP patients who received the ITB bolus injection, we also experienced that 3 ambulatory CP patients showed standing impairment and 1 ambulatory CP patient showed an impaired gait pattern, similar to a systemic review[27] reporting that some patients with cerebral origin compromised their ambulatory and transfer abilities after the ITB therapy. In addition, 1 patient showed voiding difficulty although he was satisfied with spasticity reduction of upper and lower extremities after the ITB bolus injection; therefore, they did not proceed to receive the pump implantation.
Because of the adverse negative effects, CP patients were not likely to proceed with ITB pump implantation compared to patients with acquired brain injury although the ITB bolus injection showed spasticity reduction and other beneficial effects in both groups. Since the effect on spasticity reduction was similar in both groups, difference in proportion of ITB implantation between 2 groups is more likely related to functionally negative effects such as impaired standing and gait pattern after the ITB test trials according to a different baseline functional level. Patients with acquired brain injury were functionally more severe than the CP patients in the baseline characteristics, focusing on the spasticity reduction of extremities and comfort of caregivers. This implies that baseline function rather than underlying etiology should be considered more importantly when performing ITB pump implantation.
After the ITB pump implantation, 1 patient suffered headache and 1 patient showed decreased sitting balance. Both symptoms were relieved after modifying the infusion dose of the ITB pump without altering the antispastic effect. However, 1 patient with persistent drowsiness eventually wanted to remove the ITB pump. Therefore, it is essential to effectively adjust the infusion dose to maximize the beneficial effects while minimizing the negative adverse events.[28,29] As previous studies reported equipment-related complications,[22,23,30] we also experienced that 4 patients out of 23 patients showed catheter-related or wound complications which required revision management by surgical operation. Among them, 3 patients were completely recovered from the revision surgery. However, 5 patients out of 8 complicated patients expressed no subjective satisfaction to the ITB pump implantation. Therefore, it is essential to carefully monitor the catheter-related complication or other adverse events to solve the complication as soon as possible.
In our study, we did not find a significant relationship between injury duration, spasticity, and functional improvement because there were not enough patients to compare the outcomes based on injury duration if the severity of injury was controlled. However, a recent review regarding the timing of intervention by Saulino et al[31] described that contrary to the conventional norm where physicians waited 1 year following brain damage before performing ITB therapy, early exposure to ITB therapy is safe in appropriately selected patients. In fact, if the treatment is delayed for a longer period, musculoskeletal deformities and contracture may develop. In other literature by Maneyapanda et al,[32] it was shown that severe generalized spasticity in the earlier phases following brain injury could interfere with rehabilitation programs. Taken together, patients with severe spasticity should consider early application of ITB therapy in order to ease the care, prevent complications, and improve functional outcomes.
The limitation of this research is the absence of control group who did not receive the ITB therapy. However, this study included chronic patients who had consistent spasticity which had not been effectively controlled by oral medications. In addition, patients with acquired brain injury were functionally more severe than the CP patients in the baseline characteristics. Therefore, it is difficult to directly compare the outcomes between 2 groups because main therapeutic goal might be different; for example, caregiver convenience rather than functional improvement in patients with acquired brain injury. Further research should be needed to evaluate the response of a retrial with lower dose ITB when adverse negative effects are shown after the ITB bolus injection and to evaluate the outcomes of the ITB pump treatment for longer periods.
In conclusion, ITB therapy was effective in reducing spasticity in patients with severe spasticity, although it might have reversible adverse effects or catheter-related complications and spasticity reduction did not always induce functional improvements. Before ITB pump implantation, it seems necessary to perform the ITB bolus injection to verify positive beneficial effects and negative adverse effects especially in ambulatory CP and maintain appropriate dose of ITB to control spasticity efficaciously without adverse effects after the ITB pump implantation in patients with CP and acquired brain injury.
Footnotes
Abbreviations: CP = cerebral palsy, ITB = intrathecal baclofen.
Funding: This study was supported by grants from the National Research Foundation (NRF-2014R1A2A1A11052042; 2015M3A9B4067068), the Ministry of Science and Technology, Republic of Korea, the Korean Health Technology R&D Project (HI16C1012), Ministry of Health & Welfare, Republic of Korea.
The authors have no conflicts of interest to disclose.
Young Kwon Yoon and Kil Chan Lee equally contributed to this study.
References
- [1].Skalsky AJ, Fournier CM. Intrathecal baclofen bolus dosing and catheter tip placement in pediatric tone management. Phys Med Rehab Clin N Am 2015;26:89–93. [DOI] [PubMed] [Google Scholar]
- [2].Khurana SR, Garg DS. Spasticity and the use of intrathecal baclofen in patients with spinal cord injury. Phys Med Rehab Clin N Am 2014;25:655–69. ix. [DOI] [PubMed] [Google Scholar]
- [3].Bose P, Hou J, Thompson FJ. Kobeissy FH. Traumatic brain injury (TBI)-induced spasticity: neurobiology, treatment, and rehabilitation. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Francis; 2015;Chapter 14. Frontiers in Neuroengineering. [PubMed] [Google Scholar]
- [4].Roberts A. Surgical management of spasticity. J Childrens Orthop 2013;7:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Theroux MC, DiCindio S. Major surgical procedures in children with cerebral palsy. Anesthesiol Clin 2014;32:63–81. [DOI] [PubMed] [Google Scholar]
- [6].Lynn AK, Turner M, Chambers HG. Surgical management of spasticity in persons with cerebral palsy. PM R 2009;1:834–8. [DOI] [PubMed] [Google Scholar]
- [7].Kim AR, Chang JW, Chang WS, et al. Two-year outcomes of deep brain stimulation in adults with cerebral palsy. Ann Rehab Med 2014;38:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rekand T. Clinical assessment and management of spasticity: a review. Acta Neurol Scand 2010;122:62–6. [DOI] [PubMed] [Google Scholar]
- [9].Brennan PM, Whittle IR. Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain. Brit J Neurosurg 2008;22:508–19. [DOI] [PubMed] [Google Scholar]
- [10].Penn RD, Kroin JS. Continuous intrathecal baclofen for severe spasticity. Lancet (London, England) 1985;2:125–7. [DOI] [PubMed] [Google Scholar]
- [11].Kan P, Gooch J, Amini A, et al. Surgical treatment of spasticity in children: comparison of selective dorsal rhizotomy and intrathecal baclofen pump implantation. Child's Nerv Syst 2008;24:239–43. [DOI] [PubMed] [Google Scholar]
- [12].Sampson FC, Hayward A, Evans G, et al. Functional benefits and cost/benefit analysis of continuous intrathecal baclofen infusion for the management of severe spasticity. J Neurosurg 2002;96:1052–7. [DOI] [PubMed] [Google Scholar]
- [13].Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7. [DOI] [PubMed] [Google Scholar]
- [14].Sohn MK, Cho KH, Kim YJ, et al. Spasticity and electrophysiologic changes after extracorporeal shock wave therapy on gastrocnemius. Ann Rehab Med 2011;35:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Van Schaeybroeck P, Nuttin B, Lagae L, et al. Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo-controlled, double-blind study. Neurosurgery 2000;46:603–9. discussion 609–12. [DOI] [PubMed] [Google Scholar]
- [16].Ueta T. Intrathecal baclofen for severe spasticity. Brain Nerve 2008;60:1415–20. [PubMed] [Google Scholar]
- [17].Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg 2007;107(1 suppl):32–5. [DOI] [PubMed] [Google Scholar]
- [18].Borowski A, Littleton AG, Borkhuu B, et al. Complications of intrathecal baclofen pump therapy in pediatric patients. J Pediatr Orthop 2010;30:76–81. [DOI] [PubMed] [Google Scholar]
- [19].Awaad Y, Rizk T, Siddiqui I, et al. Complications of intrathecal baclofen pump: prevention and cure. ISRN Neurol 2012;2012:575168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sgouros S, Charalambides C, Matsota P, et al. Malfunction of SynchroMed II baclofen pump delivers a near-lethal baclofen overdose. Pediatr Neurosurg 2010;46:62–5. [DOI] [PubMed] [Google Scholar]
- [21].Vidal J, Fenollosa P, Martin E, et al. Safety and efficacy of intrathecal baclofen infusion by implantable pump for the treatment of severe spinal spasticity: a Spanish multicenter study. Neuromodulation 2000;3:175–82. [DOI] [PubMed] [Google Scholar]
- [22].Huraibi HA, Phillips J, Rose RJ, et al. Intrathecal baclofen pump implantation complicated by epidural lipomatosis. Anesth Analg 2000;91:429–31. [DOI] [PubMed] [Google Scholar]
- [23].Dickerman RD, Schneider SJ. Recurrent intrathecal baclofen pump catheter leakage: a surgical observation with recommendations. J Pediatr Surg 2002;37:E17. [DOI] [PubMed] [Google Scholar]
- [24].Marquardt G, Beck J, Seifert V. An unusual complication impeding refilling of a pump used for intrathecal baclofen therapy. Rev Neurol 2008;47:557–8. [PubMed] [Google Scholar]
- [25].Phillips MM, Miljkovic N, Ramos-Lamboy M, et al. Clinical experience with continuous intrathecal baclofen trials prior to pump implantation. PM R 2015;7:1052–8. [DOI] [PubMed] [Google Scholar]
- [26].Grzegorzewski P, Kawecki Z, Swierczynska A, et al. Baclofen dose in a screening test and in a baclofen pump filling—is there any differences. Przegl Leki 2007;64(suppl 2):8–12. [PubMed] [Google Scholar]
- [27].Pin TW, McCartney L, Lewis J, et al. Use of intrathecal baclofen therapy in ambulant children and adolescents with spasticity and dystonia of cerebral origin: a systematic review. Dev Med Child Neurol 2011;53:885–95. [DOI] [PubMed] [Google Scholar]
- [28].Sansone JM, Mann D, Noonan K, et al. Rapid progression of scoliosis following insertion of intrathecal baclofen pump. J Pediatr Orthop 2006;26:125–8. [DOI] [PubMed] [Google Scholar]
- [29].Ordia JI, Fischer E, Adamski E, et al. Continuous intrathecal baclofen infusion delivered by a programmable pump for the treatment of severe spasticity following traumatic brain injury. Neuromodulation 2002;5:103–7. [DOI] [PubMed] [Google Scholar]
- [30].Bernuz B, Assier H, Bisseriex H, et al. Intrathecal baclofen pump: a foreign-body reaction case report and its solution. J Rehab Med 2012;44:184–5. [DOI] [PubMed] [Google Scholar]
- [31].Saulino M, Ivanhoe C, McGuire J, et al. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation 2016;19:607–15. [DOI] [PubMed] [Google Scholar]
- [32].Maneyapanda MB, McCormick ZL, Marciniak C, et al. Long-term dosing of intrathecal baclofen in the treatment of spasticity after acquired brain injury. Phys Med Rehab 2017;17:30020–5. [DOI] [PubMed] [Google Scholar]


