Abstract
Reducing the false negative rate of sentinel lymph node biopsy (SLNB) for breast cancer patients has always been a focus of clinical research. We aimed to map the sentinel lymph nodes (SLNs) in detail, and analyze the factors related to SLNs located at locations that are often ignored by surgeons, to reduce the rate of false negatives from SLNB. A retrospective analysis involving 545 breast cancer patients who underwent SLNB in west China hospital between August 2010 and February 2016 was performed. Blue dye, radioisotope, or combined methods were used for tracing SLNs. Using blue dye, radioisotope, and a combination of blue dye and radioisotope successfully traced SLNs in 479, 507, and 525 patients, the detection rate was 88.2%, 93.9%, and 97.4%, respectively. Among the 1559 detected SLNs, 139 (9.6%) were located at the latissimus dorsi lateral margin, and 108 (6.9%) were located at level 2. Subcutaneous injection of radioisotope (P = .004) and intradermal injection of blue dye (P = .002) were independent factors associated with SLNs distributed at level 2 and the latissimus dorsi lateral margin, respectively. It was noteworthy that 2 of 7 patients had skipping metastasis in level 2, so subcutaneous injection of the isotope is strongly recommended for tracing SLNs distributed in level 2 because of the possibility of skipping metastasis. Though intradermal injection of blue dye was superior methods for tracing SLNs located at the latissimus dorsi lateral margin, we surprisingly found those patients with metastasis to the latissimus dorsi lateral margin nodes also could have metastasis to level 1 (expect for the latissimus dorsi lateral margin) nodes, it seemed that maybe there is no need to excise SLNs at the latissimus dorsi lateral margin in SLNB, whether such nodes should be regarded as useful for SLNB still needs to be determined by further large, multicenter clinical studies.
Keywords: breast cancer, distribution of sentinel lymph node, sentinel lymph node biopsy, tracing method
1. Introduction
With the results of multi-institutional randomized studies being made available, sentinel lymph node biopsy (SLNB) has replaced axillary lymph node dissection (ALND) as the standard of care for primary treatment of early axillary lymph node negative breast cancer patients.[1–3] This has greatly reduced ALND-related morbidity such as lymphedema, and sensory and motor dysfunction.[4–6]
Nevertheless, the American Society of Clinical Oncology has reported the results of 6 trials on SLNB, in which the false-negative rate (FNR) ranged from 4.6% to 16.7%.[7,8] Because higher FNR brings a physical and psychological burden for breast cancer patients, this greatly restricts the popularity of SLNB. Consequently, more and more studies are being undertaken to minimize the FNR of SLNB as far as possible.[9,10]
Since the 1990 s, with the evolution of the lymph node tracing method, and administration and injection routes, the FNR of SLNB has been reduced to a great extent.[11,12] However, each patient's condition is different, and some subjectivity from the surgeons also affects the accuracy of SLNB. For example, some surgeons are inclined to find sentinel lymph nodes (SLNs) in familiar areas but overlook what they might consider “unimportant” anatomical positions. Thus, the main purpose of our retrospective study was to map SLNs in detail, and explore the factors related to SLNs located at locations that are often overlooked during surgery, so that to reduce the subjective factors that raise the FNR of SLNB, and provide useful guidance to trainee and inexperienced surgeons.
2. Patients and methods
2.1. Patients
From August 2010 to February 2016, a total of 545 breast cancer patients underwent SLNB at the Department of Breast Surgery, West China Hospital, Sichuan University, China. Basic clinical data of each patient, including patient age, operation method, tumor location, and pathological characteristics of the tumors, were collected. Hormone receptor positivity was defined as 1% or more of cells staining for estrogen receptor or progesterone receptor. Her2 positivity was defined as 3+ staining by immunohistochemistry or fluorescence in situ hybridization (FISH) amplification with a value greater than 2. The research was in compliance with the Declaration of Helsinki and was approved by the ethics committees of Sichuan University West China Hospital. Informed consent was obtained from each patient.
2.2. SLNB technique
Sulfur colloid (SC) labeled with technetium–99m (99mTc) was injected on the day before surgery, or at least 4 hours before surgery. Methylthioninium (1%; 1 mL) was injected 15 to 20 minutes before surgery. Patients were injected either subcutaneously above the primary tumor, intradermally, or around the areola. After general anesthesia and dye injection, a 3 to 4 cm incision was made in the axillary region. The skin, subcutaneous tissue, and mammary gland tissue were then carefully separated to locate the blue nodes and record their number. Nodes with high radioactivity (hot node) were also identified.
2.3. Identification and evaluation of SLNs
SLNs were identified intraoperatively by use of radioisotope, blue dye, or combined methods. The first lymph nodes with blue lymphatic vessels directly leading to them, and those with a radioactivity count higher than 10% of the highest radioactivity count of the lymph nodes were regarded as SLNs. Cytokeratin immunohistochemistry (IHC) was used to identify whether metastases were present in the lymph node. Metastases were classified according to the 6th criterion of the American Joint Cancer Committee. The presence of macrometastases (≥2 mm), micrometastases (0.2–2 mm), and isolated tumor cells (≤0.2 mm), all resulted in a node-positive classification.
2.4. Statistical analysis
Descriptive statistics included means, ranges, standard deviations, and proportions. Categorical data were presented as percentages, and differences between proportions were compared using the χ2 test or Fisher exact test. Continuous variables were compared using the unpaired Student t test. Multivariate analysis was performed to identify independent determinants for the SLNs located at the latissimus dorsi lateral margin and level 2. All statistical evaluations were performed using SPSS for Windows (SPSS 18.0, Chicago, IL). Results with a P < .05 were considered statistically significant.
3. Results
3.1. Tumor clinical pathological characteristics
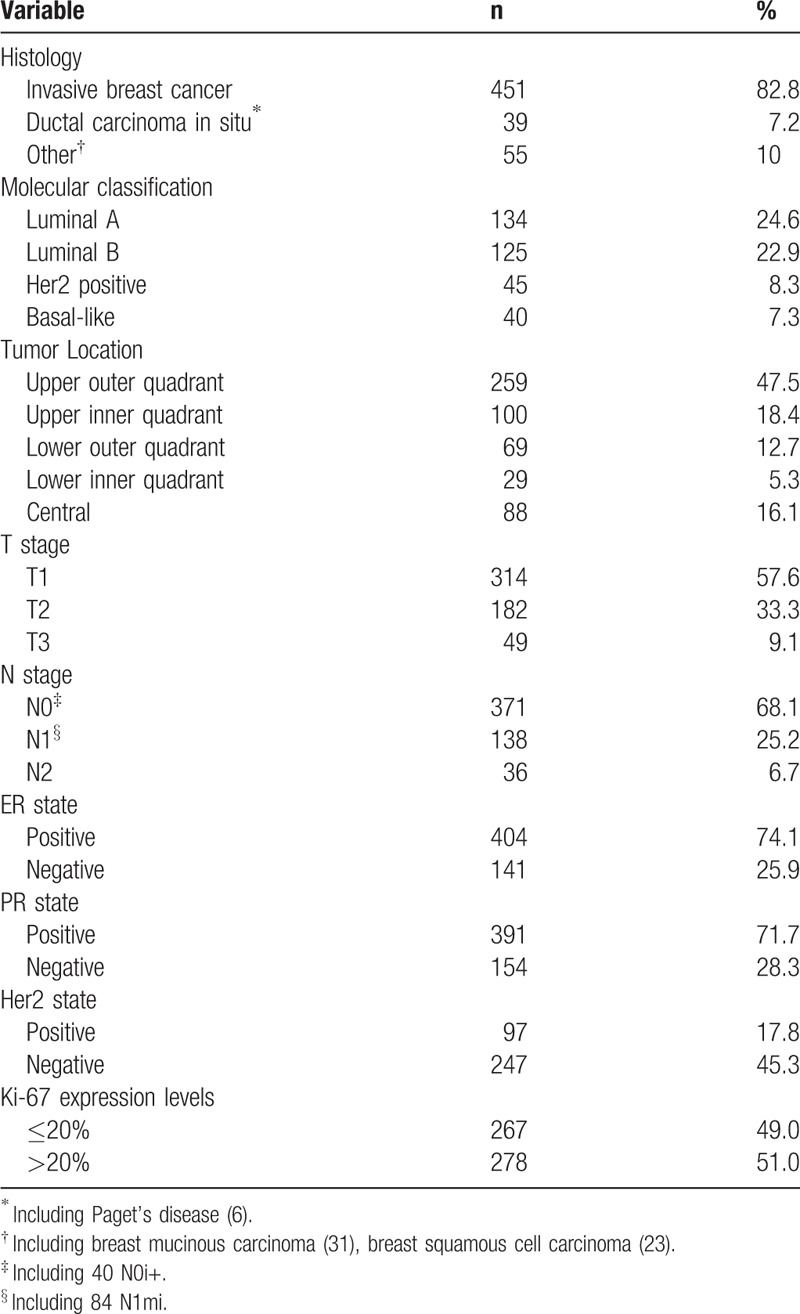
All breast cancer patients included in this study were females with a mean age of 48.7 ± 11.8 years (range: 19–85 yrs). The average size of the tumors was 2.5 ± 1.5 cm (range: 0.2–12 cm). The majority of patients were treated by mastectomy (83.8%), with the remaining patients treated by breast conserving surgery (16.2%). In a certain number of patients, whose Her2 state was 2+ by IMH testing, FISH tests were not performed for various reasons, resulting in only partial data for these patients. The pathological characteristics of the tumors are shown in Table 1.
Table 1.
Tumor pathological characteristics.
3.2. Identification of SLNs using blue dye and radioisotopes, and the mapping of axillary SLNs
SLNs were successfully found in 543 patients (99.6%). Using blue dye, radioisotope, and a combination of blue dye and radioisotope successfully traced SLNs in 479, 507, and 525 patients, the detection rate was 88.2%, 93.9%, and 97.4%, respectively. Compared with the detection rate of using combined methods to trace SLNs, the detection rate of using blue dye or the radioisotope alone to trace SLNs was significantly different (P < .05). Furthermore, we found using combined methods detected 2.42 ± 1.19 SLNs (range: 1–7), using radioisotope detected 2.07 ± 1.16 SLNs (range: 0–6), and using blue dye detected 1.47 ± 1.08 SLNs (range: 0–4). Then we described the location of the removed SLNs in detail (see Table 2 and Figure 1). The total number of removed SLNs was 1559, with the majority of them located at the roots of thoracic dorsal blood vessel or the fat surrounding the caudate lobe of the mammary gland. Of the remainder, a total of 247 (16.5%) SLNs were located at the latissimus dorsi lateral margin (n = 139, 9.6%) and level 2 (n = 108, 6.9%).
Table 2.
Axillary sentinel lymph node mapping.
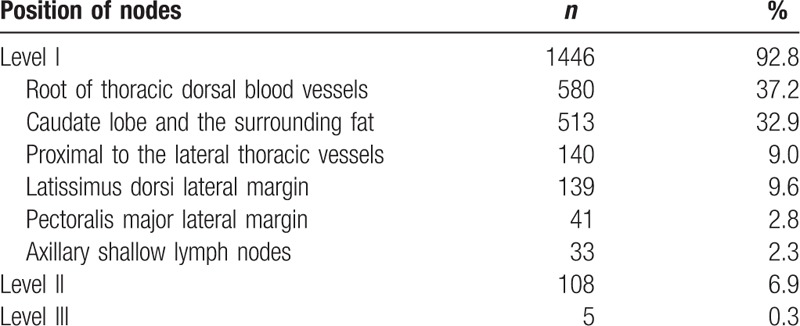
Figure 1.
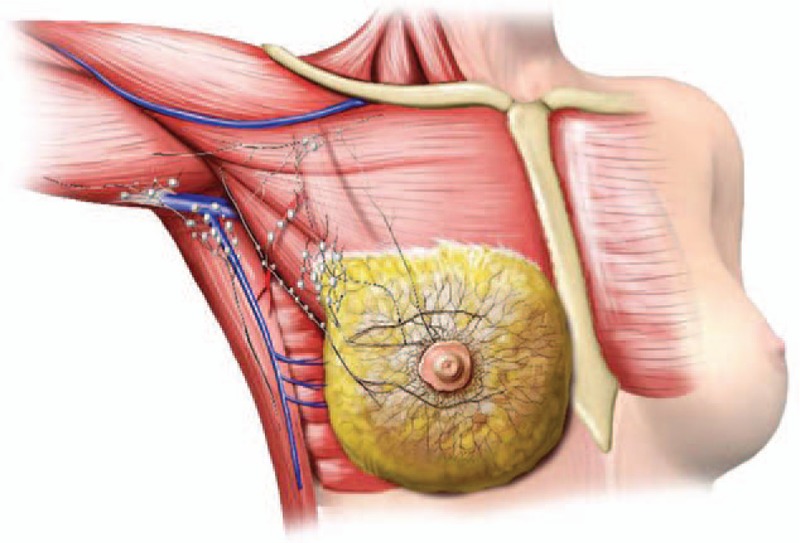
The distribution of sentinel lymph nodes in detail. A large node is equivalent to 10 small nodes.
3.3. Factors related to the latissimus dorsi lateral margin and level 2 distribution
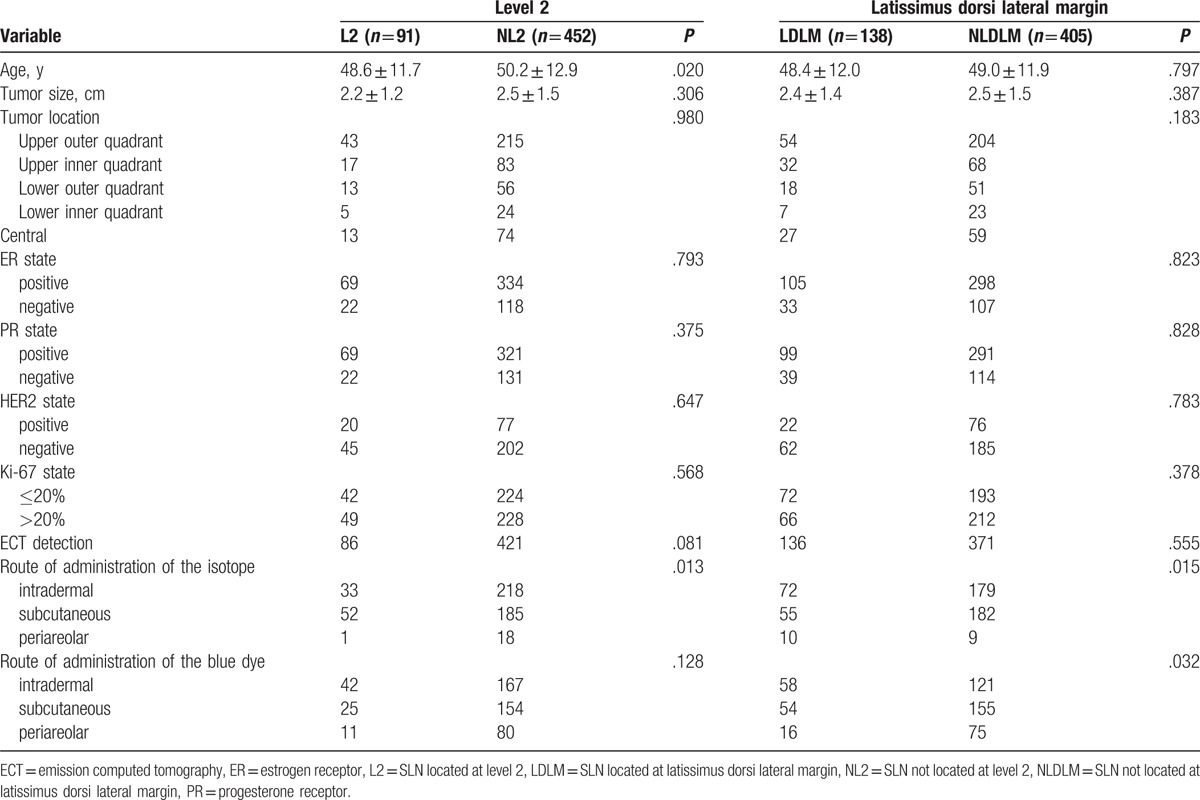
Because SLNs located at the latissimus dorsi lateral margin and level 2 were always overlooked by surgeons, then we further explored the factors related to SLNs located at these 2 anatomical positions. Results of the univariate analyses are shown in Table 3. Though univariate analyses results showed age (P = .020) and subcutaneous injection of the isotope (P = .013) were associated with SLNs distributed at level 2, intradermal injection of the isotope (P = .015) and blue dye (P = .032) were associated with SLNs distributed at the latissimus dorsi lateral margin, multivariate analysis results showed that subcutaneous injection of the isotope [P = .004; 95% confidence interval (CI) 0.335, 0.809] and intradermal injection of the blue dye (P = .002; 95% CI 1.185, 2.161) was the only independent factor associated with SLNs distributed at level 2 and the latissimus dorsi lateral margin, respectively. It was noteworthy that 2 of 7 patients had skipping metastasis in level 2. In addition, we found those patients with metastasis to the latissimus dorsi lateral margin nodes also could have metastasis to level 1 (expect for the latissimus dorsi lateral margin) nodes.
Table 3.
Univariate analysis of factors associated with SLN located at level 2 and the latissimus dorsi lateral margin.
4. Discussion
In recent decades, a majority of studies have been devoted for developing novel, consistent SLN tracing methods, to reduce the FNR of SLNB,[12] resulting in the currently used methods of tracing SLNs: blue dye,[13] radioisotope, the combination of blue dye and isotope,[14] fluorescent dye,[15,16] and nanoparticles.[17,18] Though the novel methods of lymphoscintigraphy confer their own unique advantages in reducing the FNR of SLNB in preclinical studies, their application in practice depends upon further prospective clinical studies to identify suitable protocols, and the uptake of such advanced methods might require some time.[19–21]
For now, to better reduce the FNR of SLNB, we should comprehensively analyze the related factors, to determine the most cost-effective and objective lymph node tracing methods. However, this is far from sufficient. We suspect that if inexperienced surgeons overlooked some SLNs during operations, the FNR of SLNB would increase correspondingly, such that mapping of the SLNs becomes necessary. However, we found only a few studies had described the distribution pattern of SLNs in axillary tissues.[22–24] For example, Clough et al[25] explored the distribution of SLNs in axillary tissues in 242 breast cancer patients, using blue dye or radioisotope to trace the SLNs. They found that the majority of SLNs were located at the medial part of the axilla, alongside the lateral thoracic vein.[25] However, another study showed SLNs were mainly found between the pectoralis major muscle and the lateral thoracic vein, but this study only used the anatomical method, so the false negative rate was up to 21.7%.[26] Though these studies had limitations, they all confirmed one fact that the distribution of SLNs was diversiform, so that if surgeons only gave special attention to certain areas, the FNR of SLNB would increase.[27]
In this study, we found that up to 9.6% and 6.9% of SLNs located at the latissimus dorsi lateral margin and level 2, respectively. Furthermore, by analyzing our SLN distribution map in comparison with other available maps, we found that the latissimus dorsi lateral margin and level 2 were frequently being overlooked by surgeons, so these 2 anatomical positions acquire more attentions from surgeons to reduce the FNR of SLNB. Multivariate analysis results further showed that subcutaneous injection of the isotope and intradermal injection of blue dye were related to SLNs located at level 2 and the latissimus dorsi lateral margin, respectively. To inform trainee and inexperienced surgeons of these positions, we suggest subcutaneous injection of the isotope to trace SLNs located at level 2, and intradermal injection of blue dye to trace SLNs located at the latissimus dorsi lateral margin.
Considering that excision of SLNs at the latissimus dorsi lateral margin may increase lymphedema of patients whose SLNs located at level 1 were negative, we further surprisingly found if a patient has metastasis to the latissimus dorsi lateral margin SLNs, she always has metastasis to SLNs in level 1 (expect for the latissimus dorsi lateral margin) at the same time. This result reminds us that maybe there is no need to excise SLNs at the latissimus dorsi lateral margin in SLNB. Because our study contains a relatively small number of patients, the decision regarding whether SLNs at the latissimus dorsi lateral should be excised still requires further large clinical studies.
In terms of SLNs in level 2, because we found 2 of 7 patients had skipping metastasis in level 2, so this part shouldn’t be overlooked. We hope surgeons will carefully consider the SLNs located in level 2 in the future.
5. Conclusion
Subcutaneous injection of the isotope is strongly recommended to trace SLNs distributed at level 2 because of the possibility of skipping metastasis. Intradermal injection of blue dye are superior methods for tracing SLNs located at the latissimus dorsi lateral margin; however, whether such SLNs should be removed still needs to be determined by further large multicentered clinical studies.
Footnotes
Abbreviations: 99mTc = technetium-99m, ALND = axillary lymph node dissection, ECT = emission computed tomography, ER = estrogen receptor, FISH = fluorescence in situ hybridisation, FNR = false-negative rate, Her2 = human epidermal growth factor receptor 2, IHC = cytokeratin immunohistochemistry, L2 = SLN located at level 2, LDLM = SLN located at latissimus dorsi lateral margin, NL2 = SLN not located at level 2, NLDLM = SLN not located at latissimus dorsi lateral margin, PR = progesterone receptor, SC = sulfur colloid, SLNB = sentinel lymph node biopsy, SLNs = sentinel lymph nodes.
YZ and ZD contributed equally to this study.
Contributors: ZYT, DZG, ZD, and LQ proposed the study concept and design; performed the research; and acquired the data. ZYT and DZG analyzed and interpreted the data, wrote the first draft, and provided a critical revision of the article with important intellectual content. LQ was the study supervisor.
The authors declare no conflicts of interest.
References
- [1].Dixon JM. Sentinel lymph node biopsy in breast cancer surgery. Ann Surg Oncol 2016;23:3426–8. [DOI] [PubMed] [Google Scholar]
- [2].Bao J, Donovan C, Chung A, et al. The staging value of sentinel lymph node biopsy for breast cancer: translating pathologic findings to clinical practice. Chin Clin Oncol 2016;5:36. [DOI] [PubMed] [Google Scholar]
- [3].Bao J, Giuliano AE. Sentinel lymph node biopsy: from B04 to tumor genomics. Ann Surg Oncol 2016;23:2380–2. [DOI] [PubMed] [Google Scholar]
- [4].Liu M, Wang S, Cui S, et al. The feasibility of the ACOSOG Z0011 Criteria to Chinese breast cancer patients: a multicenter study. Sci Rep 2015;5:15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beek MA, Verheuvel NC, Luiten EJ, et al. Two decades of axillary management in breast cancer. Br J Surg 2015;102:1658–64. [DOI] [PubMed] [Google Scholar]
- [6].Doscher ME, Schreiber JE, Weichman KE, et al. Update on post-mastectomy lymphedema management. Breast J 2016;22:553–60. [DOI] [PubMed] [Google Scholar]
- [7].Coromilas EJ, Wright JD, Huang Y, et al. Axillary evaluation and lymphedema in women with ductal carcinoma in situ. Breast Cancer Res Treat 2016;158:373–84. [DOI] [PubMed] [Google Scholar]
- [8].Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365–83. [DOI] [PubMed] [Google Scholar]
- [9].Jakobsen JK. Sentinel node biopsy in uro-oncology: a history of the development of a promising concept. Urol Oncol 2015;33:486–93. [DOI] [PubMed] [Google Scholar]
- [10].James TA, Coffman AR, Chagpar AB, et al. Troubleshooting sentinel lymph node biopsy in breast cancer surgery. Ann Surg Oncol 2016;23:3459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nieciecki M, Dobruch-Sobczak K, Wareluk P, et al. The role of ultrasound and lymphoscintigraphy in the assessment of axillary lymph nodes in patients with breast cancer. J Ultrason 2016;16:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nowikiewicz T, Kurylcio A, Polkowski W, et al. Imaging methods for the local lymphatic system of the axilla in early breast cancer in patients qualified for sentinel lymph node biopsy. Prz Menopauzalny 2016;15:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bakhtiar N, Jaleel F, Moosa FA, et al. Sentinel lymph node identification by blue dye in patients with breast carcinoma. Pak J Med Sci 2016;32:448–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].He PS, Li F, Li GH, et al. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer 2016;16:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Toh U, Iwakuma N, Mishima M, et al. Navigation surgery for intraoperative sentinel lymph node detection using Indocyanine green (ICG) fluorescence real-time imaging in breast cancer. Breast Cancer Res Treat 2015;153:337–44. [DOI] [PubMed] [Google Scholar]
- [16].Shinden Y, Ueo H, Tobo T, et al. Rapid diagnosis of lymph node metastasis in breast cancer using a new fluorescent method with gamma-glutamyl hydroxymethyl rhodamine green. Sci Rep 2016;6:27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu X, Lin Q, Chen G, et al. Sentinel lymph node detection using carbon nanoparticles in patients with early breast cancer. PLoS One 2015;10:e0135714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang X, Yang Z, Lin B, et al. Technetium-99m-labeled rituximab for use as a specific tracer of sentinel lymph node biopsy: a translational research study. Oncotarget 2016;7:38810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maguire A, Brogi E. Sentinel lymph nodes for breast carcinoma: an update on current practice. Histopathology 2016;68:152–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tallet A, Lambaudie E, Cohen M, et al. Locoregional treatment of early breast cancer with isolated tumor cells or micrometastases on sentinel lymph node biopsy. World J Clin Oncol 2016;7:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li CZ, Zhang P, Li RW, et al. Axillary lymph node dissection versus sentinel lymph node biopsy alone for early breast cancer with sentinel node metastasis: a meta-analysis. Eur J Surg Oncol 2015;41:958–66. [DOI] [PubMed] [Google Scholar]
- [22].Ngui NK, French J, Kilby CJ, et al. Axillary reverse mapping in patients with breast cancer: Is it oncologically safe? J Surg Oncol 2016;113:726–31. [DOI] [PubMed] [Google Scholar]
- [23].Tanaka T, Sato S, Tada A, et al. Prevalence and clinical significance of supra- or infraclavicular drainage on preoperative lymphoscintigraphy in women with breast cancer. Diagn Interv Imaging 2016;97:611–5. [DOI] [PubMed] [Google Scholar]
- [24].Han C, Yang B, Zuo WS, et al. The feasibility and oncological safety of axillary reverse mapping in patients with breast cancer: a systematic review and meta-analysis of prospective studies. PLoS One 2016;11:e0150285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clough KB, Nasr R, Nos C, et al. New anatomical classification of the axilla with implications for sentinel node biopsy. Br J Surg 2010;97:1659–65. [DOI] [PubMed] [Google Scholar]
- [26].Kang B, Jun H, Lee K, et al. Clinical application of sentinel lymph node biopsy based on axillary anatomy in breast cancer: a single institution experience. Breast 2014;23:812–5. [DOI] [PubMed] [Google Scholar]
- [27].Han C, Yang L, Zuo W. A mini-review on factors and countermeasures associated with false-negative sentinel lymph node biopsies in breast cancer. Chin J Cancer Res 2016;28:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


