Supplemental Digital Content is available in the text
Keywords: cancer, end-of-life care, hospice, quality indicators
Abstract
The aim of the study is to examine the effect of hospice care on quality of end-of-life (EOL) care for patients with advanced cancer in Taiwan between 2002 and 2011.
It is a population-based longitudinal study following National Health Insurance medical care claims of hospice and nonhospice patients with advanced cancer in their last month of life.
Utilization of hospice service doubled from 10.5% to 21.5% over the study period. Of 12,682 patients identified as having advanced cancer, 7975 (62.88%) were found to have 1 or more quality indicators (QIs) of poor EOL cancer care. After adjustments, those receiving hospice cares had a significant reduction in incidence of chemotherapy in the last 14 days of life as well as intensive care unit (ICU) admission and cardiopulmonary resuscitation (CPR) in the last month of life. The hospice care group also had significant increases in having more than 1 hospitalization and dying under hospital care, but no change in having more than 1 emergency room (ER) visit. The hospice group curve of estimated incidence rates of each QI was consistently below that of the nonhospice group in chemotherapy—with the difference between the 2 curves increasing over time—ICU admission, and CPR, and above that of the nonhospice group for dying in a hospital and having more than 1 hospitalization over the study period. The 2 groups overlapped on ER visits. Overall, hospice care was associated with less chance to have 1 or more QIs of EOL care for advanced cancer patients (RR = 0.56, 95% CI: 0.52–0.60, P < .001).
The utilization of hospice services doubled over the 10-year study period. Hospice care was associated with better EOL care in patients with advanced cancer.
1. Introduction
There were 14.1 million new cases of cancer and 8.2 million cancer deaths worldwide in 2012.[1] Although the diagnostic practices and treatments for various cancers have improved, mortality rates have not.[2] In Taiwan, cancer has been the leading cause of death since 1982, accounting for 28.4% of total deaths in 2012,[3] a rate similar to those reported for Canada (29.9%)[4] and the United States (23.3%).[5] Therefore, near end-of-life (EOL) care must be considered as an important phase of a cancer-treatment program.
With the goal of relieving the pain and suffering of terminally ill patients, interest in the hospice movement has gained momentum in recent decades. Hospice care in Taiwan has gradually progressed since 1983, where the first hospice ward was established in 1990.[6] In Taiwan, the hospice care system includes both inpatient hospice care, which predominates, and home hospice care. Both are covered by Taiwan's National Health Insurance (NHI) program. The utilization rate of hospice services has more than doubled; from 7.34% in 2000 to 16.83% in 2006.[7] In Western countries, patients receiving hospice care were reported to have greater satisfaction with this care—had better symptom control, and utilized fewer acute care services—than patients under conventional care.[8–12] However, the effect of hospice services on EOL cancer care in Taiwan is not well known.
There are 6 accepted and validated quality indicators (QIs) of EOL cancer care, each of them indicative of poor care quality. These QIs include receiving chemotherapy within 14 days of death, having more than 1 emergency room (ER) visit during the last month of life, being admitted to a hospital more than once during the last month of life, receiving care in an intensive care unit (ICU) during the last month of life, receiving cardiopulmonary resuscitation (CPR) during the last month of life, and dying in a hospital. These 6 QIs of EOL cancer care have already been adopted in the United States[13,14] and Canada.[15]
The hospice movement in Taiwan began in 1983.[6] Since then, its impact on patients with advanced cancers during EOL has been measured using these QIs. This study evaluates the effect of hospice services on these well-accepted QIs of EOL cancer care in a national representative cohort of patients with advanced cancer in Taiwan from 2002 to 2011. To do this, we utilized Taiwan's National Health Insurance Research Database (NHIRD) to collect information of medical care received in these patients during the last month of their lives.
2. Method
2.1. Study design and cohort selection
This was a population-based cohort study of all cancer patients who died in Taiwan between January 1, 2002 and December 31, 2011. Patients were excluded if they died within 30 days of cancer diagnosis, if they were younger than 20 years at the time of death, if they had no insurance claims in their last year of life, or if they had missing or inaccurate data (eg, their death date was earlier than their diagnosis date).
2.2. Data source and identification
The NHI program, implemented in Taiwan in 1995, has a unique database that covers all inpatient medical benefit claims, and includes data on approximately 99.9% of Taiwan's residents as of 2012. Moreover, the NHI has contracts with 97% of all medical providers in Taiwan.[16] Patient data were linked to Taiwan's 2000 Longitudinal Health Insurance Database (LHID2000), which contains all original claims data for 1,000,000 individuals randomly sampled from the NHIRD Registry in 2000. From this dataset, we extracted inpatient care and outpatient visit data collected from 1996 to 2011. Taiwan's Bureau of National Health Insurance verifies the accuracy of diagnosis by randomly interviewing and reviewing the charts of 1 claimant for every 100 ambulatory care claims and 1 per 20 inpatient claims.[17]
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes was used to identify the various types of cancer. These included lung cancer (162.0, 162.2, 162.3, 162.4, 162.5, 162.8, 162.9, 165.0, 165.8, and 165.9), liver cancer (155, 155.0, and 155.1), colorectal cancer (153.x–154.x), head and neck cancer (140.x–149.x, 160.xx, and 161.x), gastric cancer (151.x), breast cancer (174.x), esophageal cancer (150.x), prostate cancer (185), pancreatic cancer (157.x), hematologic malignancy (200.x–208.x), and cervical cancer (180.x). The remaining cancer types were classified into the category of “other” (140.x–239.x, except above codes). In Taiwan, patients with a definite cancer must be examined to receive what is known as a catastrophic illness certificate (CIC). Decedents in this study were identified by a record of death during the study period (2002–11). Patient comorbidity was assessed using the Charlson comorbidity index (CCI), computed by examining ICD-9-CM diagnosis and procedure codes recorded in the year before diagnosis following the Deyo method, and applied to inpatient and outpatients claims as described by Klabundle et al.[18–20]
2.3. Definitions of variables
2.3.1. QIs of EOL cancer care
This study followed 6 indicators of quality of EOL cancer care: receiving chemotherapy during the final 2 weeks of life, having more than 1 ER visit, being admitted to a hospital more than once, receiving care in an ICU during the final month of life, receiving CPR during the final month of life, and dying in a hospital. All indicators are considered indicative of poor quality care.
2.3.2. Hospice care group and nonhospice group
Patients with advanced cancer were classified as belonging to the hospice care group (H group) if they had ever received hospice care (inpatient or home hospice care) on their claim data. Patients with advanced cancer who had not received hospice care were classified as the nonhospice group (non-H group).
2.3.3. Dying in a hospital
A patient was classified as dying in a hospital if the date of discharge for the last admission was the same as the date of death.[21]
2.3.4. Socioeconomic status
Socioeconomic status (SES) is an important factor to consider in studies of health care utilization.[22,23] Following previous studies,[24,25] we classified SES into 3 groups: low, moderate, and high. Those earning less than US$ 571 per month were included in the low group, those earning between US$ 571 and US$ 1141 per month were included in the moderate group, and those earning more than US$ 1141 per month were included in the high group.
2.3.5. Urbanization
Urbanization levels, which were urban, suburban, and rural, were extracted from the postal codes used in the claims data.
This study was exempted from full ethical review by the Research Ethics Committee of the Buddhist Dalin Tzu Chi Hospital, Taiwan (No.B10301001). Informed consent was not needed as NHIRD files contained only de-identified secondary data.
2.4. Statistical analysis
Mean ± standard deviation (SD) and frequency (proportions) were used to summarize the sample characteristics. The distributional properties of continuous variables and categorical variables were compared between the hospice and nonhospice groups using the Wilcoxon rank-sum test and the Fisher exact test as appropriate. Survival was estimated using the Kaplan-Meier method. Trends in incidence rates of each QI in EOL care for both groups from 2002 to 2011 were analyzed using chi-squared trend test and fitted using Poisson regression models. The goodness-of-fit (GOF) of Poisson regression model was assessed using the deviance GOF test and the Nagelkerke R2 statistic. All statistical operations were performed using R statistical software (version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria). Two-sided P ≤ .05 was considered significant.
3. Results
3.1. Baseline characteristics
A total of 14,416 patients who died of cancer between 2002 and 2011 were included in this study. We excluded 800 patients who died within 30 days after cancer diagnosis, 24 patients younger than 20 years at the time of death, 654 patients with no insurance claims in their last year of life, and 256 patients with inaccurate or missing data, leaving us with a study cohort of 12,682 patients (Fig. 1). Of these, 7975 (62.88%) patients had at least 1 QI. A majority of patients were male (64.8%), belonged to a lower SES (69.3%), and lived in urban areas (51.9%) (Table 1). They had a mean CCI of 4.34 ± 3.99. Kaplan-Meier survival curves (Fig. 2) show that the median (mean) probability of survival after diagnosis was 0.91 (1.65) years for the non-H group and 1.07 (1.73) years for the H group (P = .021).
Figure 1.
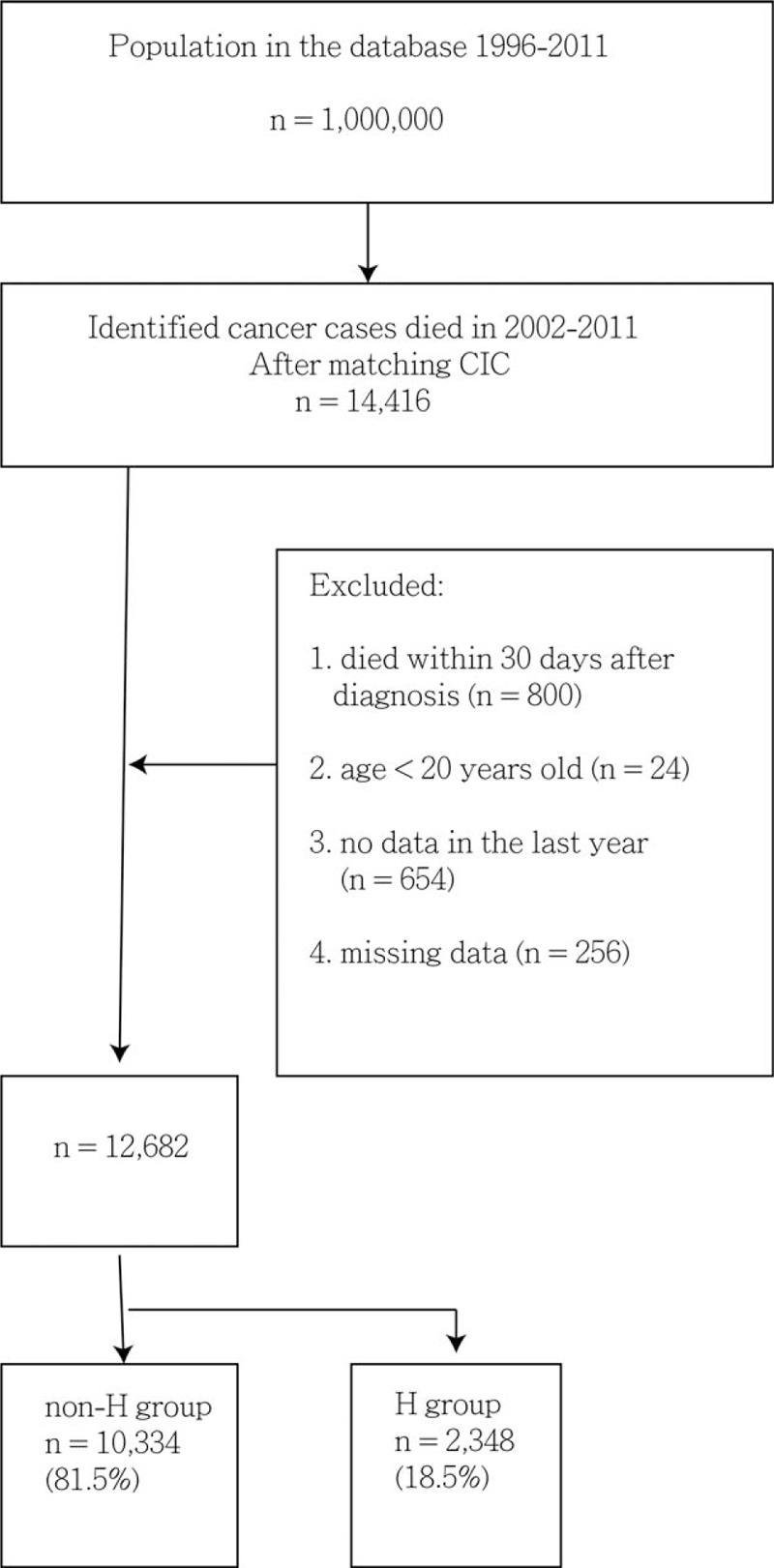
Study flow chart of patient selection. CIC = catastrophic illness certificate, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Table 1.
Baseline demographic and clinical characteristics of Taiwanese patients who had cancer-related death between 2002 and 2011.
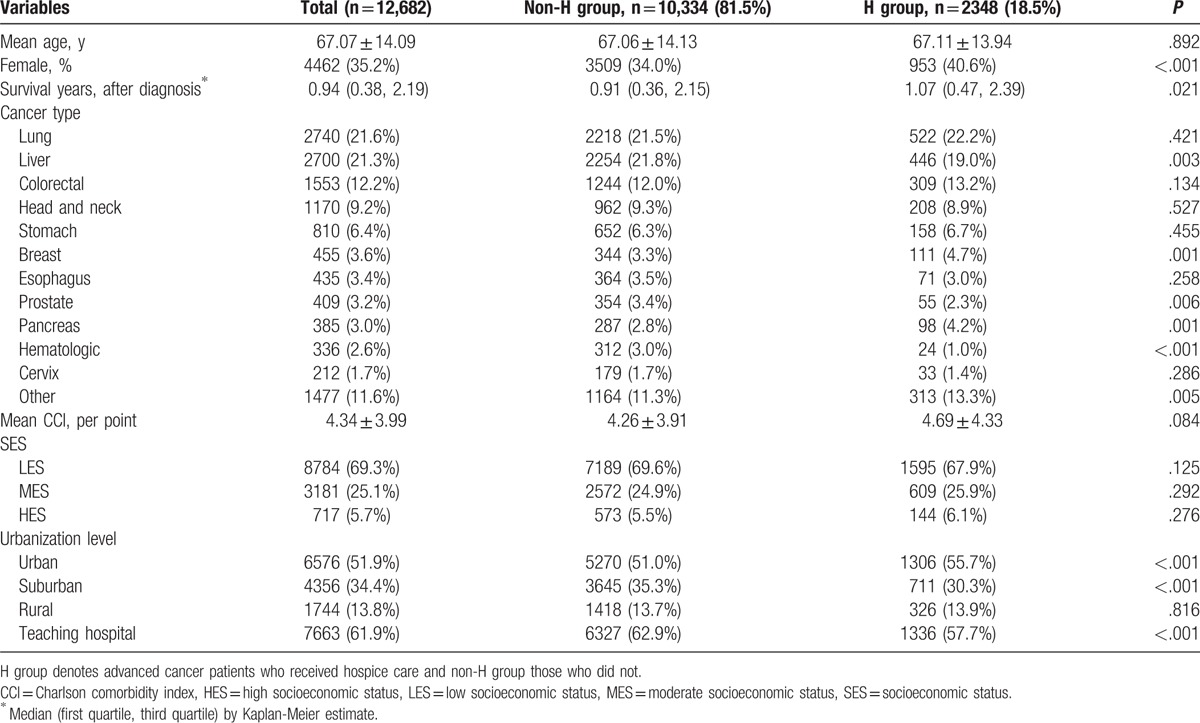
Figure 2.
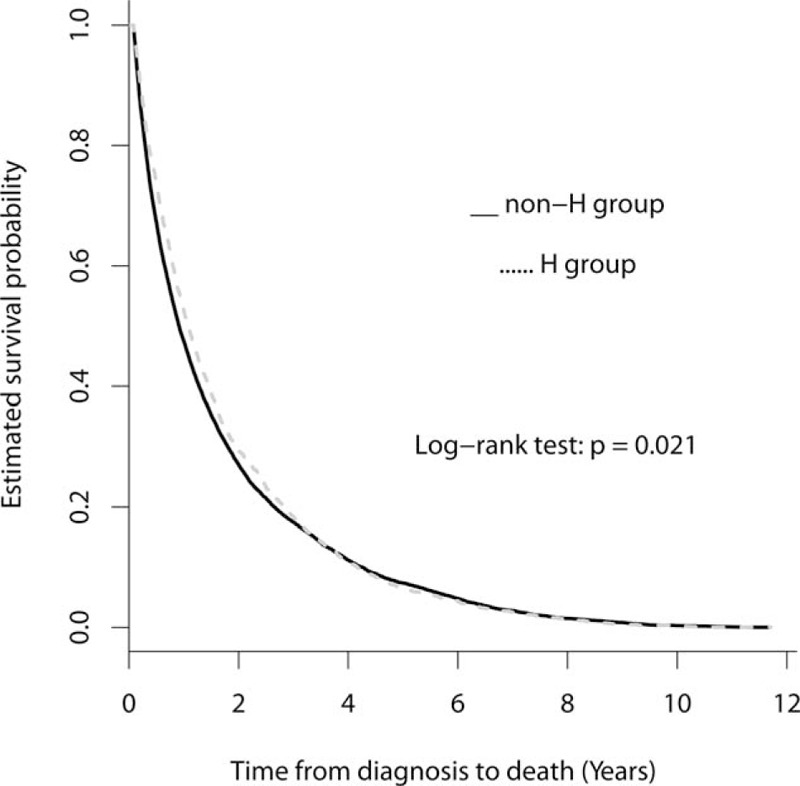
Kaplan-Meier estimates of survival curves for the non-H group and H groups. The median of survival probabilities in years after diagnosis for non-H group was 0.91 years, shorter than that for the H-group (1.07 years; P = .021).
3.2. Trends of incidence rates of the QIs in EOL cancer care over 2002–11
The highest rate for QIs was dying in a hospital, that had the average QI incidence (35.8%) over 10 years, followed by receiving CPR (24.5%), receiving hospice care (18.5%), having more than 1 hospitalization (18.3%), having at least 1 ICU admission (17.8%), having more than 1 ER visits within last month of life (17.1%), and receiving chemotherapy in the last 2 weeks of life (11.7%) (Table 2). The incidence rates of chemotherapy in the last 14 days of life increased from 4.8% in 2002 to 15.1% in 2011 (χ2 trend test, P < .001), having more than 1 ER visit in the last month of life from 8.9% in 2002 to 18.5% in 2011 (χ2 trend test, P < .001), and being admitted to a hospital more than once in the last month of life from 12.8% in 2002 to 19.8% in 2011 (χ2 trend test, P < .001), all significant. There was no significant increase in incidence of ICU visits in the last month of life (13.2% in 2002 to 16.9% in 2011; χ2 trend test, P = .189) or receiving CPR in the last month of life (20.8% in 2002 to 22.1% in 2011; χ2 trend test, P = .120). Incidence of dying in a hospital increased significantly from 20.4% in 2002 to 46.6% in 2011 (χ2 trend test, P < .001), as did having 1 or more QIs (47.5% in 2002 to 66.9% in 2011; χ2 trend test, P < .001). However, the proportion of those receiving hospice care also significantly increased from 10.5% in 2002 to 21.5% in 2011 (χ2 trend test, P < .001).
Table 2.
Incidence rates of the QIs in the EOL cancer care during 2002–11.

Poisson regression models were used to compare incidence rate trends of the H and non-H groups during 2002–11 (Fig. 3A–G). The supplement summarizes the estimated relative risks (RR), 95% confidence intervals (CI), and P values for the differences in trends (Table S1). After adjustment for the effects of the other covariates (including calendar years and the interaction terms between calendar years of the H group or non-H group), hospice care patients had a significant decrease in incidence of chemotherapy in the last 14 days of life (RR = 0.33, 95% CI: 0.27–0.41, P < .001), ICU admission (RR = 0.14, 95% CI: 0.10–0.18, P < .001), and CPR (RR = 0.16, 95% CI: 0.13–0.19, P < .001). Although there was not a significant increase in having more than 1 ER visit, there were significant increases in having more than 1 hospitalization (RR = 1.44, 95% CI: 1.30–1.59, P = .001) and dying in a hospital (RR = 1.39, 95% CI: 1.31–1.47, P < .001). Overall, hospice care was associated with less chance to have 1 or more QIs of EOL care for patients with advanced cancers (RR = 0.56, 95% CI: 0.52–0.60, P < .001) (Table 3).
Figure 3.
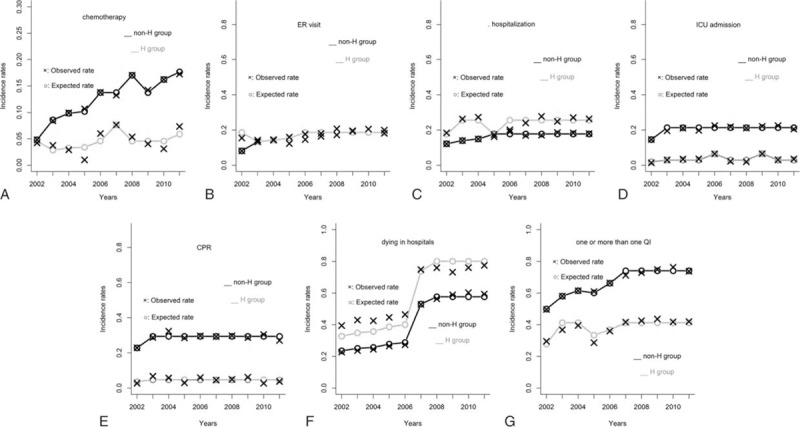
A–G. Incidence rates of different QIs over years stratified by hospice groups. The estimated curves of H group and non-H group overlapped from 2003 to 2011 (3B). × and ○ indicate the observed and estimated incidence rates, respectively. QI = quality indicator.
Table 3.
The analyses of incidence rates of the 6 QIs in EOL cancer care by fitting Poisson regression models over adjustments.
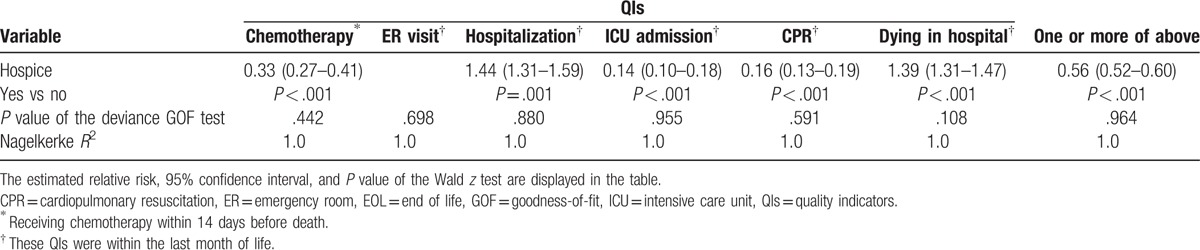
The effect of calendar year varied. For example, Figure 3 shows that compared with reference years 2002 and 2006–10, the incidence rate of chemotherapy significantly decreased in 2003 (RR = 0.62, 95% CI: 0.49–0.80, P < .001), 2004 (RR = 0.72, 95% CI: 0.58–0.89, P = .002), and 2005 (RR = 0.74, 95% CI: 0.60–0.91, P = .005), but increased in 2011 (RR = 1.29, 95% CI: 1.10–1.50, P = .001) after adjustment.
Finally, the interactive effects of calendar years and the H group or non-H group during 2002–11 also varied. Again, after adjusting for the effects of the other covariates, non-H patients had a significant decrease in incidence of chemotherapy in the last 14 days of life only in 2002 (RR = 0.35, 95% CI: 0.24–0.51, P < .001), but a significant increase in that QI in 2008 (RR = 1.24, 95% CI: 1.05–1.47, P = .012) and 2010 (RR = 1.18, 95% CI: 1.00–1.39, P = .046) (Fig. 3). Hospice patients, however, had a significant increase in incidence of the same QI only in 2007 (RR = 1.67, 95% CI: 1.03–2.71, P = .038) compared with 2003–06, 2009, and 2011.
Combining the estimated main effects of H group and calendar years, respectively, together with the estimated interactive effects of calendar years and the H group or non-H group, we found that the H and non-H groups had different incidence rate curves for each QI during 2002–11 (Fig. 3). For example, although each of the curves connecting the estimated incidence rates of chemotherapy for the H group and non-H group had its own vacillating variations during 2002–11, the H group curve was consistently below that of the non-H group and the distance between these 2 curves increased progressively from 2002 to 2011. The results for other QIs can also be interpreted in this manner.
4. Discussion
This longitudinal population-based cohort study of the effect of hospice care on quality of EOL cancer care in Taiwan found that the utilization rates of hospice services doubled from 10.5% to 21.5% during the 10-year period from 2002 to 2011. Another novel finding was that hospice care was associated with better EOL care in patients with advanced cancers. The study found a reduction in ICU care, CPR, and chemotherapy near EOL cancer care among hospice patients. These reductions may be related to the fact that on June 7, 2000 the government passed the Natural Death Act (Hospice-Palliative Care Act), which makes it possible for patients to forgo CPR, and made palliative hospice care legal for terminally ill patients.[26] Patients with advanced cancers or their families usually sign a do-not-resuscitate (DNR) form before receiving hospice care.
In this study, 11.7% of terminal cancer patients in the hospice group received chemotherapy in the last 2 weeks of life, which was lower than the 15% reported by earlier studies based on Medicare claims.[27,28] In the present study, there was a significant decrease in the estimated incidence rates of receiving chemotherapy over time among the hospice patients compared with those of the non-H group. The distance between these 2 curves increased progressively from 2002 to 2011, indicating better quality of care and life quality in hospice patients near EOL.
The average incidences of ICU admission and CPR were 17.8% and 24.5%, respectively. In the United States, being admitted to an ICU during the last month of life increased from 23.7% to 28.8% between 2003–07 and 2010.[29] In Canada, this value increased from 3.06% to 5.39% between 1993 and 2004.[2] Although these 2 indicators did not significantly increase over time in this study, hospice patients did see a significant reduction in ICU admission and CPR treatment after adjustments. The trends for ICU admission and CPR treatment slowed down after 2002, possibly because the Natural Death Act, which gives dying patients or their families the right to refuse unnecessary medical managements, was enacted in Taiwan in 2000. A previous study found improvements in medical staff decision making and quality of EOL care for the terminally ill in Taiwan after the Natural Death Act was enacted.[30]
For patients with advanced cancers and their families, ER visits could be distressing, disruptive, and exhausting.[31] Generally, frequent ER visits are considered an indicator of poor-quality cancer care. In this study, we found a trend of increasing ER-visit frequency in the last month of life among patients both with and without hospice care. Yet, the effect of palliative care interventions on reducing ER visits was not strongly substantiated.[32] However, understanding why patients were sent to ER near the EOL provided insight into the nature of the problems they experienced and the direction for possible interventions.[31] Timeliness in providing patients and their families appropriate symptom assessment and control might be a solution that warrants further research.
Previous studies have reported that patients receiving home hospice care had fewer hospitalizations in the last month of life.[33,34] However, the indicator of more than 1 hospitalization in the last month of life increased over time in this study, with an average incidence of 18.3%. After adjustments, hospice patients had a greater increase in incidence of having more than 1 hospitalization than those without hospice care. One reason for this was that terminal cancer patients were frequently hospitalized for acute problems and the treatments of symptoms.[15,21] Another possible reason for increased hospitalizations was proximity, as hospice wards are common in acute hospitals in Taiwan. Previous studies had shown that patients receiving hospice care often had been sicker than those without hospice care.[35] Although home hospice services have also been provided in Taiwan since 1995, patients receiving home hospice services still do not experience decreased trend of hospitalization in their last month of life. This might suggest the need for improvements in the quality of home hospice care.
The average rate of dying in a hospital was 35.8% in this study, which was found to increase significantly over time. The percentages of hospital death for cancer patients were 47% in South East England in 2002,[36] 40% in Canada in 2000,[37] 38.8% in Korea,[38] and 34.6% in Italy in 2000.[39] In the present study, hospice patients saw a significant increase in incidence of dying in a hospital, possibly due to increased access. Acute ward general beds increased from 69,572 in 2002 to 74,082 in 2011, with the number of hospice beds increasing from 272 beds to 692 beds over the same period of time.[40] Another possible explanation was the adoption of hospice shared care in Taiwan in 2005 to treat inpatient patients with advanced cancer in acute hospital.[41] This change may warrant investigation of the impact of hospice shared care on the life quality of dying patients and their families.
This study indicates that the life-sustaining treatments (eg, CPR and ICU admission) for patients with advanced cancer were decreasing. However, this study also shows that advanced cancer patients had more frequent ER visits, more hospitalizations, and a greater likelihood of dying in hospital. This fact might indicate that patients and their families had unmet needs. We recommend that future studies investigate how to improve the suffering and distress of advanced cancer patients during the EOL. We suggest that the indicators of ER visit frequency and hospitalizations in the last month of life and dying in a hospital might be integrated into the national hospital accreditation to monitor the quality of hospice care in Taiwan for advanced cancer patients.
Another solution for decreasing ER visits, decreasing hospitalizations, and decreasing likelihood of dying in hospital might be home-based EOL care. A previous systemic review reported that compared with usual care, home-based EOL care was associated with a 33% increased likelihood of dying at home, although with no definite conclusion about unplanned admission to hospital.[42] Our previous study reported that home hospice care gave patients with advanced lung cancer a 33.4% increased chance of dying at home and an 8-day decrease in hospital stays in the last month of life, compared with their counterparts with only inpatient hospice care.[43] Thus, an increase in home hospice care programs might increase the likelihood of dying at home and decrease the time spent in hospital in the last month of life for advanced cancer patients. With consideration of differences in culture and health care delivery, we suggest that the indicator of ER visit frequency and hospitalizations in the last month of life might be replaced by the indicator of hospital stay in the last month of life in Taiwan.
5. Limitations
Our study has some limitations. Previous studies have reported that QIs for cancer care include symptom control; information and care planning (eg, advanced directive or a surrogate decision maker); communication about chemotherapy; and psychosocial care.[44] However, data on these indicators are not found in insurance claims. Choice of EOL cancer care not only involves access to hospice but also involves patient and family attitudes about hospice and chemotherapy, as well as the relationship with their specialist physicians. The information about these attitudes is not included in insurance claims. Other confounders related to each QI (eg, clinical symptoms and signs, patient or family preferences, and DNR designation) are not recorded in insurance claims records. The information about subject's education level was not available in the NHIRD of Taiwan, which was inevitably a limitation of this study. Another limitation is that it is not possible for clinicians to accurately predict patient's survival in real-time; although clinicians often overestimate survival.[45] Another limitation is possible misclassification bias, as diagnoses in NHI claims primarily serve the purpose of administrative billing and do not undergo verification for scientific purposes.
6. Conclusions
Utilization of hospice services doubled over the 10-year study period. Hospice care was associated with better EOL care in patients with advanced cancer. Further work is still needed to better alleviate suffering and distress for advanced cancer patients and their families.
Supplementary Material
Footnotes
Abbreviations: CCI = Charlson comorbidity index, CIC = catastrophic illness certificate, CPR = cardiopulmonary resuscitation, DNR = do not resuscitate, EOL = end of life, ER = emergency room, ICU = intensive care unit, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, QI = quality indicator, SES = socioeconomic status.
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
JKC received research grants from Buddhist Dalin Tzu Chi Hospital (DTCRD104(2)-E-02).
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011;29:1587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ministry of Health and Welfare. Available at: http://www.mohw.gov.tw/cht/DOS/Statistic.aspx?f_list_no=312&fod_list_no=2747. Accessed on September 5, 2016. [Google Scholar]
- [4].Statistics Canada. Leading Causes of Death in Canada, 2011. Ottawa, Canada: Statistics Canada. [Google Scholar]
- [5].Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep 2013;62:1–96. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_06.pdf. Accessed on March 6, 2016. [PubMed] [Google Scholar]
- [6].Lai YL, Su WH. Palliative medicine and the hospice movement in Taiwan. Support Care Cancer 1997;5:348–50. [DOI] [PubMed] [Google Scholar]
- [7].Tang ST, Wu SC, Hung YN, et al. Trends in quality of end-of-life care for Taiwanese cancer patients who died in 2000–2006. Ann Oncol 2009;20:343–8. [DOI] [PubMed] [Google Scholar]
- [8].Hughes SL, Weaver FM, Giobbie-Hurder A, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA 2000;284:2877–85. [DOI] [PubMed] [Google Scholar]
- [9].Grande GE, Todd CJ, Barclay SI, et al. A randomized controlled trial of a hospital at home service for the terminally ill. Palliat Med 2000;14:375–85. [DOI] [PubMed] [Google Scholar]
- [10].Kane RL, Wales J, Bernstein L, et al. A randomised controlled trial of hospice care. Lancet 1984;1:890–4. [DOI] [PubMed] [Google Scholar]
- [11].Rabow MW, Dibble SL, Pantilat SZ, et al. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83–91. [DOI] [PubMed] [Google Scholar]
- [12].Jordhoy MS, Fayers P, Saltnes T, et al. A palliative-care intervention and death at home: a cluster randomised trial. Lancet 2000;356:888–93. [DOI] [PubMed] [Google Scholar]
- [13].Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005;17:505–9. [DOI] [PubMed] [Google Scholar]
- [14].Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 2008;26:3860–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barbera L, Paszat L, Chartier C. Indicators of poor quality end-of-life cancer care in Ontario. J Palliat Care 2006;22:12–7. [PubMed] [Google Scholar]
- [16].National Health Insurance Research Database. Available at: http://nhird.nhri.org.tw/date_01.html. Accessed on September 4, 2016. [Google Scholar]
- [17].Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 2004;27:1605–9. [DOI] [PubMed] [Google Scholar]
- [18].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [19].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [20].Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- [21].Barbera L, Paszat L, Qiu F. End-of-life care in lung cancer patients in Ontario: aggressiveness of care in the population and a description of hospital admissions. J Pain Symptom Manage 2008;35:267–74. [DOI] [PubMed] [Google Scholar]
- [22].Lemstra M, Mackenbach J, Neudorf C, et al. High health care utilization and costs associated with lower socio-economic status: results from a linked dataset. Can J Public Health 2009;100:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kangovi S, Barg FK, Carter T, et al. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood) 2013;32:1196–203. [DOI] [PubMed] [Google Scholar]
- [24].Lee CC, Su YC, Ho HC, et al. Risk of stroke in patients hospitalized for isolated vertigo: a four-year follow-up study. Stroke 2011;42:48–52. [DOI] [PubMed] [Google Scholar]
- [25].Chang CM, Huang KY, Hsu TW, et al. Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: a nationwide population-based study in Taiwan. PLoS One 2012;7:e40590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Laws & Regulations Database of the Republic of China. Available at: http://law.moj.gov.tw/Index.aspx. Accessed on September 7, 2016. [Google Scholar]
- [27].Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among medicare beneficiaries at the end of life. Ann Intern Med 2003;138:639–43. [DOI] [PubMed] [Google Scholar]
- [28].Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–21. [DOI] [PubMed] [Google Scholar]
- [29].Goodman DC, Morden NE, Chang H, et al. Trends in Cancer Care Near the End of Life A Dartmouth Atlas of Health Care Brief. Available at: http://www.dartmouthatlas.org/downloads/reports/Cancer_brief_090413.pdf. Accessed on September 7, 2016. [PubMed] [Google Scholar]
- [30].Chiu TY, Hu WY, Huang HL, et al. Prevailing ethical dilemmas in terminal care for patients with cancer in Taiwan. J Clin Oncol 2009;27:3964–8. [DOI] [PubMed] [Google Scholar]
- [31].Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ 2010;182:563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].DiMartino LD, Weiner BJ, Mayer DK, et al. Do palliative care interventions reduce emergency department visits among patients with cancer at the end of life? A systematic review. J Palliat Med 2014;17:1384–99. [DOI] [PubMed] [Google Scholar]
- [33].Riolfi M, Buja A, Zanardo C, et al. Effectiveness of palliative home-care services in reducing hospital admissions and determinants of hospitalization for terminally ill patients followed up by a palliative home-care team: a retrospective cohort study. Palliat Med 2014;28:403–11. [DOI] [PubMed] [Google Scholar]
- [34].Henson LA, Gao W, Higginson IJ, et al. Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta-analysis. J Clin Oncol 2015;33:370–6. [DOI] [PubMed] [Google Scholar]
- [35].Chiang JK, Kao YH, Lai NS. The impact of hospice care on survival and healthcare costs for patients with lung cancer: A National Longitudinal Population-Based Study in Taiwan. PLoS One 2015;10:e0138773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Davies E, Linklater KM, Jack RH, et al. How is place of death from cancer changing and what affects it? Analysis of cancer registration and service data. Br J Cancer 2006;95:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Neutel CI, Bishop ML, Harper SD, et al. Proportion of cancer deaths occurring in hospital, Canada, 1994–2000. Can J Public Health 2005;96:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yun YH, Lim MK, Choi KS, et al. Predictors associated with the place of death in a country with increasing hospital deaths. Palliat Med 2006;20:455–61. [DOI] [PubMed] [Google Scholar]
- [39].Beccaro M, Costantini M, Giorgi Rossi P, et al. Actual and preferred place of death of cancer patients. Results from the Italian survey of the dying of cancer (ISDOC). J Epidemiol Community Health 2006;60:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ministry of Health and Welfare, Statistics. Available at: http://www.mohw.gov.tw/cht/DOS/DM1.aspx?f_list_no=557&fod_list_no=4173. Accessed on September 7, 2016. [Google Scholar]
- [41].Lin WY, Chiu TY, Ho CT, et al. Hospice shared-care saved medical expenditure and reduced the likelihood of intensive medical utilization among advanced cancer patients in Taiwan-a nationwide survey. Support Care Cancer 2014;22:1907–14. [DOI] [PubMed] [Google Scholar]
- [42].Shepperd S, Goncalves-Bradley DC, Straus SE, et al. Hospital at home: home-based end-of-life care. Cochrane Database Syst Rev 2016;2:CD009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chiang JK, Kao YH. Impact of home hospice care on patients with advanced lung cancer: A Longitudinal Population-Based Study in Taiwan. J Palliat Med 2016;19:380–6. [DOI] [PubMed] [Google Scholar]
- [44].Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med 2011;14:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gwilliam B, Keeley V, Todd C, et al. Prognosticating in patients with advanced cancer—observational study comparing the accuracy of clinicians’ and patients’ estimates of survival. Ann Oncol 2012;24:482–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


