Abstract
Computer tomography-guided transthoracic needle aspiration (CT-TTNA) is a minimally invasive technique for sampling peripheral lung lesions. Radial endobronchial ultrasound-guided transbronchial biopsy (rEBUS-TBB) is an alternative. The present study analyzed and compared rEBUS-TBB and CT-TTNA in the diagnosis of peripheral pulmonary lesions (PPL).
Clinical data of 513 patients with PPL who underwent an rEBUS-TBB or CT-TTNA examination were analyzed retrospectively. The positive diagnostic rate, complication rate, and influencing factors of the 2 methods were compared.
The positive diagnostic rate and complication rate were significantly higher in CT-TTNA than rEBUS-TBB (P = .001; P < .001, respectively). The rEBUS-TBB group showed a higher positive diagnostic rate in larger lesions (>2 cm) than in smaller (≤2 cm) (P = .012), and was lower in the lesions proximal to the chest wall than those distally located (P = .046); no significant difference was observed in the different pulmonary segments (P = .109). In the CT-TTNA group, the positive diagnostic rate in larger lesions did not differ significantly than the smaller lesions (P = .05); it differed significantly in different segments (P = .044). The incidence of pneumothorax was lower in lesions proximal to the chest wall than those located distally (P = .037). In the rEBUS-TBB group, the success rate of the exploration and biopsy of the lesions was 87.4%; the rate of exploration of larger lesions and with bronchial sign was higher than smaller lesions and without bronchial sign (P < .001; P < .001, respectively) while that of lesions close to the chest wall was lower than those distally located (P = .006).
rEBUS-TBB and CT-TTNA are effective and safe in the diagnosis of PPL. The positive diagnostic rate of CT-TTNA is higher than rEBUS-TBB. The incidence of pneumothorax in CT-TTNA is higher than rEBUS-TBB. CT-TTNA is selected for smaller lesions close to the chest wall; rEBUS-TBB is used for lesions larger, distal from the chest wall or with a bronchial sign.
Keywords: biopsy, computed tomography, diagnosis, needle aspiration, radial endobronchial ultrasound, the peripheral pulmonary lesion
1. Introduction
Peripheral pulmonary lesions (PPL) are defined as lesions that are located in the subsegmental bronchi and cannot be visualized by bronchoscopy.[1,2] However, owing to the wide usage of computed tomography (CT), the discovery of PPL has greatly increased. Some of these lesions are benign (infections, inflammatory, vascular) that require drug therapy or follow-up while others are malignant tumors necessitating surgical resection or chemotherapy. Therefore, it is crucial to identify the nature of the lesions with respect to the management of these patients. A safe and efficient approach for the identification of the nature of PPL is challenging for the clinicians. Transbronchial biopsy (TBB) and CT-guided transthoracic needle aspiration (CT-TTNA) are the 2 commonly used methods for the diagnosis of PLL. The value of CT-TTNA in the diagnosis of PPL has been fully recognized for its simple operating and high diagnostic yield, with an accuracy of 76.4% to 97%.[3,4] However, the disadvantages of CT-TTNA are as follows: high incidence of adverse events (especially, pneumothorax),[5] radiation exposure, and occupation of the CT room for a prolonged period. Transbronchoscopy biopsy under x-ray is no longer recommended due to its low diagnostic accuracy.[6] In recent years, with the development of fluorescent bronchoscopy, endobronchial ultrasound (EBUS), and electromagnetic navigation technology, the positive diagnosis rate of TBB for PPL has greatly improved, especially, the rEBUS-TBB.[7] It has created a new era for the diagnosis of PPL with transbronchoscopy and considerably improved the diagnostic accuracy.[5,8–12] Nevertheless, the optimal choice for a safe and effective lesion sampling for pathological examination is yet to be defined. Therefore, we designed a retrospective study to evaluate the 2 methods for optimal choice of the diagnosis of PPL.
2. Methods
2.1. Patients
The study included 513 patients with PPL who underwent rEBUS-TBB or CT-TTNA examination in the Third Affiliated Hospital of Soochow University between October 2013 and December 2015. The inclusion criteria for the study were as follows: complete clinical and imaging data, pulmonary lesions meeting the PPL standards, and signed the informed consent for the clinical operation. The exclusion criteria consisted of incomplete information, non-peripheral lesions, ground glass nodules, and not signed the informed consent for the clinical operation. The Ethics Committee of the Changzhou First People's Hospital (Jiangsu, China) approved this retrospective study.
2.2. Definition of PPL
All cases were scanned by CT, which showed that the lesion was located in the subsegmental bronchi. All the patients who underwent rEBUS-TBB examination were those whose lesions were not visible by bronchoscopy.[1,2]
2.3. rEBUS-TBB Methods
Thin layer CT was performed to identify the target bronchi where the lesions were localized.[8] The throat of the patient was anesthetized with 2% lidocaine via oxygen jet atomization, placed on the examination table in a supine position with oxygen inhalation and monitoring of pulse oxygen. The operation was performed using an OlympusBF-P260F (Olympus, Japan) bronchoscope. First, the bronchoscopist observed the central airway, lobar bronchi, segmental bronchi, and subsegmental bronchi, and no lesion was present within the endoscopically visible bronchi. Second, the bronchoscope was made to reach the predetermined target bronchus, the ultrasonic probe (UM-S20-17S; Olympus, Japan) inserted via biopsy channel until resistance was exerted, the ultrasound opened, and then the probe was withdrawn slowly while observing the screen image until the discovery of the typical image of lesion followed by the detection of adjacent bronchi in order to define the best location. The depth of the ultrasonic probe was marked before withdrawing the probe. Subsequently, the biopsy forceps were inserted through the biopsy channel to the required length and then opened, entering furthermore to approximately 1 to 2 mm depth, and then the assembly is clipped. The above process is repeated to obtain 5 to 6 specimens, and brush cytology is performed for pathological and cytological examinations. Biopsy materials were fixed in 10% formalin solution pathologically examined. In the case of bleeding, dilute adrenaline and cold physiological saline were used for local hemostasis. After the confirmation of the absence of active bleeding, the bronchoscopy is ended. Chest radiography was performed 2 to 4 hours after the biopsy. If any typical lesion was not detected after 30 minutes, we considered it as no lesions found by ultrasound, and the operation was ceased. The adverse events during and after the operation, such as bleeding, chest pain, low oxygen, and postoperative infection were recorded.
2.4. CT-TTNA Methods
The CT-TTNA procedure was as follows[11]: according to the location of the lesion observed in the CT, the accurate position is chosen to perform a routine CT scan in order to determine the puncture point, the direction of the needle, and the depth of the needle for making a mark on the patient's skin. The puncture site was disinfected routinely with sterile towels, and layer by layer infiltrated by anesthesia with 2% lidocaine. The patient was required to hold the breath, and the needle (Tsk TOPCUT [MGN] biopsy needle, TSK Laboratory, Japan) was rapidly inserted into the lesion and retained at the predetermined angle and depth. The patient was required to breathe calmly before undergoing another CT scan to confirm that the tip of the needle was in the target lesion. Then, the operator fixed the needle core to the biopsy gun (PAJUNK, TSK Laboratory), opened the gun's insurance, squeezed the trigger, and withdrew the needle rapidly after an aspiration was obtained. The specimen was fixed in 10% formalin solution for pathological examination. The above process was repeated if necessary. Another CT scan was essential to assess the occurrence of complications such as pneumothorax and hemorrhage after the patient rested for 5 minutes.
2.5. Result determination
The specimens obtained from rEBUS-TBB or CT-TTNA were diagnosed by 2 pathologists (at least 1 was an associate chief pathologist). Those with a clear diagnosis of pathological examination were considered to be positive, and benign results were followed-up for 6 months. Those benign results such as “inflammation, necrosis” without followed-up data, were judged “not sure”. Chronic mucosal inflammation and fibroplasia were considered negative, as well as results such as “chronic inflammation” which was indicated the presence of other diseases by different methods of biopsy or after 1 to 6 months of follow-up, and other rare cases that cannot be identified.
2.6. Complications
Pneumothorax, hemoptysis, fever, pectoralgia, pleural reaction, low oxygen, respiratory failure, and death occurred in the patients after operation.
2.7. Analysis of influencing factors
The characteristics of lesions in CT were analyzed, including the relationship between the size, location, distance from the chest wall, existence of bronchial sign, positive rate, and complications. The size referred to the longest lesion diameter (LLD). The location indicated the pulmonary segment in which the lesion occurred. The distance from the chest wall described the distance from the exterior margin of the lesion to the chest wall in the layer of LLD (Dt). The bronchial sign indicated that there was bronchus directly entering the lesion or bronchography could be visualized in the lesion on the CT scan.
2.8. Statistical methods
The data were entered into a database and analyzed using SPSS statistical software package (SPSS 19.0, IBM, New York). The measurement data were expressed as means ± standard deviations. The numerical data were expressed as rates. The rates were compared using the chi-square test. P < .05 was considered statistically significant.
3. Results
From October 2013 to December 2015, 335 patients underwent rEBUS-TBB for the evaluation of lung lesions. Seventy five patients fulfilled the exclusion criteria, and thus, the remaining 260 patients were included in the study.
Consecutively, 434 patients underwent CT-TTNA, of which, 181 patients were excluded. Thus, 253 cases were included in the present study.
3.1. General data
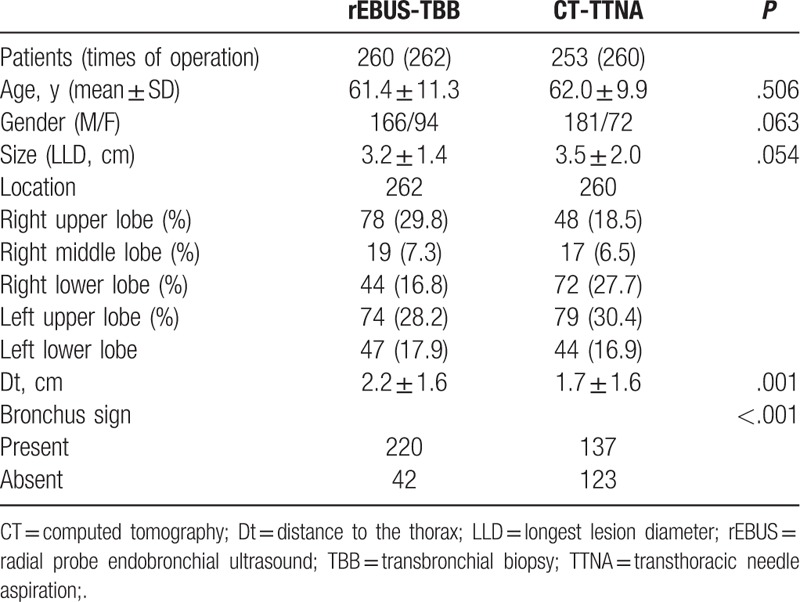
Five hundred thirteen cases were enrolled in the study, including 347 men and 166 women, aged 19 to 85 (mean, 62 ± 11) years.
3.1.1. Group rEBUS-TBB
Two hundred sixty cases were detected by ultrasound in 262 parts. Of these, 166 were men and 94 women, with a mean age 61.4 ± 11.3 (range, 31–82) years.
3.1.2. Group CT-TTNA
Two hundrd fifty three patients underwent 260 times of puncture; 181 were men and 72 women, with a mean age 62.0 ± 9.9 (range, 19–85) years (Table 1).
Table 1.
General data of the patients.
3.2. Diagnosis
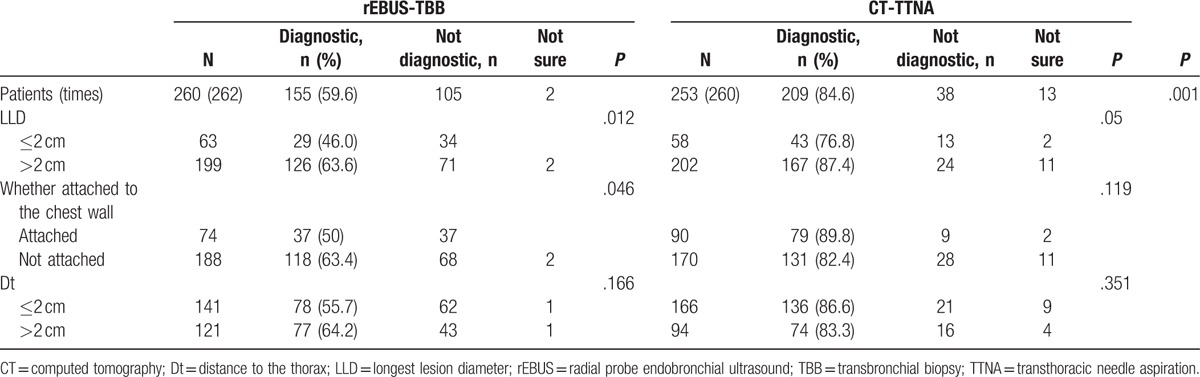
In the EBUS-TBB group, 226 patients’ lesions were detected and the specimens were obtained successfully. On the other hand, 34 patients’ lesions could not be detected. Also, 2 patients’ lesions were detected, but the specimens could not be acquired. The success rate of exploration and biopsy was 86.3%. One hundred fifty five cases were diagnosed while 69 cases were negative. Two cases were such whose diagnosis was undetermined with the positive diagnostic rate of 59.6% (155/260), of which the biopsy positive rate was 68.6% (155/226). In the CT-TTNA group, the results were positive in 209 cases while 13 cases had an undetermined diagnosis, and the positive diagnostic rate was 84.6% (209/247). Statistically significant differences were observed in the positive diagnostic rate of the 2 groups (P < .001) (Table 2).
Table 2.
The influencing factors of positive diagnostic yield.
3.3. Complications
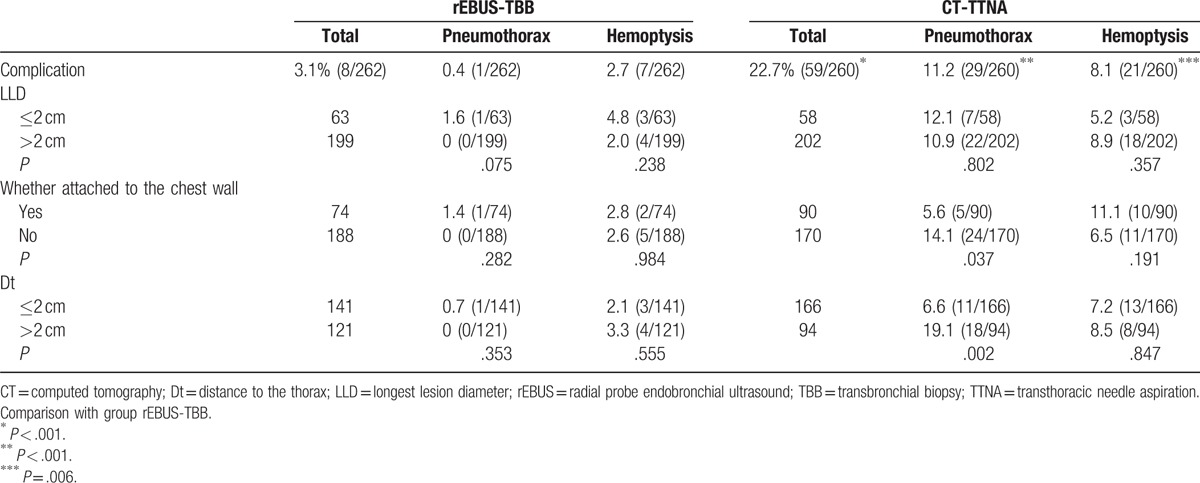
In the EBUS-TBB group, 15 cases were bleeding over 50 mL during the operation, and the bleeding ceased gradually after the injection of adrenaline and ice saline. Light hemoptysis after the operation was observed in 7 patients; 3 were administered the hemostatic treatment. Pectoralgia was noted in 6 cases during the operation. Of these, it was released in 5 cases after the operation and persisted in 1 case, which was considered as pyopneumothorax and diagnosed as pulmonary abscess after the surgery. No hypoxia or respiratory failure was noted. The incidence of postoperative complications was 3.1% (8/262), and the incidence of a complication requiring treatment was 1.5% (4/262). No serious complication occurred.
In the CT-TTNA group: The postoperative hemoptysis was noted in 21 cases. Of these, the majority were light and not severe, and only 1 patient was administered the hemostatic treatment due to excess hemoptysis. Pneumothorax was noted in 29 cases, of whom 11 required intercostals catheter drainage, fever was recorded in 4 cases, pectoralgia was noted in 4 cases, and pleural reaction in 1 case. The total incidence of complications was 22.7% (59/260), and the incidence of complications requiring treatment was 6.9% (18/260). All the complications recovered after the treatment and no severe, life-threatening complication occurred. The incidence of complications was significantly different between the 2 groups (P < .001) (Table 3).
Table 3.
The influencing factors of complications.
3.4. Influencing factors of positive diagnostic yield and complications
3.4.1. Size of the lesion
The EBUS-TBB group comprised of 199 patients with LLD >2 cm. Of these, 126 patients were diagnosed by EBUS-TBB, and 2 patients’ result could not be judged, with the positive diagnostic yield of 64.0% (126/197). Sixty three patients presented LLD ≤2 cm, of which, 29 were diagnosed using EBUS-TBB, with the positive diagnostic yield of 46.0% (29/63). The difference between the 2 groups was statistically significant (P = .012). The incidence of pneumothorax and hemoptysis did not differ significantly in the 2 groups (P = .075; P = .238).
The CT-TTNA group consisted of 202 patients with LLD >2 cm. Of these, 167 patients were diagnosed as positive by CT-TTNA, 24 patients were diagnosed as negative, and 11 patients’ result could not be judged, with the positive diagnostic yield of 87.4% (167/191). There were 58 patients with LLD ≤2 cm, of which, 43 were diagnosed as positive, 13 as negative, and 2 patients’ result could not be judged, with the positive diagnostic yield of 76.8% (43/56). The difference between the 2 groups was not statistically significant (P = .05). The incidence of pneumothorax and hemoptysis was not significantly different between the 2 groups (P = .802; P = .357) Tables 2 and 3.
3.5. Location of the lesion
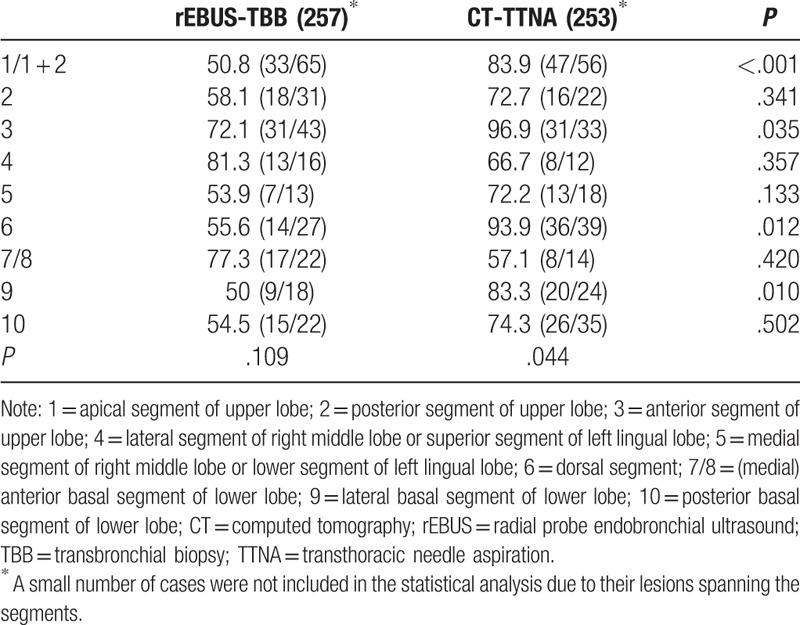
With respect to the lesion site, in the rEBUS-TBB group, the positive diagnostic yield did not differ significantly in different pulmonary segments (P = .109). In the CT-TTNA group, the positive diagnostic yield showed significant difference in different pulmonary segments (P = .044); the diagnostic yield of the anterior segment of the upper lobe and dorsal segment of the lower lobe was at a high level while the diagnostic yield was lower in the (medial) anterior basal segment and posterior segment of the lower lobe. In the apical and anterior segments of the upper lobe and the dorsal and lateral basal segments of the lower lobe, the positive diagnostic yield of CT-TTNA was higher than rEBUS-TBB. There was no significant difference in the positive diagnostic rate in other pulmonary segments (Table 4).
Table 4.
The positive diagnostic yield of different segments in rEBUS-TBB and CT-TTNA.
3.6. Whether the lesion attached to the chest wall
In the rEBUS-TBB group, the positive diagnostic accuracy of lesions attaching the chest wall was lower than that of those distal from the chest wall. The difference between the 2 groups was statistically significant (P = .046). The incidence of pneumothorax and hemoptysis had no significant difference between the 2 groups (P = .282; P = .984).
In the CT-TTNA group, the positive diagnostic accuracy did not show a statistical difference with respect to the lesion attachment to the chest wall (P = .119). The incidence of pneumothorax of lesions attaching the chest wall was lower than those not attaching with a significant difference (P = .037). The incidence of hemoptysis showed no significant difference between the 2 groups (P = .191) (Tables 2 and 3).
3.7. Distance from the lesion to the chest wall (Dt)
In the rEBUS-TBB group, whether the Dt was ≤2 cm or >2 cm, the positive rate of diagnosis and the incidence of pneumothorax and hemoptysis did not differ significantly. In the CT-TTNA group, the incidence of pneumothorax in patients whose Dt ≤2 cm was lower than those with Dt >2 cm, did not show a significant difference in the positive diagnostic yield and the incidence of hemoptysis (Tables 2 and 3).
3.8. Influencing factors of the success rate of ultrasound exploration and biopsy
3.8.1. Size of the lesion
One hundred niety nine cases presented LLD >2 cm (Table 5). Of these, the exploration and biopsy succeeded in 185 cases, and the lesions were not detected in 14 cases, with the success rate of 93% (185/199). There were 63 cases with LLD ≤2 cm. Of these, the exploration and biopsy succeeded in 45 cases, and the lesions were not detected in 18 cases, with the success rate of 71.4% (45/63). A significant difference was observed between the 2 groups (P = .001).
Table 5.
The influencing factors of the success rate of ultrasound exploration and biopsy.
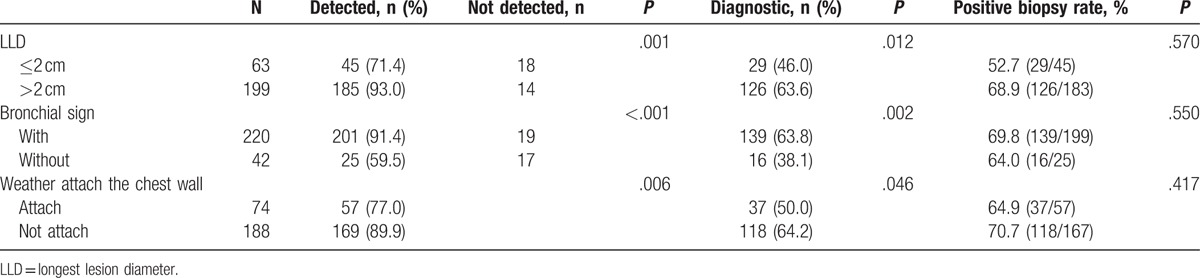
3.8.2. Bronchial sign
A total of 220 cases presented the bronchial sign. Of these, the lesions were detected, and biopsy performed successfully in 201 cases. The lesions could not be detected in 19 cases, with the success rate of 91.4% (201/220), the positive diagnostic yield of 63.8% (139/218), and the positive biopsy accuracy of 69.8% (139/199). There were 42 cases without the bronchial sign. Of these, the lesions were detected and biopsy performed successfully in 25 cases. The lesions could not be detected in 17 cases, with the success rate of 59.5% (25/42), the positive diagnostic yield of 38.1% (16/42), and the positive biopsy accuracy of 64.0% (16/25). Significant differences were observed in the success rate of exploration and biopsy between the 2 groups (P < .001). Also, significant differences were noted in the diagnostic yield (P = .002) while the positive biopsy rate did not differ remarkably between the 2 groups (P = .550).
3.8.3. Distance between the lesion and the chest wall (Dt)
Seventy four patients’ lesions were close to the chest wall. Of these, 57 patients’ lesions were detected in whom the biopsy was performed successfully, and 17 patients’ lesions were not detected, with a success rate of 77.0% (57/74) and a positive biopsy rate of 64.9% (37/57). One hundred eighty eight patients’ lesions were not close to the chest wall. Of these, 169 patients’ lesions were detected and subjected to a successful biopsy, and 19 patients’ lesions were not detected, with a success rate of 89.9% (169/188) and a positive biopsy rate of 70.7% (118/167). Significant differences were observed in the success rate of exploration and biopsy between the 2 groups (P = .006) while the positive biopsy rate did not show a significant difference (P = .417).
4. Discussion
The present study shows that both rEBUS-TBB and CT-TTNA have certain value in the diagnosis of PPL, with the diagnostic yield of 84.6% and 59.6%, respectively, which is similar to previous studies.[9,13,14] The positive diagnostic yield was significantly higher in CT-TTNA than in rEBUS-TBB. This could be attributed to the mechanism that in CT-TTNA, the biopsy is performed after the confirmation by CT scan that the puncture needle tip is inside the lesion and the specimens in CT-TTNA are larger than those in rEBUS-TBB. The rEBUS-TBB is not a real-time guided biopsy. Thus, we cannot completely guarantee that the biopsy site is the location detected by the ultrasound, and the specimens are relatively small, especially, from smaller lesions. All these make the diagnostic yield in CT-TTNA higher than in rEBUS-TBB.
Pneumothorax and hemorrhage are the main complications in CT-TTNA and rEBUS-TBB. In this study, the incidences of pneumothorax and hemorrhage in CT-TTNA were 11.2% and 8.1% while those in rEBUS-TBB were 0.4% and 2.7%. The incidences of complications were higher in the CT-TTNA group than in the rEBUS-TBB group, especially, the incidence of pneumothorax, and there was a significant difference between the 2 groups. A significant difference was not observed in the incidence of hemoptysis between the 2 groups. Also, no severe life-threatening complications occurred in the 2 groups. These findings were similar to the previous studies.[9,13] In CT-TTNA, the puncture needle crosses through the lung tissue and needs to be adjusted several times if it is not positioned accurately in the first attempt, which causes a significant increase in the incidence of pneumothorax. In rEBUS-TBB, the biopsy is performed through the bronchi without touching the lung tissue. Thus, pneumothorax rarely occurs in rEBUS-TBB. In this study, pneumothorax occurred in 1 patient in the rEBUS-TBB group, and its pathological result was striated muscle and fibrous tissue, which was diagnosed as pulmonary abscess after the surgery. It might be plausible that the bronchoscope slipped to the distance, crossed through the lesion, and penetrated the visceral pleura to the chest wall while performing the biopsy. Subsequently, the bronchoscope was fixed by an assistant in the entrance of the nose in case it slipped, and then, no pneumothorax occurred later.
Further analysis showed that in the CT-TTNA group, the positive biopsy rate was not related to the lesion size; nevertheless, it was related to the segments where lesions were localized irrespective of the distance between the lesion and the chest wall. The positive biopsy rate was higher in the anterior segment of the upper lobe and dorsal segment of the lower lobe, whereas it was lower in the anterior (medial) basal segment of the lower lobe and posterior segment of the upper lobe. The incidence of pneumothorax was related to the distance between the lesion and the chest wall. The incidence of pneumothorax in lesions close to the chest wall was significantly lower than those further away. There is less injury to the lung when the puncture biopsy is performed on the lesion close to the chest wall. In the rEBUS-TBB group, the positive diagnistic rate was associated with the lesion size and the distance between the lesion and the chest wall. The positive diagnostic yield of lesions that were >2 cm and distal from the chest wall was higher than the lesions that were ≤2 cm and close to the chest wall, and the results were consistent with the other studies worldwide.[14,15] In the EBUS-TBB procedure, the larger lesions are located in larger bronchi, and hence, the bronchoscope can easily reach and detect the lesions by ultrasound. There is less probability that the biopsy site was not consistent with the location detected by rEBUS. The lesions that are smaller and close to the chest wall are located in smaller bronchi, rendering difficulty for the bronchoscope to reach the target location, and the biopsy forceps are more likely to stray into the wrong bronchi. The biopsy forceps also cannot be fully opened in the smaller bronchi, and thus, the specimens are smaller, which leads to lower positive diagnostic yield. The incidence of complications including pneumothorax and hemoptysis was not significantly different between the 2 groups, which may be related to the lower complication rate of the whole rEBUS-TBB group. The positive rate of different lung segments, an apical, anterior, and dorsal segment of the lower lobe, and the lower lobe basal segment is higher by CT-TTNA than rEBUS-TBB. We compared the positive diagnostic yield of different segments in the 2 groups, and we found that the positive diagnostic yield was higher in the CT-TTNA than the rEBUS-TBB groups in the apical segment, anterior segment of the upper lobe, dorsal segment, and the lateral basal segment of the lower lobe. Due to the specific anatomical characteristics of the apical segment of the upper lobe and the dorsal segment of the lower lobe, the bronchoscope needed to go through 2 rotations while entering; even if the ultrasonic probe has detected the lesion, it is hard for the biopsy forceps to enter and are more likely to stray into another bronchus, resulting in the low positive diagnostic rate in the rEBUS-TBB group. The lesions in the anterior segment of the upper lobe and lateral basal segment of the lower lobe are close to the anterior chest wall and the lateral chest wall; thus, it is easy to perform the transthoracic needle aspiration, resulting in high positive diagnostic rate in the CT-TTNA group. However, the sample size of this study was small and not adequate for the comparison and analysis between different segments. Therefore, a larger sample size study is essential.
In this study, the success rate of exploration and biopsy was 87% in the rEBUS-TBB group. Comparing the characteristics of the lesions detected by rEBUS with those not detected, we found that the rate of exploration of lesions that were >2 cm and with the bronchial sign was higher than lesions that were ≤2 cm and without a bronchial sign, which was similar to the previous studies.[16] Larger lesions are located in the larger bronchi, and by CT, the bronchoscopist can directly predict the bronchi where the lesions with the bronchial sign are localized. This renders it easy for the bronchoscope to enter and probe the lesion; however, the positive biopsy rate had no significant difference, which indicated that the improvement of the diagnostic positive rate benefited from higher ultrasound exploration rate. Similarly, the exploration rate, and the positive diagnostic yield of lesions not close to the chest wall were higher than those of the lesions close to the chest wall; the biopsy rate between the 2 groups had no significant difference. This indicated that lesions close to the thoracic wall were not easy to be probed by ultrasound, resulting is lower positive diagnostic rate than those not close to the thoracic wall.
In summary, both rEBUS-TBB and CT-TTNA are effective and safe in the diagnosis of PPL. The positive diagnostic rate of CT-TTNA is higher than rEBUS-TBB; however, the incidence of pneumothorax of CT-TTNA is higher than rEBUS-TBB. The positive diagnostic rate is correlated with the lesion size and the localization of the lesions in the segments. No correlation was found between the distance from the lesion to the thoracic wall and the positive diagnostic rate of CT-TTNA; however, the incidence of pneumothorax is lower in lesions close to the thoracic wall. In the rEBUS-TBB group, the rate of exploration and the positive diagnostic rate of lesions >2 cm and with the bronchial sign was higher than that ≤2 cm and without a bronchial sign. The rate of exploration of lesions close to the chest wall was lower than those distally located from the chest wall.
Therefore, in the selection of the 2 methods, CT-TTNA is the first choice for the lesions ≤2 cm and close to the chest wall while rEBUS-TBB is a better choice for the lesions that are >2 cm, not close to the chest wall or with a bronchial sign. Moreover, the operators’ proficiency in the 2 techniques is pivotal for an efficient and safe qualitative diagnosis of PPL.
The limitation of this study was its retrospective nature and that the sample size for the comparison and analysis between different segments was insufficient. Thus, a randomized and controlled study with larger sample size is imperative.
Both rEBUS-TBB and CT-TTNA are effective and safe in the diagnosis of PPL. The positive diagnostic rate of CT-TTNA is higher than rEBUS-TBB; however, the incidence of pneumothorax of CT-TTNA is higher than rEBUS-TBB. CT-TTNA is the first choice for the lesions which are ≤2 cm and close to the chest wall while rEBUS-TBB is a better choice for the lesions which are >2 cm, not close to the chest wall, or show a bronchial sign.
Acknowledgments
The authors thank Drs. Shi Liangrong and Wei Xiaohua for their contributions to the study.
Footnotes
Abbreviations: CT = computed tomography, CT-TTNA = computer tomography-guided transthoracic needle aspiration, LLD = longest lesion diameter, PPL = peripheral pulmonary lesions, rEBUS-TBB = radial endobronchial ultrasound-guided transbronchial biopsy, TBB = transbronchial biopsy.
QZ and SZ have contributed equally to this work.
Authors’ contributions: QZ, SZ, and JZ conceived the study, participated in the designing, the progress of the study, and collected the data. QZ drafted the manuscript and conducted the statistical analysis. XX and QX participated in the study. All the authors read and approved the final manuscript.
Conflict of interest: None of the authors have any conflict of interest to disclose.
References
- [1].Radke JR, Conway WA, Eyler WR, et al. Diagnostic accuracy in peripheral lung lesions. Factors predicting success with flexible fiberoptic bronchoscopy. Chest 1979;76:176–9. [DOI] [PubMed] [Google Scholar]
- [2].Shiner RJ, Rosenman J, Katz I, et al. Bronchoscopic evaluation of peripheral lung tumours. Thorax 1988;43:887–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123(Suppl):115s–28s. [DOI] [PubMed] [Google Scholar]
- [4].Tsukada H, Satou T, Iwashima A, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000;175:239–43. [DOI] [PubMed] [Google Scholar]
- [5].Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(Suppl):131s–48s. [DOI] [PubMed] [Google Scholar]
- [7].Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36–41. [DOI] [PubMed] [Google Scholar]
- [8].Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972–4. [DOI] [PubMed] [Google Scholar]
- [9].Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902–10. [DOI] [PubMed] [Google Scholar]
- [10].Fuso L, Varone F, Magnini D, et al. Role of ultrasound-guided transbronchial biopsy in the diagnosis of peripheral pulmonary lesions. Lung Cancer 2013;81:60–4. [DOI] [PubMed] [Google Scholar]
- [11].Steinfort DP, Liew D, Irving LB. Radial probe EBUS versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions: an economic analysis. Eur Respir J 2013;41:539–47. [DOI] [PubMed] [Google Scholar]
- [12].Chan A, Devanand A, Low SY, et al. Radial endobronchial ultrasound in diagnosing peripheral lung lesions in a high tuberculosis setting. BMC Pulm Med 2015;15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang C, Zeng D, Lei W. Diagnostic value of computer tomography-guided percutaneous needle biopsy versus radial probe endobronchial ultrasound-guided transbronchial biopsy in peripheral pulmonary lesions. Chin J Tuberc Respir Dis 2015;38:897–900. [PubMed] [Google Scholar]
- [14].Li M, Peng A, Zhang G, et al. Endobronchial ultrasound transbronchial lung biopsy with guide-sheath for the diagnosis of peripheral pulmonary lesions. Chin J Tuberc Respir Dis 2014;37:36–40. [PubMed] [Google Scholar]
- [15].Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788–93. [DOI] [PubMed] [Google Scholar]
- [16].Guvenc C, Yserbyt J, Testelmans D, et al. Computed tomography characteristics predictive for radial EBUS-miniprobe-guided diagnosis of pulmonary lesions. J Thorac Oncol 2015;10:472–8. [DOI] [PubMed] [Google Scholar]


