Abstract
Medication errors may lead to adverse drug events (ADEs), which endangers patient safety and increases healthcare-related costs. The on-ward deployment of clinical pharmacists has been shown to reduce preventable ADEs, and save costs. The purpose of this study was to evaluate the ADEs prevention and cost-saving effects by clinical pharmacist deployment in a nephrology ward.
This was a retrospective study, which compared the number of pharmacist interventions 1 year before and after a clinical pharmacist was deployed in a nephrology ward. The clinical pharmacist attended ward rounds, reviewed and revised all medication orders, and gave active recommendations of medication use. For intervention analysis, the numbers and types of the pharmacist's interventions in medication orders and the active recommendations were compared. For cost analysis, both estimated cost saving and avoidance were calculated and compared.
The total numbers of pharmacist interventions in medication orders were 824 in 2012 (preintervention), and 1977 in 2013 (postintervention). The numbers of active recommendation were 40 in 2012, and 253 in 2013. The estimated cost savings in 2012 and 2013 were NT$52,072 and NT$144,138, respectively. The estimated cost avoidances of preventable ADEs in 2012 and 2013 were NT$3,383,700 and NT$7,342,200, respectively. The benefit/cost ratio increased from 4.29 to 9.36, and average admission days decreased by 2 days after the on-ward deployment of a clinical pharmacist.
The number of pharmacist's interventions increased dramatically after her on-ward deployment. This service could reduce medication errors, preventable ADEs, and costs of both medications and potential ADEs.
Keywords: clinical pharmacist, cost saving, medication error, nephrology, preventable adverse drug event
1. Introduction
The definition of adverse drug events (ADEs) is any injuries to a patient resulting from medication use, including any harm or loss of function.[1] Medication errors refer to any mistakes occurring during the medication use process such as prescription, transcription, dispensing, administration, or monitoring, regardless of whether an injury occurred or whether the potential for injury was present.[2] ADEs in hospitalized patients will result in longer hospital stays and extra medical costs, even though they are sometimes preventable. According to Bates et al,[1] the incidence of ADEs was 6.5 per 100 admissions, and 28% of them were judged preventable. The ADEs and preventable ADEs contributed to longer hospital stays of 2.2 and 4.6 days, and to an increase in costs of US$2595 and US$4685, respectively.[3] In another study, by Classen et al,[4] an ADE was associated with an increased length of stay of 1.91 days, an increased cost of US$2262, and an increased risk of death of 1.88-fold.
Preventable ADEs could be related to medication errors and are avoidable by many methods such as pharmacist intervention.[5] In several studies, clinical pharmacists’ participation in hospital was shown to prevent medication errors, to reduce preventable ADEs, and to save costs.[6–10] These studies included both adults and children, and they were usually held in intensive care units.
Medication errors may be more common among patients with chronic kidney disease and those on dialysis because of altered pharmacokinetics, high susceptibility to medication toxicity, multiple comorbidities, and polypharmacy.[11–13] Studies also have shown that clinical pharmacists’ contributions helped to identify drug-related problems and to prevent medication errors, and may have reduced medication costs in end-stage renal disease patients.[14–19]
In Taiwan, clinical pharmacists usually set up in intensive care units rather than in general wards. However, there is no local data on the cost-saving effect of clinical pharmacists or the estimated cost of preventable ADEs in Taiwan.
A unit-based clinical pharmacist was assigned in the nephrology ward of a teaching hospital in Taiwan from January 2013 to assist physicians in reducing medication errors from prescriptions. The purpose of this retrospective study was to evaluate the differences before and after a clinical pharmacist was deployed in the nephrology ward. The primary endpoint was to evaluate the cost-saving effect after the clinical pharmacist's participation. We also explored the different types of pharmacist interventions.
2. Methods
This was a retrospective study conducted in a nephrology ward of the National Taiwan University Hospital (NTUH), which is a teaching hospital serving both inpatients and outpatients. There are more than 2500 inpatient beds in NTUH with approximately 50 pharmacists working in the inpatient pharmacy. The average number of patients cared for by each inpatient pharmacist is around 70. The routine work of an inpatient pharmacist consists of dispensing and reviewing medication orders. This study compared the number of pharmacist interventions 1 year before a clinical pharmacist was deployed in a nephrology ward with 37 beds, and at the end of her 12-month term. From January 1 to December 31, 2012, pharmacists taking care of the nephrology ward only stayed in the inpatient pharmacy and reviewed all medication orders by a computer system. Pharmacists advised physicians by telephone to modify medication orders. Physicians and nurses could also contact the pharmacists the same way. For 1 full day (8 hours) the clinical pharmacist spent most of the time on the NTUH nephrology ward, and joined the medical team on their rounds from January 1, 2013, Monday to Friday, for 12 months. Along with reviewing all medication orders through the computer system, the clinical pharmacist revised medication orders, gave recommendations of medication use actively, and accepted direct consultations from physicians and nurses—usually face to face and sometimes by telephone. Ethical approval was waived for this study because the pharmacist interventions belonged to daily practice, and patient identification was removed in this study.
All the reactive and active interventions made by all the hospital pharmacists were documented in the computer system.
2.1. Intervention analysis
Pharmacist interventions in medication orders were classified into 4 groups: order modification, therapeutic drug monitoring, key-in error, and violation of National Health Insurance (NHI) or NTUH regulations. There were 12, 3, and 11 subgroups in medication order modification, therapeutic drug monitoring, and key-in error categories, respectively (Table 1).
Table 1.
Subgroups of pharmacist interventions in 2012 and 2013.
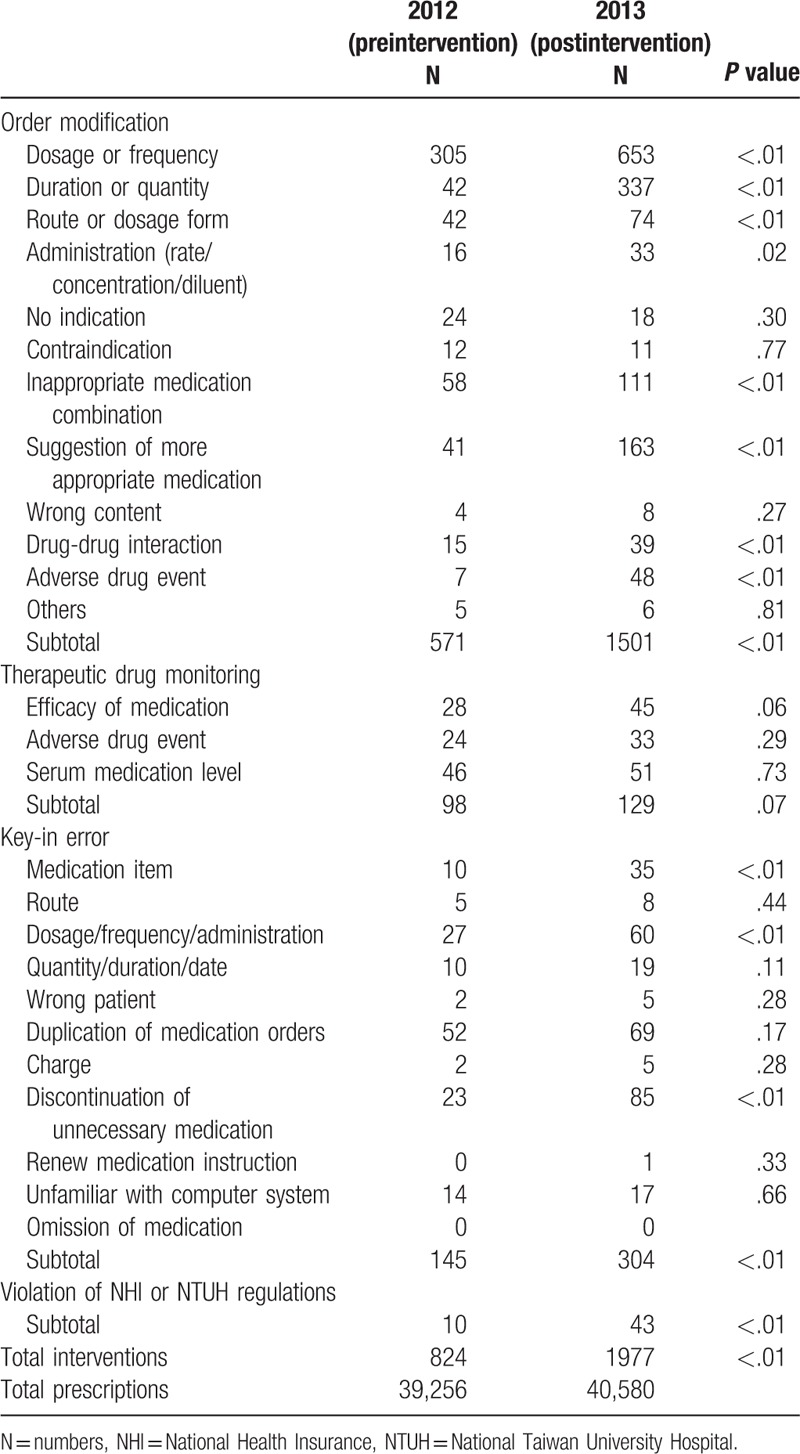
Active recommendations made by all pharmacists included suggestions of medication use, therapeutic drug monitoring, medication reconciliation, and others.
The numbers and types of pharmacist interventions in 2012 were compared with those in 2013.
2.2. Cost analysis
For cost analysis, estimated cost savings and cost avoidance were calculated.
The subgroups of pharmacist interventions belonged to discontinuing unnecessary medications (order modifications of duration or quantity, no medication indication, and inappropriate medication combination), switching medications from intravenous form to oral form, and correcting dosage or frequency were assumed that medication costs could be saved in these areas. The estimated cost savings were calculated conservatively by the 1-day medication costs of the intervened medications if the interventions belonged to any of the aforementioned types.
Estimated cost avoidance was calculated based on the probability of an ADE in the absence of pharmacist interventions. The methodology used here was described by Nesbit et al.[20] A panel consisting of 10 clinical pharmacists in NTUH independently evaluated each type of pharmacist intervention to estimate the probability of an ADE by voting. The probability score of 0.00/0.01/0.10/0.40/0.60 meant that the probability of an ADE is no harm expected/very low/low/medium/high. The probability score (P) multiplied by the number of pharmacist interventions (N) resulted in the number of preventable ADEs. For each preventable ADE, we conservatively assumed it would result in a longer hospital stay (D) by 2 days based on previous studies. The cost of a preventable ADE (C) here was estimated to be NT$5000 for 1 day's admission by NHI data, roughly including the fees of diagnosis, nursing care, pharmaceutical care, hemodialysis, and bed. Based on above, cost avoidance could be calculated by the formula P×N×D×C.
After acquiring cost saving and avoidance, the benefit/cost ratio was calculated. The definition of the benefit/cost ratio was the total number of cost savings plus avoidance divided by the annual wage of the pharmacists, which was around NT$800,000.
2.3. Statistical analysis
The χ2 test was used for binomial data. A P value of .05 or lower was considered statistically significant. The statistical analysis was performed by SPSS 18 (SPSS Inc, Chicago, IL).
3. Results
In 2012 (preintervention period), there were 813 patient-times of admission with average admission duration of 13.22 days and total 39,256 medication orders in the nephrology ward. The total number of pharmacist interventions in medication orders was 824 in 2012. A total of 571, 98, 145, and 10 belonged to order modifications, therapeutic drug monitoring, key-in error, and violation of NHI or NTUH regulations, respectively. The top 3 subtypes of interventions in order of modification group were dosage or frequency, inappropriate medication combination, duration or quantity, and route or dosage form (Table 1). The numbers of active recommendations of suggestions for medication use, therapeutic drug monitoring, medication reconciliation, and others in 2012 were 26, 4, 4, and 6, respectively (Table 2).
Table 2.
Active recommendations in 2012 and 2013.
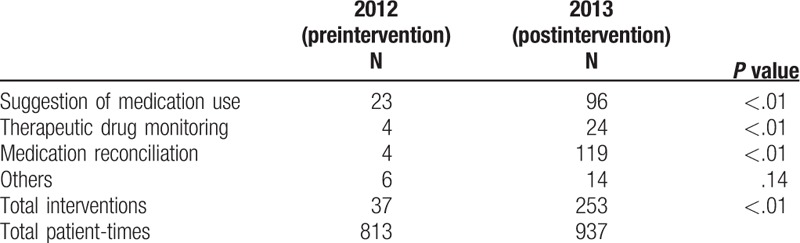
In 2013 (postintervention period), there were 937 patient-times of admission with the average admission duration of 11.10 days and total 40,580 medication orders. After the on-ward deployment of a clinical pharmacist, the number of pharmacist interventions in medication orders was 1977 in 2013. These interventions consisted of 1501 order modifications, 129 therapeutic drug monitoring, 304 key-in errors, and 43 violation of NHI or NTUH regulations. The top 3 subtypes of interventions in order of modification were dosage or frequency, duration or quantity, and the suggestion of more appropriate medication (Table 1). The numbers of active recommendations of suggestions for medication use, therapeutic drug monitoring, medication reconciliation, and others in 2013 were 96, 24, 119, and 14 respectively (Table 2). There were significantly more pharmacist interventions and active recommendations in 2013 compared with those in 2012 (P < .01).
The estimated cost saving by the discontinuation of unnecessary medications, switching medications from intravenous form to oral form, and correcting dosage or frequency was NT$2984, NT$5469, and NT$43,619 in 2012, and NT$40,092, NT$30,543, and NT$73,503 in 2013, which added up to NT$52,072 and NT$144,138, respectively.
The probability score of each kind of intervention is shown in Table 3. The estimated cost avoidance of preventable ADEs in 2012 and 2013 was NT$3,383,700 and NT$7,342,200, respectively (Table 3).
Table 3.
Cost avoidance in 2012 and 2013.
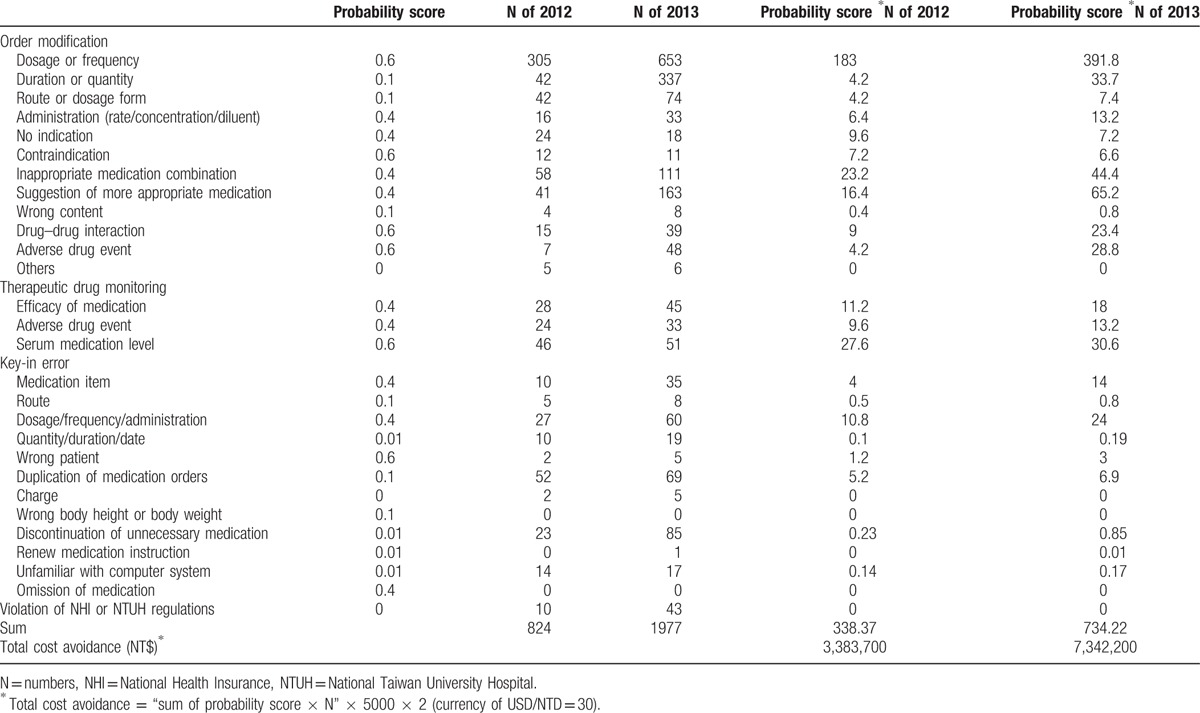
The sum of cost savings and avoidance were estimated to be NT$3,435,772 and NT$7,486,338 in 2012 and 2013, respectively. On the basis of these data, the benefit/cost ratio was 4.29 in 2012 and 9.36 in 2013.
4. Discussion
Clinical pharmacists participating in medical rounds in hospital were found to reduce preventable ADEs by 66% to 78%.[7–9] The cost avoidance generated by clinical pharmacist interventions resulted in a benefit/cost ratio of 3.1 to 13.33.[9,20] The pharmacist interventions were most commonly related to dosage, frequency, and drug omissions.[8,20] In nephrology wards and outpatient hemodialysis units, clinical pharmacist interventions helped in the detection of medication errors and in the identification of potential drug-related problems. Their recommendations were thought to be clinically important and possibly led to better therapeutic outcomes. With clinical pharmacists’ interventions, patients’ direct medication costs were reduced by 4.3%.[14–19] Manley and Carroll[11] found that for every dollar spent on pharmaceutical care of end-stage renal disease patients, the healthcare system saved an estimated US$3.98.
This is the first study to demonstrate the cost benefit and prevention of medication errors due to the intervention of a clinical pharmacist in a nephrology ward in a Taiwanese hospital. The number of pharmacist interventions in medication orders greatly increased after the on-ward deployment of a clinical pharmacist, which resulted in a significant cost benefit, fewer admission days, and probably fewer ADEs. There were obviously more interventions about medication duration or quantity, suggestions of more appropriate medications, and the detection of ADEs after the clinical pharmacist's participation. On the basis of these interventions, the preventable ADEs significantly increased from 338 to 734. The benefit/cost ratio doubled after the on-ward deployment of a clinical pharmacist.
The cost benefit in this study seems to be similar to or lower than previous studies.[3,21] However, this discrepancy may result from various calculations of cost analysis. The estimated cost saving was calculated conservatively by a 1 day cost of the intervened medications. Furthermore, the estimated cost avoidance was also calculated conservatively based on only 2 extra days of admission if ADEs occurred, although this may have resulted in more than 2 days of admission according to the literature.[3] The expenditure of 1 day's admission in patients who received hemodialysis was around NT$5000 (∼US$167) based on reimbursement of the NHI. Since there were no data on how much a preventable ADE would cost in Taiwan, this is how we did the estimation. Although we adopted such a conservative method, the benefit/cost ratio still doubled. However, it may not be practical to compare the cost benefit of this study with the studies in different countries because the medication costs and medical service costs differ greatly. Preventable ADEs were reported to result in additional lengths of hospital stay of 4.6 days, and an increase in cost of US$4685 in the USA (averagely US$1018/d).[3] Another study in Germany found each ADE cost US$1033 per patient.[21] However, in the present study, US$167 for each preventable ADE was used conservatively.
In our study, we found the average admission days decreased from 13.22 to 11.10 days after the deployment of a clinical pharmacist. There was no significant change in the nephrology ward such as new medical device use or medication formula change during this period except the deployment of a clinical pharmacist. Therefore, we speculate that this difference could be attributed to the effort of potential ADEs prevention by the clinical pharmacist. Previous study showed that ADEs contributed to longer hospital stays of 2.2 to 2.9 days, which further support our speculation.[3,21]
The number of active recommendations also increased dramatically from 37 to 253 after the participation of the clinical pharmacists. It is worth mentioning that the composition of active recommendations also changed. There were only 4 medication reconciliations in 2012, but the number increased to 119 in 2013. Medication reconciliation is a tool to reduce medication errors and is important for patient safety.[22,23] Clinical pharmacists play an important role in this service, which we have demonstrated in this study. Although active recommendations were not included in the cost analysis in this study, they might help to reduce preventable ADEs and to save costs.
Potentially, the costs of clinical pharmacist participation described in this study are significantly outweighed by the savings resulting from more appropriate drug therapy. Once the monitoring of medication orders by a nephrology pharmacist is well set up, a night-fold return on investment and shorter hospital admission seems feasible. This investment would be very attractive from a societal perspective, especially when the budget is very tight as in the Taiwan NHI. Moreover, the cost savings and avoidance are likely to be underestimated as they have been calculated conservatively. However, the current reimbursement structure for hospital pharmaceutical care by the Taiwan NHI is based on a fixed price per day structure. It is obvious that this kind of structure would impede care quality improvements, like the on-ward deployment of clinical pharmacists. Under the current reimbursement structure for pharmaceutical care in Taiwan, it is not possible to acquire the benefit of additional cost savings by the on-ward deployment of clinical pharmacists.
This study was performed in a nephrology ward; therefore, this may limit the application of our results to other clinical settings. However, because the reduction of prescribing errors and related harm was significant in this study, and the results corresponded to previous findings, it is highly probable that the beneficial effects could be copied in other clinical settings by the on-ward deployment of clinical pharmacists.
5. Conclusion
According to this retrospective study, the number of pharmacist interventions increased dramatically after a clinical pharmacist's participation in the nephrology ward. The number of pharmacist interventions increased 140% from the previous year when there was no clinical pharmacist on the ward. The clinical pharmacist also made significantly more active recommendations, with a 6-fold increase. The benefit/cost ratio of on-ward deployment of a clinical pharmacist is 9.36, which was calculated conservatively. The average admission days reduces by 2 days after clinical pharmacist interventions. The on-ward deployment of a clinical pharmacist is thought to prevent ADEs, and reduce medication errors and the cost of medications and potential ADEs. Clinical pharmacists play a very important role, not only in medication safety, but also in cost saving.
Footnotes
Abbreviations: ADE = adverse drug events, NHI = National Health Insurance, NTUH = National Taiwan University Hospital.
Reprints will NOT be ordered.
This study did not receive any grant from the governmental and/or private agencies.
The authors have no conflicts of interest to disclose.
References
- [1].Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29–34. [PubMed] [Google Scholar]
- [2].Wittich CM, Burkle CM, Lanier WL. Medication errors: an overview for clinicians. Mayo Clin Proc 2014;89:1116–25. [DOI] [PubMed] [Google Scholar]
- [3].Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997;277:307–11. [PubMed] [Google Scholar]
- [4].Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277:301–6. [PubMed] [Google Scholar]
- [5].Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug Saf 2006;29:1011–22. [DOI] [PubMed] [Google Scholar]
- [6].Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med 1994;22:1044–8. [DOI] [PubMed] [Google Scholar]
- [7].Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999;282:267–70. [DOI] [PubMed] [Google Scholar]
- [8].Kucukarslan SN, Peters M, Mlynarek M, et al. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med 2003;163:2014–8. [DOI] [PubMed] [Google Scholar]
- [9].Klopotowska JE, Kuiper R, van Kan HJ, et al. On-ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: an intervention study. Crit Care 2010;14:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kaushal R, Bates DW, Abramson EL, et al. Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm 2008;65:1254–60. [DOI] [PubMed] [Google Scholar]
- [11].Manley HJ, Carroll CA. The clinical and economic impact of pharmaceutical care in end-stage renal disease patients. Semin Dial 2002;15:45–9. [DOI] [PubMed] [Google Scholar]
- [12].Manley HJ, Cannella CA, Bailie GR, et al. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney DisV 46 2005;669–80. [DOI] [PubMed] [Google Scholar]
- [13].Jones SA, Bhandari S. The prevalence of potentially inappropriate medication prescribing in elderly patients with chronic kidney disease. Postgrad Med J 2013;89:247–50. [DOI] [PubMed] [Google Scholar]
- [14].Tang I, Vrahnos D, Hatoum H, et al. Effectiveness of clinical pharmacist interventions in a hemodialysis unit. Clin Ther 1993;15:459–64. [PubMed] [Google Scholar]
- [15].Kaplan B, Mason NA, Shimp LA, et al. Chronic hemodialysis patients. Part I: characterization and drug-related problems. Ann Pharmacother 1994;28:316–9. [DOI] [PubMed] [Google Scholar]
- [16].Kaplan B, Shimp LA, Mason NA, et al. Chronic hemodialysis patients. Part II: Reducing drug-related problems through application of the focused drug therapy review program. Ann Pharmacother 1994;28:320–4. [DOI] [PubMed] [Google Scholar]
- [17].Grabe DW, Low CL, Bailie GR, et al. Evaluation of drug-related problems in an outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol 1997;47:117–21. [PubMed] [Google Scholar]
- [18].Vessal G. Detection of prescription errors by a unit-based clinical pharmacist in a nephrology ward. Pharm World Sci 2010;32:59–65. [DOI] [PubMed] [Google Scholar]
- [19].Gharekhani A, Kanani N, Khalili H, et al. Frequency, types, and direct related costs of medication errors in an academic nephrology ward in Iran. Ren Fail 2014;36:1268–72. [DOI] [PubMed] [Google Scholar]
- [20].Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm 2001;58:784–90. [DOI] [PubMed] [Google Scholar]
- [21].Rottenkolber D, Hasford J, Stausberg J. Costs of adverse drug events in German hospitals—a microcosting study. Value Health 2012;15:868–75. [DOI] [PubMed] [Google Scholar]
- [22].Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care 2003;18:201–5. [DOI] [PubMed] [Google Scholar]
- [23].Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 2013;158:397–403. [DOI] [PubMed] [Google Scholar]


