Abstract
In recent years, lymphocyte-to-monocyte ratio (LMR) has become a novel indirect marker of inflammation, which has been demonstrated to be associated with poor prognosis of oncology and cardiovascular disease. The aim of the study was to assess the relationship between LMR on admission and in-hospital and long-term major adverse cardiac and cerebrovascular events (MACCE) in patients with ST-elevated myocardial infarction (STEMI) after primary percutaneous coronary intervention (PCI).
A total of 306 STEMI patients were enrolled and grouped according to tertiles of LMR from the blood samples obtained in the emergency room on admission. Total white blood cell count, differential count of neutrophil, lymphocyte, monocyte, and other factors were evaluated.
The median follow-up period was 21 months (1–36 months). As the LMR decreased, in-hospital nonfatal myocardial infarction and cardiovascular mortality increased (P = .002, P = .009, respectively). And long-term stroke/TIA, TVR, nonfatal myocardial infarction, and cardiovascular mortality also increased with decreasing LMR (P = .012, P = .001, P = .003, P = .002, respectively). The receiver operating characteristic (ROC) curve of LMR for predicting MACCE showed the sensitivity of 76% and specificity of 78% and the optimal cut-off value was determined as 2.62. In multivariate analysis, after adjusting for confounders, LMR was an independent predictor of in-hospital and long-term MACCE (odds ratio [OR] 1.192 [1.069–1.315] P < .001, OR 1.239 [1.125–1.347] P < .001, respectively).
The LMR is an independent predictor of in-hospital and long-term MACCE in patients with STEMI after primary PCI. Our results suggest that this simple, inexpensive, relatively available inflammatory marker may have significant effects on the treatment and prognosis in patients with STEMI.
Keywords: lymphocyte-to-monocyte ratio, major adverse cardiac and cerebrovascular events, primary percutaneous coronary intervention, ST-elevated myocardial infarction
1. Introduction
Acute myocardial infarction (AMI) is a serious type of coronary atherosclerotic heart disease, with high prevalence and high mortality worldwide.[1] Previous studies have shown that inflammation and immune cells play a critical role in the onset, development, and prognosis of the patients with ST-elevated myocardial infarction (STEMI).[2] The discovery of novel inflammatory biomarkers has become significant so as to help diagnose accurately and provide information of prognosis and treatment in the disease. Changes of white blood cell have been used as an independent risk factor for predicting cardiovascular events, but the number of white blood cell is susceptible to be interfered from other noncardiac factors. It has been demonstrated that preoperative neutrophil-to-lymphocyte ratio (NLR) has good predictive value of mortality and recanalization of impaired coronary blood flow in patients with STEMI after percutaneous coronary intervention (PCI).[3,4]
Compared with neutrophils, monocytes have a more important effect on the pathophysiological process of infarcted myocardium repair and cardiac functioning.[5] Lymphocytes and monocytes are important immune cells associated with the development of the pathogenesis of atherosclerosis. It has been investigated that there were a relative low lymphocyte count and a relative high monocyte count in cardiovascular diseases, which showed predictive and prognostic value in myocardial infarction (MI).[6,7] In addition, lymphocyte-to-monocyte ratio (LMR) has been recently studied that it was a novel marker of inflammation related to coronary artery disease.[8] Major adverse cardiac and cerebrovascular events (MACCE), which comprise stroke/transient ischemic attack (TIA), target vessel revascularization (TVR), nonfatal MI, and cardiovascular mortality, are very important adverse events associated with prognosis in patients with MI. In this study, we evaluated the relations between LMR and in-hospital and long-term MACCE in STEMI patients who underwent primary PCI to assess the prognostic value of LMR for STEMI.
2. Methods
2.1. Study population
From April 2013 through December 2016, a total number of 403 patients who diagnosed with STEMI and treated with primary PCI were screened at the First Affiliated Hospital of Zhengzhou University. Diagnostic criteria were referred to the American College of Cardiology (ACC) and the American Heart Association (AHA) 2009 STEMI Diagnostic and Therapeutic Guidelines: continuous typical chest pain lasting >30 minutes and ST-segment elevation >1 mm in 2 or more standard leads or at least 2 contiguous leads or new left bundle branch block on the electrocardiogram (ECG).[9] Patients with acute and chronic infection, hematological diseases, trauma or surgical operation within 2 weeks, recent use of steroid drugs, severe liver or renal failure, autoimmune diseases were excluded. Finally, the study population included 306 patients. The ethics committee of the First Affiliated Hospital of Zhengzhou University has approved this study protocol. Informed consent was obtained from all participants.
2.2. Study procedures
Baseline characteristics including age, sex, body mass index were collected. The history of hypertension (reference to the 2007 European guidelines for the prevention and treatment of hypertension), diabetes mellitus (reference to the 2012 American Diabetes Association Diabetes Medical Treatment Standard), smoking (according to the WHO standard, the average number of cigarettes smoked per day was more than 1 and lasted for 1 year or more), and other clinical characteristics were also recorded. Blood samples of the patients were obtained from the antecubital vein in the emergency room on admission. Complete blood count (including neutrophils, lymphocytes, monocytes, platelets, and total white blood cell) were accessed by Beckman Coulter UniCelDxH800. High sensitivity C-reactive protein (hs-CRP), maximum creatine kinase-MB, low density lipoprotein (LDL), high density lipoprotein (HDL), and other biochemical indicators were evaluated by standard laboratory methods at the biochemistry laboratory. The LMR was a ratio computed as the lymphocyte count to the monocyte count. The patients were stratified into tertiles according to the LMR on admission. And we also recorded NLR and platelet-to-lymphocyte ratio (PLR) of patients. All patients were measured the left ventricular ejection fraction (LVEF) by color Doppler ultrasonography using the modified Simpson's method. The standard techniques were used to perform primary PCI for all patients. The patients received aspirin and clopidogrel with a loading dose before PCI, and a daily dose was given for a period after the operation. The choices of stents balloon and whether apply predilatation were determined by operators.
2.3. Follow-up and MACCE
The follow-up data of prognostic information about the included patients were obtained through hospital records, patients’ primary care physicians, patient interviews (in person or by telephone), or their families. Total MACCE include stroke/TIA, TVR, nonfatal MI, and cardiovascular mortality during hospitalization and long-term period. Stroke/TIA was defined as an acute neurologic deficit consistent with recent ischemic or hemorrhagic events. TVR was determined as PCI or surgical bypass attaching to any segment of the target vessel, including any branch of the proximal and distal to the index lesion of the entire coronary artery, as well as the index lesion itself underwent primary intervention. Nonfatal MI was defined as the development of chest pain recurrence lasting for 30 minutes or more, accompanied by new ECG changes. Cardiovascular mortality was described as death owing to apparent cardiac causes or any death associated with PCI. We recorded MACCE of patients as in-hospital MACCE, which happened during hospitalization, and long-term MACCE, which happened during follow-up period.
2.4. Statistical analysis
Categorical variables were represented as percentages (%) and compared by chi square test between groups. Continuous variables were expressed as mean ± standard deviation and compared using the one-way Analysis of Variance model. Considering that LMR, NLR, and PLR are non-normal variables, we transformed LMR, NLR, and PLR into logarithmic function and represented as lnLMR, lnNLR, and lnPLR. The relationship between LMR on admission and other variables was assessed by Spearman correlation analysis. The receiver operating characteristic (ROC) curve analysis was used to determine the best cut-off value for predicting MACCE and the sensitivity and specificity of LMR. The survival curves were analyzed using Kaplan–Meier estimation based on MACCE-free cumulative incidence, and the log-rank test was used to compare the differences. And we performed log-rank test with Bonferroni correction to compare every 2 groups among tertiles. The univariate analysis was applied to evaluate the effects of different variables on in-hospital and long-term MACCE. Any variables tested in the univariate analysis for which the P value was <.10 indicated a correlation with the MACCE and were contained in the full multivariate model. All data analysis was performed by SPSS software (version 17.0, SPSS Chicago, IL). If it was not specifically stated, the P value <.05 was regarded as statistically significant.
3. Results
The baseline and clinical characteristics of the patients were shown in Table 1. Total 306 consecutive patients were included in our study. There were 244 (79.7%) men and mean age of patients was 63.5 ± 10.6 years. At the time of admission, the study population was stratified into 3 groups based on the level of LMR (the first tertile: the ratio of >3.2; the second tertile: the ratio of 2.1–3.2; the third tertile: the ratio of <2.1). The age of T3 group was higher than other 2 groups. The patients in the T2 and T3 group had a lower proportion of current smoker than T1 group. The biomaker level of hs-CRP was higher in T3 group than the other. Higher LVEF on admission was found in T1 group. Pain-to-balloon time and the percentage of multivessel disease were significantly increased as the tertile decreased. Additionally, baseline total neutrophil and monocyte count were significantly higher in T3 group compared with the other groups, while the baseline total lymphocyte count was lower in T3 group. No differences were found between the groups concerning body mass index, sex, hypertension, hyperlipidemia, diabetes mellitus, the history of previous MI, previous medications (aspirin, statins, insulin, and ACE/angiotensin II receptor antagonists/calcium channel blockers/Diuretics), maximum creatine kinase-MB, troponin-I, LDL, HDL, triglyceride, total cholesterol, glomerular filtration rate, glycoprotein IIb/IIIa antagonist, total white blood cell, and platelet.
Table 1.
Baseline and clinical characteristics of study population.
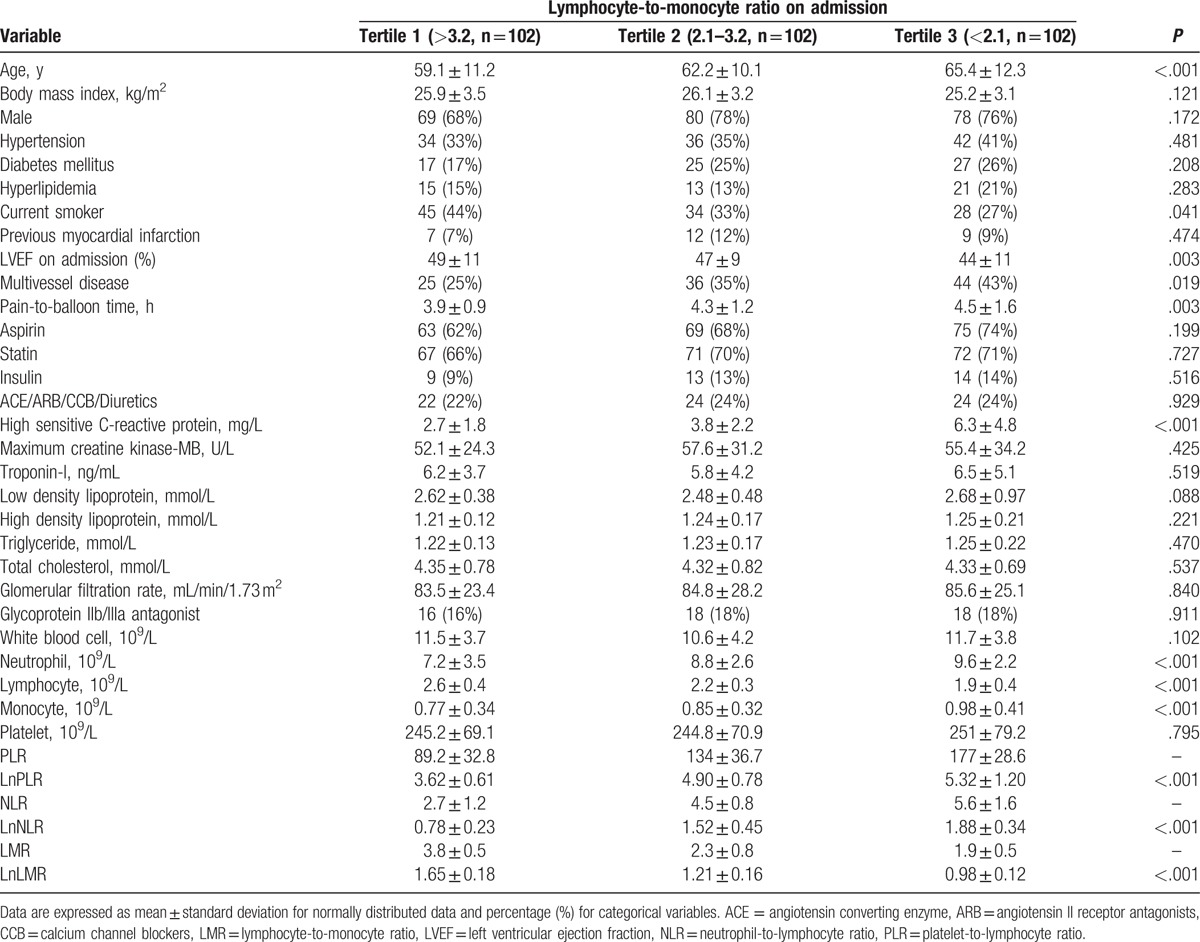
The median follow-up period was 21 months (1–36 months). A total of 67 (22%) and 84 (27%) MACCE were recorded during in-hospital and follow-up period, respectively. MACCE according to LMR tertiles in the in-hospital and follow-up period after primary PCI were showed in Table 2. Among in-hospital MACCE, nonfatal MI rate was 3% in the T1 group, 13% in the T2 group, and 19% in the T3 group (P = .002). Cardiovascular mortality rate was 1% in the T1 group, 5% in the T2 group, and 11% in the T3 group (P = .009). The T3 group had a higher frequency of nonfatal MI and cardiovascular mortality than the other group. The frequencies of stroke/TIA and TVR were no difference between groups (P = .517; P = .952, respectively). Among long-term MACCE, nonfatal MI rate was 9% in the T1 group, 11% in the T2 group, and 25% in the T3 group (P = .003). Cardiovascular mortality rate was 5% in the T1 group, 13% in the T2 group, and 22% in the T3 group (P = .002). The T3 group had a higher frequency of nonfatal MI and cardiovascular mortality than the other group. The frequencies of stroke/TIA and TVR also had a significant difference between groups (P = .012; P = .001, respectively). In general, there was a significant difference in in-hospital and long-term MACCE between groups according to LMR tertiles on admission (P < .001; P < .001, respectively).
Table 2.
Major adverse cardiac and cerebrovascular events according to lymphocyte-to-monocyte ratio.
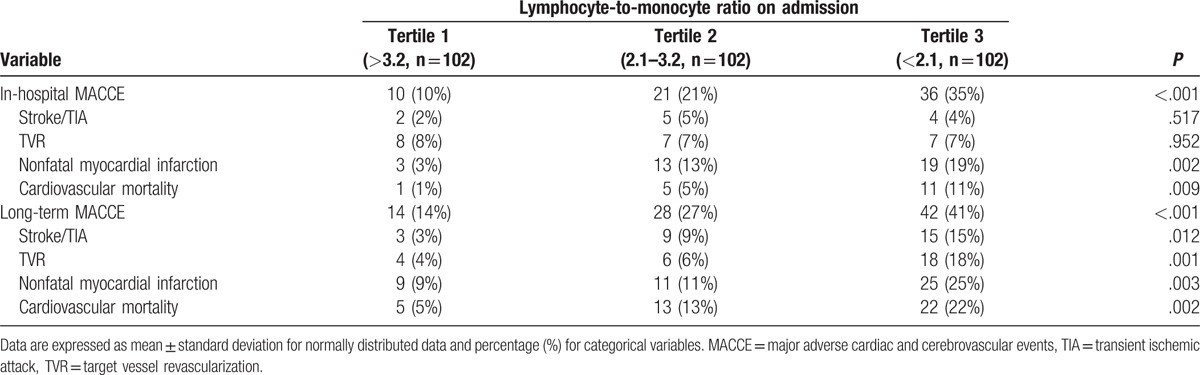
As showed in Table 3, LMR on admission and LVEF had some positive relevance (r = 0.206; P = .017). And LMR was negatively correlated with age, hs-CRP, NLR, and PLR to an extent (r = −0.107, −0.405, −0.592, −0.423, respectively; P < .001).
Table 3.
Association between LMR on admission and variables.
Figure 1 shown the ROC curves of LMR for predicting MACCE in the entire study population. The optimal cut-off of LMR was determined as 2.62 with 76% sensitivity and 78% specificity in its association with in-hospital and long-term MACCE. Figure 2 represented the cumulative survival curves for long-term MACCE-free with LMR tertiles by the Kaplan–Meier method (the log-rank P < .001). In order to identify which 2 Kaplan–Meier curves differ, we applied log-rank test with Bonferroni correction. The results showed that P value of T1 and T2 was .012, P value of T1 and T3 was less than .0001, and P value of T2 and T3 was .043 (P value <.017 was regarded as statistically significant).
Figure 1.
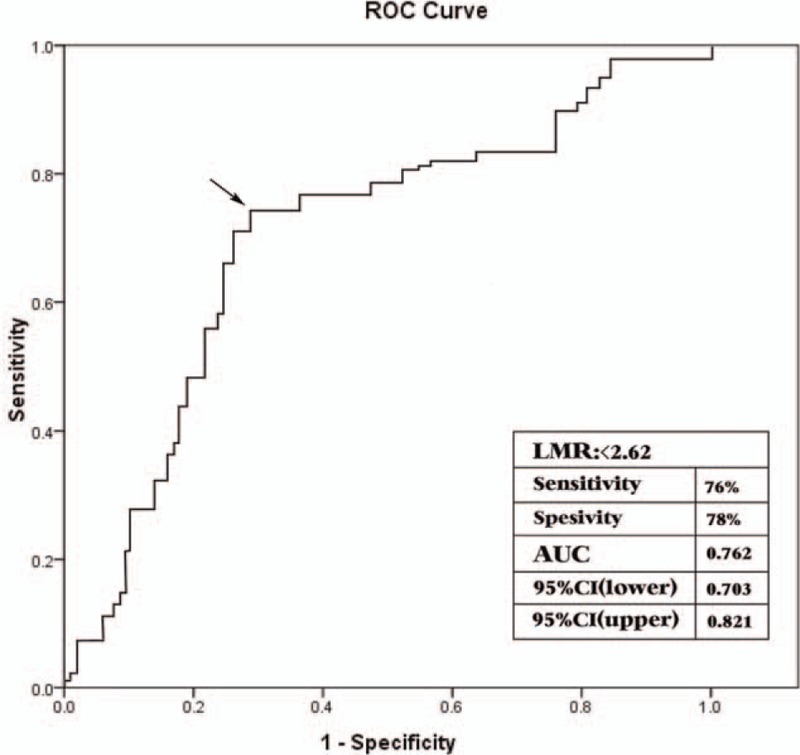
The receiver operating characteristic curve of LMR for predicting MACCE. The cut-off value was 2.62 with sensitivity of 76% and specificity of 78% for in-hospital and long-term MACCE. AUC = area under roc curve, CI = confidence interval, LMR = lymphocyte-to-monocyte ratio, MACCE = major adverse cardiac and cerebrovascular events, ROC = receiver operating characteristic.
Figure 2.
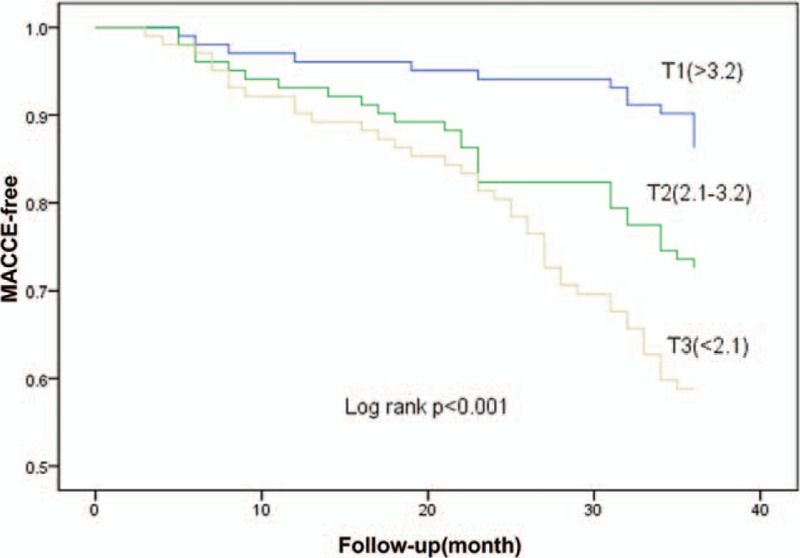
Kaplan–Meier curves for long-term MACCE-free cumulative incidence according to tertiles of LMR. The P value of log-rank test was <.001. MACCE = major adverse cardiac and cerebrovascular events.
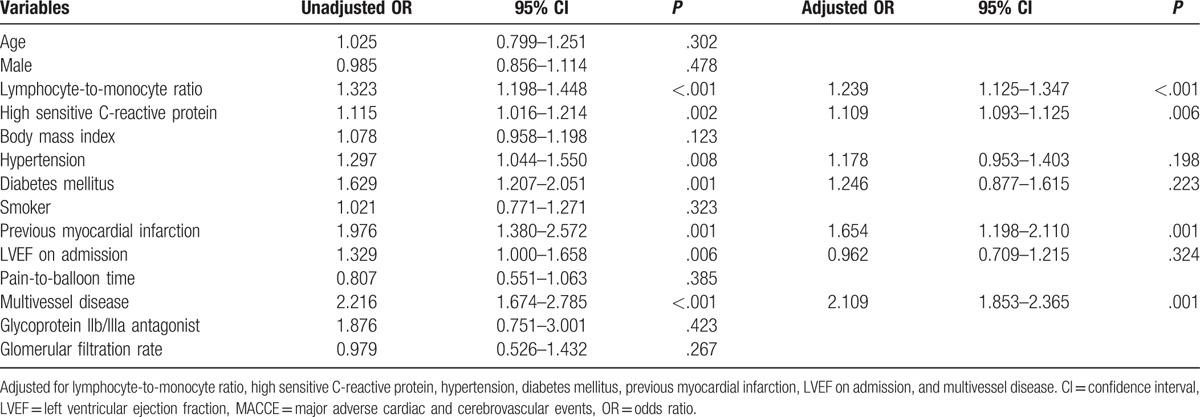
The effects of age, sex, body mass index, LMR, hs-CRP, diabetes mellitus, hypertension, smoker, previous MI, LVEF on admission, pain-to-balloon time, multivessel disease, glomerular filtration rate, and glycoprotein IIb/IIIa antagonist on MACCE were evaluated using univariate and multivariate logistic regression for in-hospital and long-term MACCE (Tables 4 and 5). In the univariate analysis, if unadjusted P value of the variables was <.10, they were identified as potential risk factors and analyzed in the multivariate logistic regression model. In multivariate logistic regression analyses for the in-hospital MACCE, LMR, hs-CRP, previous MI, LVEF on admission, multivessel disease, and glycoprotein IIb/IIIa antagonist were evaluated. LMR (odds ratio [OR] 1.192, 95% confidence interval [CI]: 1.069–1.315, P < .001), hs-CRP (OR 1.111, 95% CI: 1.043–1.179, P = .022), and LVEF on admission (OR 1.092, 95% CI: 1.027–1.157, P = .006) remained independent predictors of in-hospital MACCE after primary PCI (Table 4). In multivariate logistic regression analyses for the long-term MACCE, LMR, hs-CRP, hypertension, diabetes mellitus, previous MI, LVEF on admission, and multivessel disease were evaluated. LMR (OR 1.239, 95% CI: 1.125–1.347, P < .001), hs-CRP (OR 1.109, 95% CI: 1.093–1.125, P = .006), previous MI (OR 1.654, 95% CI: 1.198–2.110, P = .001), and multivessel disease (OR 2.109, 95% CI: 1.853–2.365, P = .001) remained independent predictors of long-term MACCE after primary PCI (Table 5).
Table 4.
Effects of multiple variables on in-hospital MACCE in univariate and multivariate logistic regression analyses.
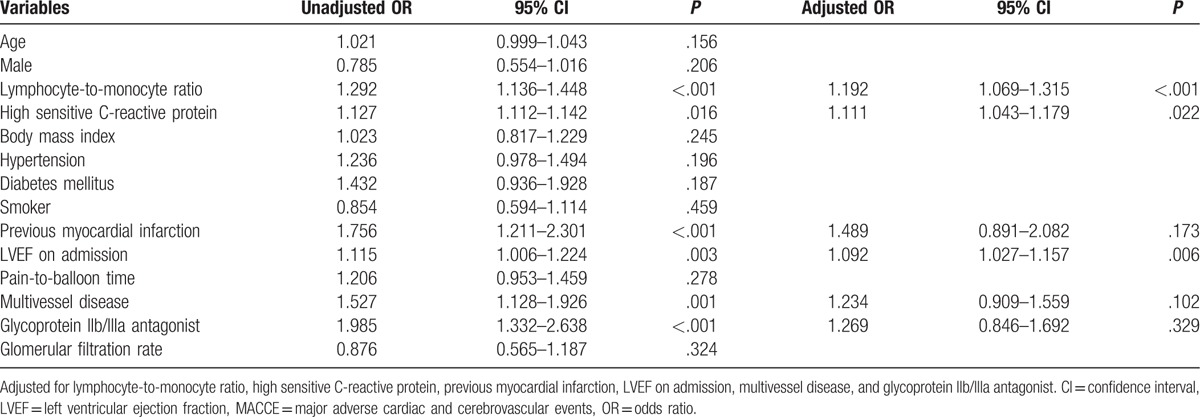
Table 5.
Effects of multiple variables on long-term MACCE in univariate and multivariate logistic regression analyses.
4. Discussion
In this study, we evaluated the value of LMR on admission in predicting the in-hospital and long-term MACCE in patients with STEMI who underwent primary PCI. As far as we know, this study appears to be the first to access the predictive value of LMR for MACCE in STEMI patients. The result of this study showed that the level of LMR on admission acted as an important prognostic indicator of in-hospital and long-term MACCE among STEMI patients. Even after adjusting other risk factors in the univariate and multivariate logistic regression, this relation remained also significant.
AMI is still one of the leading causes of death worldwide. Despite the improvement of treatment in the AMI patients, the incidence of MACCE remains still high and long-term mortality is equally depressing. Therefore, there is an urgent need to understand the progress of prognostic mechanisms after AMI and in addition to find new prognostic indicators. During a few days after AMI, the infarcted myocardium experiences rapid update, including digestion and fibrosis of the extracellular matrix.[10] Ventricular remodeling is an important factor of prognosis after AMI.[11] Studies have shown that atherosclerotic plaques were accompanied with the infiltration of inflammatory cells and ventricular remodeling after MI started from inflammatory response which triggered by acute ischemic. A strong inflammatory response to the ischemic myocardium contributes to the pathological process of ventricular remodeling after MI.[12]
In the past few decades, numerous studies have accumulated a lot of evidence to show a significant relation between inflammatory markers and MACCE in patients with STEMI. Previously, atherosclerosis was thought as a consequence of lipid and complex carbohydrate accumulation in the vascular wall, but now it is thought to be inflammatory factor aggregation leading to the formation of atherosclerotic plaques that can corrode or rupture, which results in acute coronary events. Immune cells, including congenital and adaptive immune system, have a significant impact on the pathogenesis of atherosclerosis.[13] The conversion of the immune cells into pro- and anti-inflammatory chemokine- and cytokine-producing units with the interaction between different immune cells can decisively affect the tendency of plaque to rupture, leading to clinical cardiovascular and cerebrovascular complications.[14]
The relation of the monocyte, neutrophil, and lymphocyte with the mortality of MI have been demonstrated lately.[15,16] In the early stage of MI, neutrophils gathered at the injured myocardium, which caused further damage to the myocardium and made inflammatory cells continue to gather.[17] However, despite the neutrophil count in MI increased, the neutrophils would be removed from the myocardial tissue after devouring necrotic substances, not participating in the follow-up pathophysiological process of ventricular remodeling.[5] Recently, LMR has appeared as a novel marker of inflammation that can be easily calculated from complete blood count. Several studies have showed that LMR is an effective maker of systemic inflammatory response and has been demonstrated to be associated with various types of malignancies and vascular diseases, such as peripheral arterial disease and coronary artery disease.[18–20] Kurtul et al[21] have found that there was an association of LMR with the no-reflow phenomenon in STEMI patients after primary PCI. And there have been the study indicated that LMR was significantly correlated with mortality in STEMI.[22] In pathological study of the recurrent MI, the infiltration of monocytes and lymphocytes was found in the restenosis site, which might indicate that monocytes and lymphocytes have a possible important influence on the adverse outcomes of MI.
Lymphocytes and monocytes are the major cells of systemic inflammatory response. Numerous studies have shown that the injured myocardium promoted the activation of innate immunity while innate immunity aggravated ischemic injury and blocked the remodeling after MI. But immunity also could contribute to the healing of the injured myocardium.[23] Low lymphocyte count measured in MI patients could predict long-term recurrent MI and was related with adverse outcomes and mechanical complications.[24] Azab et al[25] have found that the decrease of CD4+ T cells was closely related to the cardiac ejection dysfunction, increased incidence of MI and mortality in patients with STEMI. CD4+ T cells, especially CD4+ T regulatory cells, were beneficial to the infarcted myocardium after MI, while depletion of B cells was useful.[18,26]
Monocytes, which generated in spleen and bone marrow, entered the circulatory system after MI and were raised to the infarcted myocardium.[27] They linked to the adhesion molecules, moved into the subendothelial space of the valve reacting to several cytokines which produced locally such as insulin-like growth factor, TGF-α and-β, TNF-α, IL-6, IL-1, macrophage colony-stimulating factor and transformed into macrophages ultimately.[28] Some clinical studies have proved that the increased monocyte count were related with left ventricular dysfunction after PCI in STEMI patients, suggesting that monocytes might have a possible important effects on the process of left ventricular remodeling after reperfused MI.[29] Tsujioka et al[30] have shown that the increase of mononcyte in the pathophysiology process would have adverse impact on repairing myocardium. Inhibition of monocytes gathering to infarcted myocardium may improve ventricular function.[15]
The results of our study indicated that there was an association between LMR and in-hospital and long-term MACCE in patients with STEMI after primary PCI. However, there are some limitations in this study. It was a single-center study and the number of patients enrolled was relatively small. Most of the enrolled patients in the study are man, which may have a bias on study population. And we did not assess the level of LMR afterwards. Although a large number of experimental and clinical evidences suggest an interaction between inflammation and MI, there are no use of standard and specific inflammatory markers in the prognosis of STEMI patients. Another cohort of similar patients followed prospectively will be needed to validate the observations in this study.
5. Conclusion
According to our results, LMR on admission has a strong and independent value in predicting the in-hospital and long-term MACCE in patients with STEMI after primary PCI. Even after adjustment for confounders, LMR can independently predict MACCE, which may have significant effects on the treatment and prognosis in patients with decreased LMR on admission.
Footnotes
Abbreviations: AMI = acute myocardial infarction, CI = confidence interval, HDL = high density lipoprotein, hs-CRP = high sensitivity C-reactive protein, LDL = low density lipoprotein, LMR = lymphocyte-to-monocyte ratio, LVEF = left ventricular ejection fraction, MACCE = major adverse cardiac and cerebrovascular events, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, PCI = percutaneous coronary intervention, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic, STEMI = ST-elevated myocardial infarction, TIA = transient ischemic attack, TVR = target vessel revascularization.
Funding: This work was supported by grants from the Key Project of the Scientific and Technological Department of Henan (No.152102410067; No. 162102310142).
The authors report no conflicts of interest.
References
- [1].Velibey Y, Erbay A, Ozkurt E, et al. Acute myocardial infarction associated with blood transfusion: case report and literature review. Transfus Apher Sci 2014;50:260–2. [DOI] [PubMed] [Google Scholar]
- [2].Chia S, Nagurney JT, Brown DF, et al. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2009;103:333–7. [DOI] [PubMed] [Google Scholar]
- [3].Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012;110:621–7. [DOI] [PubMed] [Google Scholar]
- [4].Kaya MG, Akpek M, Lam YY, et al. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol 2013;168:1154–9. [DOI] [PubMed] [Google Scholar]
- [5].Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241–6. [DOI] [PubMed] [Google Scholar]
- [6].Nunez J, Nunez E, Bodi V, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis 2010;21:1–7. [DOI] [PubMed] [Google Scholar]
- [7].van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J 2012;163:57.e2–65.e2. [DOI] [PubMed] [Google Scholar]
- [8].Murat SN, Yarlioglues M, Celik IE, et al. The relationship between lymphocyte-to-monocyte ratio and bare-metal stent in-stent restenosis in patients with stable coronary artery disease. Clin Appl Thromb Hemost 2017;23:235–40. [DOI] [PubMed] [Google Scholar]
- [9].Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009;120:2271–306. [DOI] [PubMed] [Google Scholar]
- [10].Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 2009;81:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].White HD, Norris RM, Brown MA, et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44–51. [DOI] [PubMed] [Google Scholar]
- [12].Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012;110:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salisbury D, Bronas U. Inflammation and immune system contribution to the etiology of atherosclerosis: mechanisms and methods of assessment. Nurs Res 2014;63:375–85. [DOI] [PubMed] [Google Scholar]
- [14].Legein B, Temmerman L, Biessen EA, et al. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci 2013;70:3847–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 2015;35:1066–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erkol A, Oduncu V, Turan B, et al. Neutrophil to lymphocyte ratio in acute ST-segment elevation myocardial infarction. Am J Med Sci 2014;348:37–42. [DOI] [PubMed] [Google Scholar]
- [17].Dreyer WJ, Michael LH, West MS, et al. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation 1991;84:400–11. [DOI] [PubMed] [Google Scholar]
- [18].Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gary T, Pichler M, Belaj K, et al. Lymphocyte-to-monocyte ratio: a novel marker for critical limb ischemia in PAOD patients. Int J Clin Pract 2014;68:1483–7. [DOI] [PubMed] [Google Scholar]
- [20].Hu P, Shen H, Wang G, et al. Prognostic significance of systemic inflammation-based lymphocyte-monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One 2014;9:e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kurtul A, Yarlioglues M, Celik IE, et al. Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis 2015;26:706–12. [DOI] [PubMed] [Google Scholar]
- [22].Kiris T, Celik A, Varis E, et al. Association of lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology 2017;68:707–15. [DOI] [PubMed] [Google Scholar]
- [23].Frantz S, Bauersachs J, Kelly RA. Innate immunity and the heart. Curr Pharm Des 2005;11:1279–90. [DOI] [PubMed] [Google Scholar]
- [24].Widmer A, Linka AZ, Attenhofer Jost CH, et al. Mechanical complications after myocardial infarction reliably predicted using C-reactive protein levels and lymphocytopenia. Cardiology 2003;99:25–31. [DOI] [PubMed] [Google Scholar]
- [25].Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470–6. [DOI] [PubMed] [Google Scholar]
- [26].Ke D, Fang J, Fan L, et al. Regulatory T cells contribute to rosuvastatin-induced cardioprotection against ischemia-reperfusion injury. Coron Artery Dis 2013;24:334–41. [DOI] [PubMed] [Google Scholar]
- [27].Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012;209:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014;115:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jugdutt BI. Monocytosis and adverse left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol 2002;39:247–50. [DOI] [PubMed] [Google Scholar]
- [30].Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 2009;54:130–8. [DOI] [PubMed] [Google Scholar]


