Abstract
Objective:
To describe the time course of α4-integrin receptor desaturation and disease activity return in patients with relapsing-remitting MS who discontinued natalizumab and to investigate baseline and on-study predictors for the recurrence of disease activity.
Methods:
In the course of TOFINGO, a 32-week, patient- and rater-blinded multicenter, parallel-group study, we performed MRI, counted relapses, and measured α4-integrin receptor occupancy (RO) at baseline and 8, 12, 16, 20, and 24 weeks. The relationship between RO and total number of new T1 gadolinium-enhancing (Gd+) lesions was modeled using Poisson linear regression.
Results:
Patients (N = 142) were randomized (1:1:1) to 8-, 12-, or 16-week washout (WO) groups. At randomization, the median RO in the 8-, 12-, and 16-week WO groups was 94.5%, 92.4%, and 90.9%, which declined to 79.8%, 30.7%, and 8.7% after 8, 12, and 16 weeks of WO, respectively. The percentage of patients with new T1 Gd+ lesions increased with longer WO period before commencing fingolimod: 2.1% (8 weeks), 9.1% (12 weeks), and 50.0% (16 weeks). Overall, 71% of patients with first relapse between weeks 6 and 18 had RO values below the time-matched population median. Higher T2 lesion volume (LV) at baseline predicted a higher number of new T1 Gd+ lesions.
Conclusions:
A faster decline in natalizumab RO, longer WO period, and higher T2 LV at baseline were associated with an increased risk for return of inflammatory disease activity. These results provide a mechanistic rationale and, together with the main outcomes of the TOFINGO study, support initiation of fingolimod within 8 weeks of natalizumab discontinuation.
ClinicalTrials.gov identifier:
Patients with relapsing-remitting MS (RRMS) often discontinue natalizumab treatment, most frequently due to the risk of developing progressive multifocal leukoencephalopathy.1 Natalizumab discontinuation leads to recurrence of clinical and radiologic disease activity within 4 months of discontinuation.2 Natalizumab primarily acts via blocking the α4-integrin receptor and preventing potentially autoaggressive immune cell transition through the blood-brain barrier.2 Hence, α4-integrin receptor desaturation should be a direct measure of its therapeutic activity. No systematic studies have reported the relationship between α4-integrin receptor occupancy (RO) and recurrence of disease activity following natalizumab discontinuation.
In daily practice, fingolimod 0.5 mg is frequently considered a suitable option for treatment of patients with RRMS after natalizumab discontinuation, but the optimal washout (WO) period remains unknown.3 TOFINGO, a randomized double-blind study, addressed the relationship between the duration of natalizumab WO before fingolimod initiation and recurrence of disease activity.4 The results of TOFINGO suggested that better control of disease activity is achieved if fingolimod is initiated within 8–12 weeks after natalizumab discontinuation, compared with 16 weeks.4 In the course of TOFINGO, blood samples were collected to determine α4-integrin RO. We investigated the time course of α4-integrin receptor desaturation and its temporal relation to MRI and clinical disease activity. Other possible baseline and on-study predictors for the recurrence of disease activity were also assessed. The analysis was aimed to provide a mechanistic rationale for the timing of switch from natalizumab to fingolimod in patients with RRMS.
METHODS
Study design and patients.
TOFINGO (ClinicalTrials.gov identifier: NCT01499667), a 32-week, patient- and rater-blinded, randomized, multicenter, parallel-group study, included 142 patients with RRMS who received their last natalizumab infusion (LNI; 300 mg IV) within ±7 days of randomization. Details of the study have been published previously.4 Briefly, patients with RRMS aged 18–65 years who had received natalizumab treatment for ≥6 months immediately before screening were randomized 1:1:1 to one of the following 3 natalizumab WO groups before fingolimod therapy was initiated: 8-week WO (no treatment for 8 weeks since LNI followed by 24 weeks of treatment with oral fingolimod 0.5 mg); 12-week WO (no treatment for 8 weeks since LNI and placebo for 4 weeks followed by 20 weeks of treatment with fingolimod); or 16-week WO (no treatment for 8 weeks since LNI and placebo for 8 weeks followed by 16 weeks of treatment with fingolimod). All patients in the study population provided written informed consent. TOFINGO involved 44 study centers and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Study assessments.
Natalizumab serum concentration and α4-integrin RO assessment.
Patient visits were scheduled at screening, randomization, and weeks 8, 12, 16, 20, 24, and 32. At each visit, the serum concentration of natalizumab was determined using a validated ELISA method (lower quantification limit, 0.1 μg/mL). Venous blood samples were collected at all visits to determine α4-integrin RO (%) using a fluorescence-assisted cell sorter (FACS) analysis. Cells were stained with matched isotype control antibodies to determine the specificity of α4 integrin and natalizumab staining. The signal obtained with matched isotype controls was used to set the threshold for CD49d-specific α4-integrin staining and/or antinatalizumab staining with anti-IgG4 reagent. Natalizumab (final concentration, 10 μg) was spiked in 150 μL whole blood samples. The samples were incubated with detection antibodies, CD49d-specific α4-integrin staining, CD3 staining, and/or antinatalizumab staining with anti-IgG4 reagent, for 15 minutes in the dark at 23°C. BD FACS lysing solution (2 mL) was added to the sample and incubated for 15 minutes in the dark at 23°C. The samples were then pelleted by centrifugation and washed twice with 2 mL of FACS Flow Sheath Fluid. One hundred thousand nucleated events were then analyzed on FACS Calibur, using FSC/CD3 staining for T-lymphocyte gating and determination of α4-integrin and natalizumab-positive CD3 cells and median fluorescence intensity of α4-integrin and natalizumab staining.
Assessment of disease recurrence.
MRI assessments (T1 gadolinium-enhancing [Gd+] lesion counts; new T2 lesions) were conducted at baseline and at weeks 8, 12, 16, 20, and 24. All relapses during the study (both confirmed and not confirmed) were counted.
The association between RO and freedom from T1 Gd+ lesions was explored using median-effect analysis.5 The relationship between RO, patient-specific covariates, and total number of T1 Gd+ lesions was modeled using Poisson linear regression (e-Methods at Neurology.org/nn).
RESULTS
Patient disposition and baseline characteristics.
Overall, 142 patients were randomized (8-week WO: n = 50; 12-week WO: n = 42; and 16-week WO: n = 50) and analyzed according to the WO group assigned at randomization. As reported previously, baseline demographic and MS characteristics were well balanced across the WO groups.4 Of the 142 patients, 137 (96.5%) provided ≥1 evaluable RO measurement. The percentage of patients providing evaluable RO values at each visit averaged 72% (range, 58%–83%). The median serum concentration of natalizumab at the end of WO for the 8-, 12-, and 16-week WO groups was 0 µg/mL, with a range of 0–1.07, 0–0.383, and 0–0.308 µg/mL, respectively (table e-1), indicating that in most patients, the drug was eliminated from blood in the first 2 months after LNI.
RO and first report of T1 Gd+ lesions or first relapse.
At randomization, the median RO was 94.5%, 92.4%, and 90.9% in the 8-, 12-, and 16-week WO groups, respectively. Overall, the median RO declined to 79.8%, 30.7%, and 8.7% at weeks 8, 12, and 16, respectively (figure 1). MRI scans were available for 121 patients. As the length of the WO period before fingolimod initiation increased, the percentage of patients free from T1 Gd+ lesions decreased, although the relationship did not reach statistical significance (median-effect analysis, r2 = 0.891, p = 0.214; figure 2A). Up to week 8, the median RO was relatively high (79.4%), and the incidence of new T1 Gd+ lesions was 2.1% (n = 2). Both these patients with new T1 Gd+ lesions had ROs below the population median. By week 12, the median RO had declined to 17.9%, and the percentage of patients with new T1 Gd+ lesions was 9.1%. At week 16, the median RO was 9.35%, and the percentage of patients with new T1 Gd+ lesions was the highest (50.0%; figure 2B). Similar results were observed with RO and the first reporting of new/enlarging T2 lesions (data not shown). Overall, 31 patients had at least 1 relapse, 26 of whom had RO data available. Of these, 17 had the first relapse between weeks 6 and 18. The majority (n = 12; 71%) of these 17 patients had RO values below the time-matched population median (figure e-1).
Figure 1.

Receptor desaturation after the last natalizumab dose. Median α4-integrin receptor occupancy by the WO group at screening (−4 weeks), randomization (week 0), and during drug WO from weeks 8 to 32. Bars represent the IQR. A total of 137 patients (96.5%) provided at least 1 evaluable receptor occupancy measurement. IQR = interquartile range; WO = washout.
Figure 2.
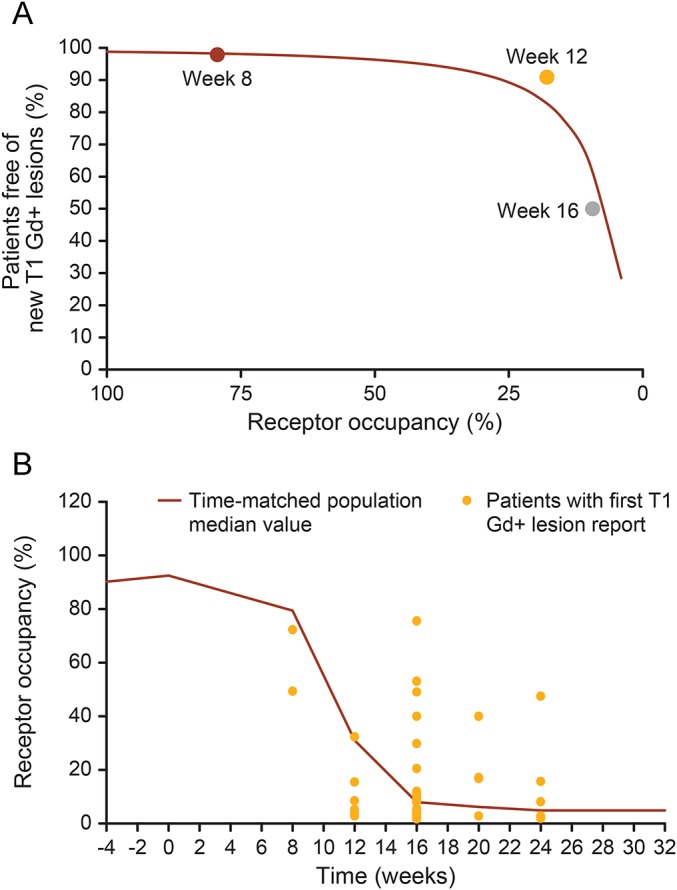
(A) Median-effect modeling of receptor occupancy and freedom from T1 Gd+ lesions. Scatter plot of the percentage of patients free from T1 Gd+ lesions vs median α4-integrin receptor occupancy for the 8-, 12-, and 16-week WO groups. Only patients not yet on fingolimod were included in the analysis. The curve represents the fit of the median-effect model to the data. (B) Median receptor occupancy and first reporting of T1 Gd+ lesions. Scatter plot showing the α4-integrin receptor occupancy at the visit when first T1 Gd+ lesions were reported in the context of the population median receptor desaturation curve. Gd+ = gadolinium enhancing; WO = washout.
Model-based prediction of T1 Gd+ lesions and new/enlarging T2 lesions.
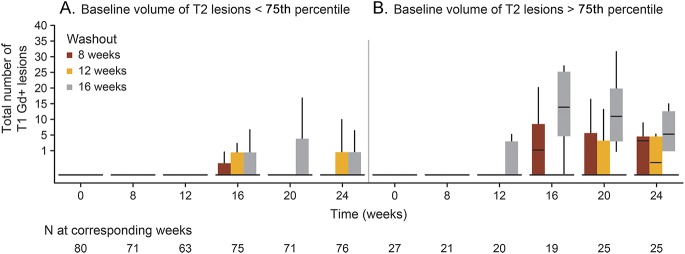
High T2 lesion volume (T2 LV) at baseline was associated with a higher number of T1 Gd+ lesions after LNI. As shown in figure 3, patients with baseline T2 LV < 75th percentile experienced fewer new T1 Gd+ lesions than did those with a baseline T2 LV > 75th percentile. This was observed in all WO groups, most noticeable in the 16-week WO group (e-Results).
Figure 3.
Total number of T1 Gd+ lesions when the baseline volume of T2 lesions was less than (A) and greater than (B) the 75th quartile. Bottom of box is Q1 and the top of the box is Q3; upper whiskers are 1.5 × IQR of the hinge; lower whiskers are 1.5 × IQR of the hinge. Gd+ = gadolinium enhancing; IQR = interquartile range.
DISCUSSION
Patients with RRMS frequently switch to fingolimod after natalizumab discontinuation to maintain adequate disease control.6,7 Several observational studies and the randomized rater- and patient-blinded TOFINGO study have suggested that a natalizumab WO period of >12 weeks is associated with higher risk of disease reactivation,4,6 and that a WO period of <12 weeks is associated with lower MRI activity without being associated with increased risks.4,6,8 Our study also suggests that in ~80% of patients, disease recurrence (new T1 Gd+ lesions) is low up to week 12 during the WO period before fingolimod initiation. Significant disease return is observed between weeks 12 and 16 of the WO period at an RO of <20% (figure 2A). This might indicate that an RO lower than 90% is still sufficient to suppress disease activity. However, recent small studies have shown that some patients may relapse or have recurrence of MRI activity within 8 weeks of LNI, indicating possibilities of an early switch to fingolimod.9,10 While a long WO period between LNI and fingolimod initiation may increase the risk of disease recurrence, early initiation of fingolimod may result in additive effects on protective immune functions.
Despite a small sample size, the TOFINGO study showed distinct numerical trends in pharmacodynamics, imaging, and clinical outcomes, which can help determine the risk of disease recurrence after natalizumab discontinuation in individual patients.
Our results showed that while natalizumab is cleared from the serum within 8–12 weeks after discontinuation, it still occupies α4-integrin receptors, thus contributing to disease activity control. In view of the association of declining RO with recurring disease activity, RO may emerge as a more plausible measure for an individualized choice of the optimal WO period. Ideally, decisions regarding the optimal WO period would also take the individual risk profile into account. In addition to prenatalizumab relapse rate and MRI activity, which have previously been identified as risk factors, our study suggests that a high baseline T2 LV defines a patient population with an increased risk of recurrence after discontinuation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients from whom data were taken for analysis. They thank Rebecca Gottschalk (Novartis Pharmaceuticals Corporation, East Hanover, NJ) for coordination of the study. All authors edited the manuscript for intellectual content, provided guidance during manuscript development, and approved the final version submitted for publication.
GLOSSARY
- FACS
fluorescence-assisted cell sorter
- Gd+
gadolinium enhancing
- LNI
last natalizumab infusion
- LV
lesion volume
- RO
receptor occupancy
- RRMS
relapsing-remitting MS
- WO
washout
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
The study was designed by the sponsor, Novartis Pharma AG, in collaboration with the steering committee. Data were collected by the investigators and analyzed by the sponsor. Novartis contributed to the interpretation of the study. All authors had full access to the data and had final responsibility for the contents and decision to submit for publication. T. Derfuss and J.M. Kovarik: design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. L. Kappos: analysis and interpretation of the data and revising the manuscript. M. Savelieva: design and conceptualization of the study, statistical analysis and interpretation of the data, and revising the manuscript. H. Wiendl: design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Y. Zhang: design and conceptualization of the study, statistical analysis and interpretation of the data, and revising the manuscript. D. Tomic: design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. R. Chhabra; drafting and revising the manuscript. A. Thakur: drafting and revising the manuscript.
STUDY FUNDING
The study was sponsored by Novartis Pharma AG.
DISCLOSURE
T. Derfuss serves on scientific advisory boards for Novartis Pharmaceuticals, Merck Serono, Biogen, Sanofi Genzyme, GeNeuro, Octapharma, MedDay, Mitsubishi Pharma, Roche, and Bayer Schering Pharma; has received funding for travel and/or speaker honoraria from Biogen, Sanofi Genzyme, Novartis Pharmaceuticals, Merck Serono, Roche, and Bayer Schering Pharma; is on the editorial board for PLoS One; receives research support from Biogen, Novartis Pharma, the European Union, the Swiss National Foundation, and the Swiss MS Society; is a member of steering committee for Mitsubishi Pharma and GeNeuro; and his spouse is an employee of and owns stock options in Novartis Pharma. J.M. Kovarik is on the editorial board for Journal of Clinical Pharmacology and is employed by and holds stock in Novartis. L. Kappos' institution (University Hospital Basel) has received in the last 3 years and used exclusively for research support: steering committee, advisory board, and consultancy fees (Actelion, Addex, Bayer HealthCare, Biogen Idec, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB, and Xenoport); speaker fees (Bayer HealthCare, Biogen Idec, Merck, Novartis, Sanofi, and Teva); support for educational activities (Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen Idec, European Union, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation). L. Kappos is on the editorial board for Multiple Sclerosis Journal, Multiple Sclerosis and Related Disorders, and Journal of Neurology. M. Sevelieva is employed by Novartis Pharma. R. Chhabra is employed by Novartis Healthcare. A. Thakur is employed by Novartis Healthcare. Y. Zhang is employed by Novartis Pharma. H. Wiendl served on the scientific advisory board for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono, Novartis, Roche, and Teva; is on the editorial board for PLoS One, Neurotherapeutics, and Recent Patents on Inflammation and Allergy Drug Discovery; has received compensation for serving as a consultant or speaker for, or has received research support from Bayer Schering Pharma, Biogen Idec/Elan Corporation, CSL Behring, EMD Serono, Fresenius Medical Care, OmniaMed, GlaxoSmithKline, GW Pharmaceuticals, Bayer Vital, Sanofi Genzyme, Roche, Merck Serono, Novartis, Novo Nordisk, Sanofi Aventis, Teva Pharmaceutical Industries, German Ministry for Education and Research, European Union, Interdisciplinary Centre of Clinical Research, and PML Consortium; and Else Kroner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, and RE Children's Foundation. D. Tomic holds a patent on fingolimod's activity on T1 hypointense lesions; is employed by Novartis; and holds stock in Novartis. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880. [DOI] [PubMed] [Google Scholar]
- 2.Rasenack M, Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert Rev Neurother 2016;16:587–594. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L, Grutzke B, Eveslage M, et al. Assessment of immune functions and MRI disease activity in relapsing-remitting multiple sclerosis patients switching from natalizumab to fingolimod (ToFingo-Successor). BMC Neurol 2015;15:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 2015;85:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 6.Jokubaitis VG, Li V, Kalincik T, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology 2014;82:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naegelin Y, Rasenack M, Blatti C, et al. Switching from natalizumab to fingolimod: recurrence of disease activity is closely related to duration of wash out and alpha-4 integrin desaturation—a prospective study in RRMS. Mult Scler J 2015;21(11 suppl):556–557. [Google Scholar]
- 8.Cohen M, Maillart E, Tourbah A, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 2014;71:436–441. [DOI] [PubMed] [Google Scholar]
- 9.Fox RJ, Cree BA, De Seze J, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology 2014;82:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naegelin Y, Rasenack M, Sanderson N, et al. An observational study evaluating disease control, safety, and immunological changes in patients with relapsing remitting multiple sclerosis switching from previous treatment with natalizumab to fingolimod (SWITCH-UHBS). Mult Scler J 2013;19(11 Suppl):460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.