TO THE EDITOR
Islet transplantation can restore euglycemia and eliminate severe hypoglycemia in patients with type 1 diabetes.1 Limitations of intrahepatic islet transplantation include a restricted transplant-tissue volume, bleeding with placement of the transplant, exposure to high levels of immunosuppressive drugs after transplantation, and the triggering of an immediate blood-mediated inflammatory response.2 The omentum has a dense vascularized surface for islet implantation, drains into the portal system, and is easily accessible.3,4
We report on a 43-year-old woman with a 25-year history of type 1 diabetes. Her weight was 53.4 kg (117.7 lb), and her body-mass index (the weight in kilograms divided by the square of the height in meters) was 21.5. She received insulin at a mean (±SD) daily dose of 32.9±1.3 units. In this patient, diabetes was complicated by unawareness of hypoglycemia and episodes of severe hypoglycemia. The stimulated C-peptide level was less than 0.3 ng per milliliter (<0.1 nmol per liter), and the glycated hemoglobin level was 6.8%.
As part of an ongoing study (Allogeneic Islet Cells Transplanted onto the Omentum; ClinicalTrials.gov number, NCT02213003), the patient underwent islet transplantation onto the omentum. A total of 602,395 islet equivalents (an islet equivalent is the standard unit for reporting variations in the volume of islets, with the use of a standard islet diameter of 150 μm) from one deceased donor (total packed-tissue volume, 6.5 ml) were combined with autologous plasma (in a 1:2 ratio) and laparoscopically layered onto the omentum. A total of 20 ml of recombinant thrombin (Recothrom), reconstituted in a solution containing 1000 units per milliliter, was layered over the islets, followed by another layer of autologous plasma to generate a degradable biologic scaffold. The induction immunosuppression regimen consisted of antithymocyte globulin and etanercept, and the maintenance immunosuppression regimen consisted of mycophenolate sodium and tacrolimus. Tacrolimus was switched to sirolimus 8 months after transplantation because the patient had hair loss. No surgical complications were observed.
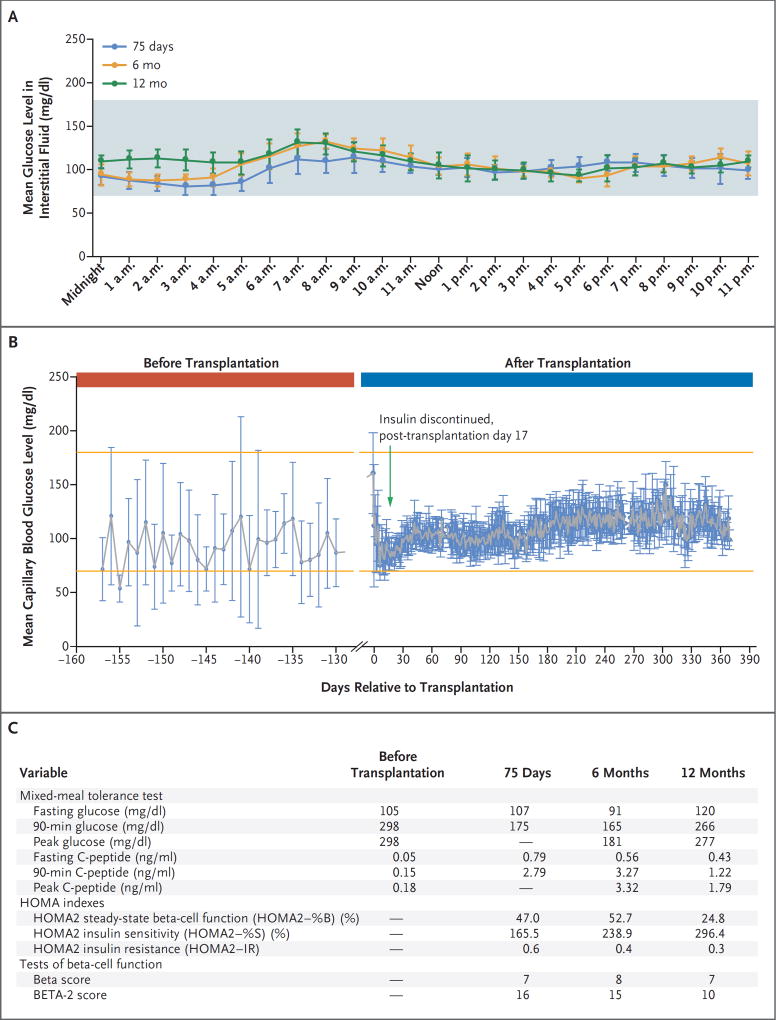
Insulin was discontinued 17 days after the transplantation. The capillary blood glucose level, data from continuous glucose monitoring, results of a mixed-meal tolerance test, homeostasis model assessment (HOMA2) indexes, and Beta and BETA-2 scores (composite scores of beta-cell function after islet transplantation)5 are shown in Figure 1, and details of the monitoring of the patient are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
Figure 1. Results of Glucose Monitoring and Mixed-Meal Tolerance Tests, Homeostasis Model Assessment (HOMA2) Index Values, and Beta and BETA-2 Scores in the Patient.
Panel A shows the results of hourly continuous glucose monitoring (G4 Platinum, Dexcom) on the 7 days before each study visit. I bars indicate standard deviations. The shaded area shows the glucose range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter). The 7-day mean (±SD) glucose level in 1986 interstitial glucose measurements obtained every 5 minutes was 99±15 mg per deciliter (range, 60 to 169) (5.5±0.8 mmol per liter [range, 3.3 to 9.4]) at 75 days; in 1985 interstitial glucose measurements, it was 105±17 mg per deciliter (range, 71 to 158) (5.8±0.9 mmol per liter [range, 3.9 to 8.8]) at 6 months; and in 1966 interstitial glucose measurements, it was 109±15 mg per deciliter (range, 73 to 151) (6.0±0.8 mmol per liter [range, 4.1 to 8.4]) at 12 months. To convert the values for glucose to millimoles per liter, multiply by 0.05551. Panel B shows 2012 capillary blood glucose values obtained before transplantation and through postoperative day 365. I bars indicate standard deviations. The upper and lower orange lines show the glucose range of 70 to 180 mg per deciliter. Panel C shows variables derived from the mixed-meal tolerance test and HOMA2 indexes. Samples obtained in the mixed-meal tolerance test at 75 days for measurements of glucose and C-peptide levels included only 0 and 90 minutes. The HOMA2 estimates steady-state beta-cell function (HOMA2–%B) and insulin sensitivity (HOMA2– %S) as percentages of a normal reference population. HOMA2–IR is the reciprocal of HOMA2–%S. HOMA2 indexes were calculated with the use of the HOMA2 calculator, version 2.2.3 (Diabetes Trials Unit, University of Oxford), available at www.dtu.ox.ac.uk/homacalculator. Beta scores range from 0 to 8, with higher scores indicating better islet function, and BETA-2 scores range from 0 to 42, with lower scores indicating higher risks of glucose intolerance and insulin dependence. To convert the values for C-peptide to nanomoles per liter, multiply by 0.331.
At 12 months, in response to a 5-hour mixed-meal tolerance test, the 90-minute glucose level was 266 mg per deciliter (14.6 mmol per liter); this level decreased to 130 mg per deciliter (7.1 mmol per liter) at 300 minutes. On continuous glucose monitoring, the 7-day mean glucose level in 1966 interstitial glucose readings was 109±15 mg per deciliter (6.0±0.8 mmol per liter), the glycated hemoglobin level was 6.0%, the Beta score (based on the fasting glucose level, the 90-minute C-peptide level after a mixed-meal tolerance test, the glycated hemoglobin level, and the use of either insulin or glucose-lowering therapies) was 7 (on a scale from 0 to 8, with higher scores indicating better islet function), and the BETA-2 score (calculated from the fasting C-peptide level, the fasting glucose level, and the glycated hemoglobin level measured in a single fasting blood sample) was 10 (on a scale from 0 to 42, with lower scores indicating higher risks of glucose intolerance and insulin dependence). After evaluation at 6 months, insulin secretion decreased and glucose levels increased, although these levels remained below those that would constitute diabetes. After transplantation, the patient exercised regularly and followed a low-carbohydrate diet, which probably contributed to her stable glycemic control.
In this patient, islet transplantation onto the omentum restored euglycemia and insulin independence. A functional decline was observed at 12 months with an increase in insulin sensitivity, which we speculate may have been due to the switch from tacrolimus to sirolimus. The patient continued to have stable glycemic control without exogenous insulin and without episodes of hypoglycemia.
Data from long-term follow-up and regarding additional omental islet transplantations are lacking. The current study is ongoing to determine the safety and long-term feasibility of this strategy of islet transplantation.
Supplementary Material
Acknowledgments
Supported by the Juvenile Diabetes Research Foundation and the Helmsley Charitable Trust, the Diabetes Research Institute Foundation, and a grant (1UL1TR000460, to the University of Miami Clinical and Translational Science Institute) from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities.
Footnotes
The views expressed in this letter are those of the authors and do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the U.S. government.
Disclosure forms provided by the authors is available with the full text of this letter at NEJM.org.
References
- 1.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39:1230–40. doi: 10.2337/dc15-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74. doi: 10.1007/s11892-011-0216-9. [DOI] [PubMed] [Google Scholar]
- 3.Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9:91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman DM, Molano RD, Fotino C, et al. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65:1350–61. doi: 10.2337/db15-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes S, Oram RA, Smith A, et al. Validation of the BETA-2 score: an improved tool to estimate beta cell function after clinical islet transplantation using a single fasting blood sample. Am J Transplant. 2016;16:2704–13. doi: 10.1111/ajt.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.