Abstract
Bone deformities and fractures are common consequences of renal osteodystrophy in the dialysis population. Persistent hypophosphatemia may be observed with more frequent home hemodialysis regimens, but the specific effects on the skeleton are unknown. We present an end-stage renal disease patient treated with frequent home hemodialysis who developed severe bone pain and multiple fractures, including a hip fracture and a tibia-fibula fracture complicated by non-union, rendering her non-ambulatory and wheelchair-bound for over a year. A bone biopsy revealed severe osteomalacia, likely secondary to chronic hypophosphatemia and hypocalcemia. Treatment changes included addition of phosphate to the dialysate, a higher dialysate calcium concentration, and increased calcitriol dose. Several months later, the patient no longer required a wheelchair and was able to ambulate without pain. Repeat bone biopsy revealed marked improvements in bone mineralization and turnover parameters. Also, with increased dialysate phosphate and calcium, as well as increased calcitriol, circulating FGF23 levels increased.
Index Words: Hypophosphatemia, Osteomalacia, Frequent hemodialysis, Fibroblast growth factor 23 (FGF-23), Home hemodialysis, serum phosphate, impaired bone mineralization, bone fracture, bone biopsy, end-stage renal disease (ESRD), case report
Although hyperphosphatemia is common in end-stage renal disease (ESRD), hypophosphatemia may also occur. Indeed, ESRD patients treated with frequent home hemodialysis often have such high rates of phosphate removal so as to require the addition of phosphate to the dialysate solution in order to prevent hypophosphatemia1; however, the long-term skeletal consequences are unknown. In individuals with normal kidney function, chronic hypophosphatemia may lead to impaired bone mineralization and osteomalacia, which may present clinically as bone pain and/or fractures. Here, we present a case of symptomatic osteomalacia in a patient on frequent home hemodialysis, treated with the addition of phosphate to the dialysate solution, calcium supplementation, and active vitamin D sterols. Furthermore, we demonstrate the response of circulating fibroblast growth factor 23 (FGF-23) levels to such therapy changes.
Case Report
A 52-year-old Caucasian female ESRD patient presented to the orthopedic clinic for a poorly-healing tibia-fibula fracture. She sustained the left leg fracture with minimal trauma eight months prior to presentation, and the fracture had yet to heal, requiring a short leg cast that was serially changed. She had an extensive fracture history, including a fractured hip twelve months prior to presentation, a pubic ramus stress fracture, and metatarsal and metacarpal fractures. She also reported right tibia pain. Her bone pain and multiple fractures rendered her non-ambulatory and wheelchair-bound for 17 months prior to presentation. She was referred to our Bone Clinic, where X-rays revealed a non-union fracture of the left tibia and fibula, with significant osteopenia.
The patient developed ESRD secondary to suspected Alport’s syndrome at 14-years-old and started dialysis. She underwent three failed kidney transplants, the last of which occurred 12 years prior to presentation, and had been dialysis-dependent for a total of 30 years. Her past medical history also included subtotal parathyroidectomy 35 years prior to presentation and sensorineural hearing loss. The patient was on home hemodialysis via a left upper extremity arteriovenous fistula, four sessions per week, seven hours per session, using a Fresenius 2008K home machine, with a blood flow rate of 220 mL/minute, a dialysate flow rate of 300 mL/minute, and a dialysate composition of 2 mEq/L potassium, 2.5 mEq/L calcium, and 35 mEq/L bicarbonate. She was anuric. The patient took ergocalciferol 50,000 IU weekly and calcitriol 0.25 mcg daily. She was not treated with calcium supplements or phosphate binders. Her diet included dairy products, and her daily dietary phosphate intake was approximately 600 mg.
Review of the patient’s laboratory results revealed chronic hypophosphatemia, with serum phosphate ranging from 1.9–2.9 mg/dL for several months prior to the clinic visit, and a nadir value of 1.5 mg/dL during the year prior to presentation. Serum calcium levels were low-normal in the months prior to the clinic visit (8.5–9.0 mg/dL), and several ionized calcium measurements during this period were <1.10 mmol/L. In the setting of this mild hypocalcemia, intact parathyroid hormone ranged from 24–53 pg/mL; more remote levels were in the high 30 to low 40 pg/ml range. Serum 25(OH) vitamin D and 1,25(OH)2 vitamin D levels were sufficient—76 ng/mL and 52 pg/mL, respectively. Plasma carboxy-terminal (total) FGF-23 was 152 RU/mL, and intact (bioactive) FGF-23 was 55 pg/mL, low values for a dialysis patient, likely reflecting the consequences of hypophosphatemia.
Given the history of fractures, bone pain, and persistent hypophosphatemia, a bone biopsy after double tetracycline labeling was performed, which revealed markedly increased osteoid volume, surface, and thickness; zero percentage mineralizing surface; a mineral apposition rate of zero; an infinite mineralization lag time; and a bone formation rate of zero (Table 1). These findings were consistent with a severe mineralization defect, and osteomalacia was diagnosed.
Table 1.
Bone histomorphometry before and after addition of phosphate to the dialysate
| Histologic variable | Reference rangea | Before treatment | After treatment |
|---|---|---|---|
| Bone volume/tissue volume (%) | 21.8 ± 7.2 | 29.0 | 38.3 |
| Osteoid volume/bone volume (%) | 1.6 ± 1.9 | 35.4 | 47.9 |
| Osteoid surface/bone surface (%) | 9.2 ± 8.4 | 83.4 | 76.5 |
| Osteoid thickness (μm) | 10.8 ± 3.2 | 27.3 | 29.2 |
| Bone formation rate/bone surface (μm2/mm2/day) | 25.2 ± 10.8 | 0 | 14.1 |
| Mineralizing surface/bone surface (%) | 12.0 ± 5.0 | 0 | 3.3 |
| Mineral apposition rate (μm/day) | 0.7 ± 0.1 | 0 | 1.1 |
| Adjusted apposition rate (μm/day) | 0.5 ± 0.2 | 0 | 0.1 |
| Mineralization lag time (days) | 23.7 ± 2.7 | ∞ | 577.5 |
mean ± SD for adult females.
Given the diagnosis of osteomalacia in the setting of hypophosphatemia and hypocalcemia, several changes were made to the patient’s treatment regimen. First, phosphate was added to the dialysate solution. With every hemodialysis treatment, one Fleet® enema solution (118 mL, with a phosphate concentration of 1.38 mmol/mL) was instilled directly to the dialysate, resulting in a dialysate phosphate concentration of 1.3 mmol/L. Additionally, the dialysate calcium concentration was increased from 2.5 to 4.0 mEq/L, and the calcitriol dose was increased from 0.25 mcg daily to 0.5 mcg daily.
Within a few weeks, the patient’s pain markedly diminished. Serum phosphate and calcium increased (although there were temporary decreases, most values were higher), and alkaline phosphatase steadily decreased, suggestive of improving bone mineralization (Fig 1). Over time, carboxy-terminal FGF-23 levels increased 57%, and intact FGF-23 levels increased 222%. Six months after the clinic visit, there was some radiographic evidence of fracture healing, so the patient was transitioned from a cast to a lower leg walking boot. Repeat bone biopsy, performed eighteen months later, revealed marked improvements in mineralization parameters, including normalized mineral apposition rate, improved percentage mineralizing surface, improved mineralization lag time, and improved bone formation rate (Table 1). By then, the patient was pain-free, no longer required the lower leg boot, and was walking without difficulty.
Figure 1.
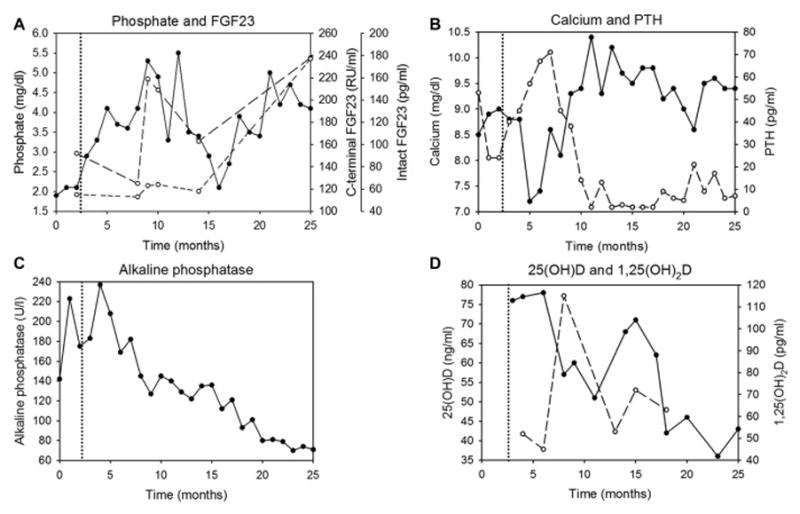
Biochemical parameters over time. The initial bone biopsy and clinic visit occurred at the 2-month time point. Phosphate was added to the dialysate just after the 3-month time point (dotted vertical line), and repeat bone biopsy was performed at the 25-month time point. (A) phosphate (solid line), C(arboxy)-terminal (total) fibroblast growth factor 23 (FGF-23) (long-dashed line), intact (bioactive) FGF-23 (short-dashed line); (B) calcium (solid line) and intact parathyroid hormone (PTH) (dashed line); (C) alkaline phosphatase; (D) 25-hydroxyvitamin D (25(OH) vitamin D) (solid line) and 1,25-dihydroxyvitamin D (1,25(OH)2 vitamin D) (dashed line).
Discussion
This case demonstrates the consequences of long-term hypophosphatemia and hypocalcemia in a patient treated with frequent home hemodialysis. Bone fragility is common in dialysis patients and may precipitate fractures. Indeed, in dialysis populations, fracture prevalence ranges from 10–40%, with even higher percentages observed in patients over 502. Hip fractures3,4 and long bone fractures5 in dialysis patients are associated with increased mortality. Children with chronic kidney disease, in whom mineralization defects are common6, are also at risk for fractures7.
Renal osteodystrophy is defined as alterations in bone morphology associated with chronic kidney disease; it is quantifiable by bone biopsy histomorphometry8. The five subtypes of renal osteodystrophy (mild, osteitis fibrosa, osteomalacia, adynamic, mixed) are classified on the basis of bone turnover and mineralization2. Turnover is quantified by the bone formation rate, and mineralization, which reflects the amount of unmineralized osteoid, is measured by the mineralization lag time2. A large retrospective study of dialysis patients found a higher frequency of fractures in patients with osteomalacia as compared to those with other types of renal osteodystrophy9.
Our patient developed severe osteomalacia, characterized by a major decrease in osteoid mineralization. As bone matrix mineralization requires normal concentrations of phosphate and calcium, any process causing hypophosphatemia and/or hypocalcemia may engender suboptimal mineralization. Our patient had both chronic hypophosphatemia and relative hypocalcemia, despite sufficient vitamin D levels. Although both phosphate and calcium deficiencies are potentially associated with the development of osteomalacia, we believe that prolonged and persistent hypophosphatemia played a predominant role in the pathogenesis of this patient’s severe mineralization defect. Indeed, within weeks after the addition of phosphate to the dialysate, the patient’s bone pain markedly diminished.
The amount of hemodialysis the patient received likely contributed to the hypophosphatemia10,11. A recent study of home hemodialysis patients receiving 17.5 hours of dialysis per week found that an average of 4.16 grams of phosphate was removed weekly12. This is nearly double the average amount of phosphate removed (2.36 grams) in a study of traditional thrice weekly hemodialysis patients who received an average of 11.5 hours of dialysis per week13. Our patient received 28 hours of hemodialysis weekly and thus likely had even greater phosphate removal. As shown in the Frequent Hemodialysis Network Nocturnal Trial, compared to patients assigned to conventional thrice weekly hemodialysis, patients assigned to hemodialysis sessions of at least 6 hours six times per week had a mean decrease in serum phosphate of 1.24 mg/dl1 Furthermore, 73% of these patients were able to discontinue phosphate binders, and 42% required phosphate supplementation of the dialysate to prevent hypophosphatemia1.
Our patient’s FGF-23 levels were very low for a dialysis patient. As phosphate has a large volume of distribution, and equilibration between the intracellular and extracellular compartments is slow, increased phosphate removal from the body may not result in lower serum phosphate levels. However, circulating levels of FGF-23, a key regulator of phosphate metabolism, may better reflect total body phosphate burden14,15. Indeed, in a study of short daily vs. conventional hemodialysis, although serum phosphate levels were similar between the two groups, the short daily hemodialysis group had significantly lower levels of carboxy-terminal FGF-23 (823 vs. 2521 RU/mL), likely secondary to increased phosphate removal15. Similarly, our patient’s markedly low FGF-23 levels likely reflected total body phosphate deficiency. After phosphate was added to the dialysate, the FGF-23 levels increased; notably, the concentration of intact (biologically active) FGF-23 tripled. Whereas oral phosphate loading increases circulating FGF-23 levels16, acute intravenous phosphate infusion does not17. Here, we demonstrate that chronic phosphate loading via the dialysate affects FGF-23 levels.
Although the development of hypophosphatemia, and the need to add phosphate to the dialysate, has been described in frequent hemodialysis patients1,18,19, the specific skeletal consequences have not been defined. The present case illustrates the utility of bone biopsy in such patients in order to define the long-term consequences. The addition of Fleet® enema solution stably increases the dialysate phosphate concentration, without affecting the concentrations of other dialysate solutes20. Furthermore, no bacteria or endotoxin have been detected in samples of Fleet®-enriched dialysate20. As such, this simple intervention may safely be able to maintain normophosphatemia in patients on extended-hours hemodialysis, and would be especially beneficial for patients with biopsy-proven osteomalacia.
Acknowledgments
Support:
This work was supported in part by USPHS Grants DK-67563, DK-35423, DK-80984; CTSI Grant UL1 TR-000124; and NIH Grants K12 HD-034610 and K08 DK-111980.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review:
Evaluated by an external peer reviewer, an Associate Editor, and Deputy Editor Berns.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daugirdas JT, Chertow GM, Larive B, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;23(4):727–738. doi: 10.1681/ASN.2011070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDIGO Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;(76):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Tentori F, McCullough K, Kilpatrick RD, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85(1):166–173. doi: 10.1038/ki.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44(4):672–679. [PubMed] [Google Scholar]
- 5.Kaneko TM, Foley RN, Gilbertson DT, Collins AJ. Clinical epidemiology of long-bone fractures in patients receiving hemodialysis. Clin Orthop Relat Res. 2007;457:188–193. doi: 10.1097/BLO.0b013e318031465b. [DOI] [PubMed] [Google Scholar]
- 6.Wesseling-Perry K, Pereira RC, Tseng CH, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denburg MR, Kumar J, Jemielita T, et al. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J Am Soc Nephrol. 2016;27(2):543–550. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 9.Araújo SM, Ambrosoni P, Lobão RR, et al. The renal osteodystrophy pattern in Brazil and Uruguay: an overview. Kidney Int Suppl. 2003;(85):S54–56. doi: 10.1046/j.1523-1755.63.s85.13.x. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman DL, Nesrallah GE, Chan CT, et al. Dialysate calcium concentration and mineral metabolism in long and long-frequent hemodialysis: a systematic review and meta-analysis for a Canadian Society of Nephrology clinical practice guideline. Am J Kidney Dis. 2013;62(1):97–111. doi: 10.1053/j.ajkd.2013.02.357. [DOI] [PubMed] [Google Scholar]
- 11.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohn OF, Coe FL, Ing TS. Solute kinetics with short-daily home hemodialysis using slow dialysate flow rate. Hemodial Int. 2010;14(1):39–46. doi: 10.1111/j.1542-4758.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y. Superior dialytic clearance of beta(2)-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int. 2006;70(4):794–799. doi: 10.1038/sj.ki.5001640. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M. Fibroblast growth factor 23 and the future of phosphate management. Curr Opin Nephrol Hypertens. 2009;18(6):463–468. doi: 10.1097/MNH.0b013e328331a8c8. [DOI] [PubMed] [Google Scholar]
- 15.Zaritsky J, Rastogi A, Fischmann G, et al. Short daily hemodialysis is associated with lower plasma FGF23 levels when compared with conventional hemodialysis. Nephrol Dial Transplant. 2014;29(2):437–441. doi: 10.1093/ndt/gft382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vervloet MG, van Ittersum FJ, Büttler RM, Heijboer AC, Blankenstein MA, ter Wee PM. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6(2):383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito N, Fukumoto S, Takeuchi Y, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25(6):419–422. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 18.Pierratos A. Nocturnal home haemodialysis: an update on a 5-year experience. Nephrol Dial Transplant. 1999;14(12):2835–2840. doi: 10.1093/ndt/14.12.2835. [DOI] [PubMed] [Google Scholar]
- 19.Sam R, Vaseemuddin M, Leong WH, Rogers BE, Kjellstrand CM, Ing TS. Composition and clinical use of hemodialysates. Hemodial Int. 2006;10(1):15–28. doi: 10.1111/j.1542-4758.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 20.Su WS, Lekas P, Carlisle EJ, et al. Management of hypophosphatemia in nocturnal hemodialysis with phosphate-containing enema: a technical study. Hemodial Int. 2011;15(2):219–225. doi: 10.1111/j.1542-4758.2011.00533.x. [DOI] [PubMed] [Google Scholar]


