Abstract
Hodgkin Lymphoma (HL) is a lymphoproliferative disorder of B cells that commonly has a favorable prognosis when treated with either combination chemotherapy and radiation therapy, or chemotherapy alone. However, the prognosis for patients who relapse, or have evidence for refractory disease, is poor and new treatments are needed for patients with progressive disease. HL has a unique tumor microenvironment consisting of a predominance of inflammatory cells and a minority of malignant Hodgkin and Reed-Sternberg (HRS) cells. This unique biology provides an opportunity for novel therapy approaches that either specifically target the malignant HRS cell or target the inflammatory tumor microenvironment. New therapies including antibody drug conjugates targeting CD30, small molecule inhibitors that inhibit critical cell signaling pathways, monoclonal antibodies that block immune checkpoints, or agents that modulate the immune microenvironment have all recently been tested in HL with significant clinical activity. Multiple clinical trials are currently ongoing testing these agents in the relapsed and refractory setting but also in earlier phases of therapy often in combination with more standard treatment.
Keywords: Brentuximab vedotin Pembrolizumab, Nivolumab, Everolimus, Hodgkin lymphoma
1. Introduction
Hodgkin lymphoma (HL) is a rare disease that accounts for approximately 9,000 new patients each year [1]. This population represents approximately 12% of all of the patients with lymphoma seen in the United States annually. HL has two distinct subtypes, classical HL and nodular lymphocyte-predominant HL. Classical HL includes four histologic subtypes: nodular sclerosis, mixed cellularity, lymphocyte depletion, and lymphocyte-rich HL [2]. Most patients diagnosed with HL respond well to initial treatment resulting in an approximately 75% cure rate. A subset of the patients are either resistant to initial therapy or relapse after initial treatment, requiring additional therapy typically in the form of second-line regimens with autologous stem cell transplantation. For those patients who progress after autologous stem cell transplantation, the outcome is poor. Novel therapies are clearly needed for patients with relapsed and refractory disease. Furthermore, novel treatments are also needed for patients with newly diagnosed HL to improve the cure rate while reducing treatment-related toxicity.
2. Biological targets in HL
HL has a unique histological appearance with a very small number of malignant Hodgkin and Reed-Sternberg (HRS) cells present among an overwhelming number of reactive and inflammatory cellular infiltrate [3]. The inflammatory infiltrate includes T cells, histiocytes, eosinophils, B cells, and plasma cells that appear to have been attracted to by a network of cytokines and chemokines that are secreted by the malignant HRS cells [4]. These cytokines include thymus and activation regulated chemokine (TARC/CCL17), interleukin (IL)-6, IL-13, or soluble IL-2 receptor [5,6]. The intratumoral immune cells that have been attracted by these cytokines appear to provide survival and growth support to the HRS cells. While many cells present in the tumor appear to be immune effector cells, these cells appear unable to effectively eradicate the tumor or mount an effective anti-tumor immune response. While initial studies suggested that intratumoral T cells have a TH2 phenotype, recent data suggest that they may in fact be TH1 cells. This is due to an abundant expression of TH1-associated TBET seen on gene expression profiling, while TH2-associated GATA3 was seen at substantially lower levels [7]. Despite the presence of TH1 cells in the tumor, these cells do not appear to efficiently target the malignant clone.
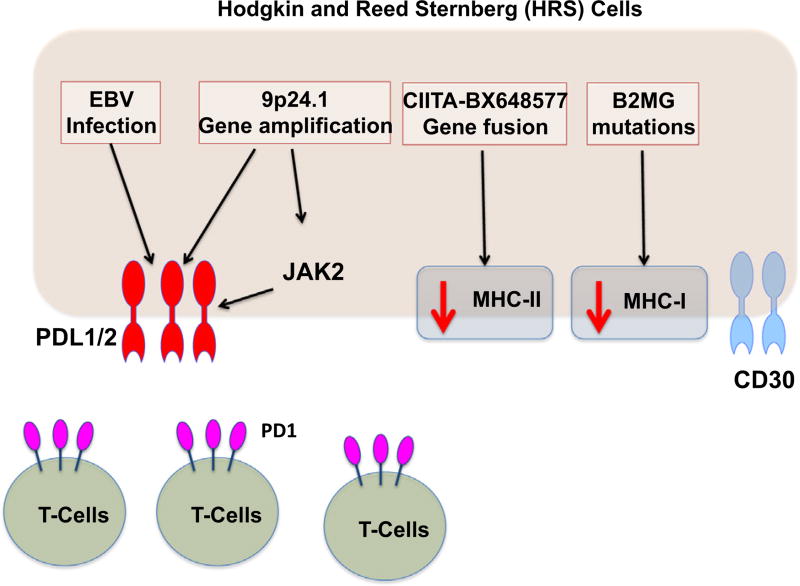
A potential reason for the lack of an effective anti-tumor immune response may be increased signaling through program death 1 (PD1). PD1 is physiologically expressed on activated T cells, and PD1 signaling has been shown to suppress T-cell function. HRS cells express high levels of the PD1 ligands (PD-L1 and PD-L2), which is linked to genetic amplification at the PD-L1 and PD-L2 locus on chromosome 9p24.1. Epstein-Barr virus (EBV) infection, which is commonly seen in HL, may also account for increased expression of the PD1 ligands [8]. Furthermore, the genetic alterations on chromosome 9p24.1results in activation of JAK-STAT signaling, further increasing the expression of PD-L1 and PD-L2 (Fig. 1).
Fig. 1.
Regulation of PD-L1 and PD-L2 expression in Hodgkin lymphoma.
An additional tumor-associated factor that has been associated with a poor outcome includes the presence of increased numbers of tumor-associated macrophages. Previous studies have reported that increased numbers of CD163+ macrophages are present in HL and this is associated with a poorer outcome and a decreased overall survival [9]. Further immune factors that have been associated with patient outcome include increased levels of circulating serum cytokines and increase monocytes in the peripheral blood that skews the absolute lymphocyte to monocyte count ratio [6,10]. Both of these factors may be associated with the proficiency of the patient’s immune response. All of these features confirm the fact that the active immune microenvironment and inadequate immune response seen in HL represent potential targets for novel therapies.
Furthermore, the unique expression of CD30 on the malignant HRS cells is an additional therapeutic opportunity [11,12]. CD30 is not typically expressed on normal human tissue under physiologic conditions. CD30 is predominantly expressed on thymocytes during thymus development and occasionally on pancreatic exocrine cells as well as cells in the uterus and endometrium during pregnancy. Some activated T cells can also transiently express CD30. This restricted expression of CD30 makes CD30 a target to specifically treat HRS cells present in HL.
3. Novel therapies in HL
Due to the unique composition of lymph nodes in HL, new treatments have been developed that either directly target HRS cells, target cells in the inflammatory infiltrate, or reverse the suppressed immune response. Many of the agents discussed below have showed promising clinical activity in patients with HL; particularly in those who have progressed after standard initial therapy and subsequent salvage autologous stem cell transplantation.
3.1. Brentuximab vedotin
Brentuximab vedotin is an antibody drug conjugate that specifically targets CD30. Initial clinical trials included significant numbers of HL patients due to the selective expression of CD30 on the HRS cell. In the initial phase I trial, patients received brentuximab vedotin on a three weekly schedule. The initial studies showed objective responses in 17 patients with HL, 11 of who had a complete response to treatment [13]. Of the 12 patients with HL who were treated at the maximum tolerated dose, an overall response rate of 50% was observed. A subsequent pivotal phase II trial was performed to confirm these results. Brentuximab vedotin was given to patients with relapsed and refractory HL, all of whom had progressed after a previous autologous stem cell transplant [14]. In 102 patients treated on this trial, the overall response rate was 75%; 34% of the patients had a complete response to treatment. The median progression-free survival for all patients was 5.6 months; however, the median duration of response for those attaining a complete response to treatment was 20.5 months. Subsequent long-term follow-up of patients on this trial confirmed the durability of the responses [15]. Of the 34 patients who had a complete response to treatment initially, 16 (47%) remained in remission without evidence of progression at a median follow-up of 53.3 months.
Based on these encouraging results, brentuximab vedotin has been incorporated into combination treatment approaches. An initial clinical trial combined brentuximab vedotin with standard ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, and dacarbazine) as initial treatment for patients with advanced stage HL [16]. The initial cohort of patients showed that brentuximab vedotin when combined with bleomycin in the ABVD regimen resulted in significant pulmonary toxicity and the study was subsequently revised to combine brentuximab vedotin with AVD (bleomycin omitted). The maximum tolerated dose of brentuximab vedotin in combination with this chemotherapy regimen was found to be 1.2 mg/kg. The study showed that the combined approach was highly effective with complete responses seen in 96% of the patients treated. Due to these very promising clinical responses, a comparative frontline randomized trial is being conducted and accrual to the study has been completed. In this trial, AVD chemotherapy plus brentuximab vedotin is being compared to standard ABVD chemotherapy and the results of this study are now awaited.
3.2. Anti-PD1 antibodies
PD1 signaling regulates the immune response by decreasing T-cell activation and suppressing T-cell proliferation and cytokine production. In the tumor microenvironment in HL, expression of the PD1 ligands including PD-L1 and PD-L2 is significantly increased with very high expression of PD-L1 and PD-L2 on HRS cells. The expression of PD-L1 and PD-L2 inhibits the intratumoral T-cell response that may be directed at the malignant cell. Overexpression of PD-L1 and PD-L2 are due to copy number gains at chromosome 9p24.1 resulting in overexpression of the PD1 ligands. Furthermore, as mentioned above, incorporation of EBV virus into the malignant cell genome can result in upregulation of PD-L1. The PD1/PD1 ligand axis provides a unique target for therapy in HL. Two recent clinical trials that have blocked the PD1/PD-L1/PD-L2 interaction have been reported and both have shown remarkable clinical results. In the clinical trial using nivolumab in relapsed and refractory HL patients, 23 patients received 3 mg/kg of nivolumab every 2 weeks [17]. The majority of patients in the trial had previously undergone an autologous stem cell transplant and most had also received brentuximab vedotin. In this group of heavily pretreated patients an overall response rate of 87% was seen with 20 of 23 patients responding. The complete response rate was 17% and the partial response rate was 70%. The progression-free survival at 24 weeks was 86% and 11 patients have continued on therapy. Recent reports have confirmed that the responses have been durable.
In a second clinical trial utilizing the PD1 antibody pembrolizumab (MK3475), patients received pembrolizumab 10 mg/kg every 2 weeks [18]. This group of patients was also heavily pretreated, with the majority of patients having been treated with an autologous stem cell transplant or brentuximab vedotin. A similar high response rate was seen in this trial. The overall response rate was 53%, with 20% of patients having a complete response and the remainder having a partial response. Similar to the study with nivolumab, the responses have appeared durable. Furthermore, in both studies correlative studies done on tumor specimens from a subset of patients confirmed very high expression of PD-L1 suggesting that this interaction is effectively blocked by these antibodies. Ongoing trials are incorporating PD-1 anti- bodies with brentuximab vedotin, and in combination with chemotherapy.
3.3. Everolimus
Signaling through the PI3-kinase/mTOR pathway has been shown to be active in patients with HL and everolimus is an oral antineoplastic agent that specifically targets mTOR complex 1 (mTORC1). Everolimus not only inhibits the signaling pathways within the HRS cells but appears to also modulate signaling within the tumor microenvironment and the production of cytokines and chemokines may be regulated by this therapy. A clinical trial of everolimus has been conducted in patients with relapsed and refractory HL. In this study, 19 patients were treated with 10 mg of everolimus daily [19]. The majority of these patients had received multiple previous lines of therapy and 84% of patients had previously undergone an autologous stem cell transplant. In this small study, the overall response rate was 47% with one complete response and eight partial responses. The median time to disease progression was 7.2 months and four patients continue to respond with a progression free survival that is greater than 12 months. Overall, the treatment was well tolerated and confirmed that everolimus has single patient activity in patients with relapsed and refractory HL. This further confirmed that targeting the mTOR pathway in HL is clinically useful. However, despite this data, it is unclear how to further develop these agents to gaining the approval of regulatory agencies.
3.4. Panobinostat and mocetinostat
Several oncogenic pathways including cell cycle progression, angiogenesis, cell survival, and tumor immunity may be targeted by inhibiting acetylases with agents such as panobinostat and mocetinostat. These agents target histone deacetylase and have efficacy in patients with HL. Additionally, these agents modulate serum cytokine levels and have also been shown to regulate the expression of PD1 on intratumoral T cells. Panobinostat has shown activity in relapsed and refractory HL. In a large clinical trial of 129 patients with relapsed and refractory disease, patients received 40 mg of panobinostat orally three times per week [20]. The therapy was effective due to the fact that tumor reductions were seen in 74% of patients in the trial and the overall response rate was 27% (35 patients). Thirty of the patients (23%) had a partial response and five patients (4%) had a complete response. The median duration of response was 6.9 months and the overall progression-free survival was 6.1 months. The study confirmed that panobinostat is well tolerated and also confirmed that panobinostat modulates TARC levels, as the levels decreased in patients who responded to treatment.
Mocetinostat has also shown efficacy in patients with relapsed and refractory classical HL [21]. Fifty-one patients were treated in an initial clinical trial and patients received either 110 mg or 85 mg in this study. The patients who received 110 mg had increased toxicity and therefore 85 mg was selected as the optimal dose. In this study, two of the 51 patients had a complete response and 12 had a partial response, suggesting that the agent has activity in this disease. Serum cytokines were also tested in this trial and multiple cytokines decreased after treatment was initiated. Serum TARC levels were significantly decreased and correlated with clinical benefit, confirming that these therapies definitely modulate cytokine signaling within the tumor microenvironment.
In addition to histone deacetylase (HDAC) inhibitors role in modulating chemokine levels, they have been shown to play a role in regulating the immune response by downregulating PD-1. Therefore, HDAC inhibitors may be suitable partners for new combination strategies.
3.5. JAK inhibitors
JAKs are a family of tyrosine kinases that transduce signals from cell surface receptors including cytokine and growth factor receptors. Phosphorylation of the JAK molecule results in recruitment of STAT proteins and STAT proteins translocate to the nucleus resulting in transcription of target genes involved in cell survival, cell proliferation, and immune response.
The JAK-STAT pathway has been found to be aberrantly activated in HL and therefore presents a potential target for therapy in this disease. SB1518 is a JAK2 inhibitor which has clinical activity in various lymphoid malignancies. This JAK inhibitor has been tested in a phase 1 clinical trial that included patients with refractory HL [22]. Doses between 100 and 600 mg/d were tested and the agent was found to be well tolerated. In this study of 34 patients, the overall response rate was 14% and all of the responses were partial responses. In the HL patients, objective responses were not documented, but five of the HL patients did have clinical improvement in their symptoms and appeared to benefit from treatment with a decrease in the sites of active disease.
A subsequent study has targeted the JAK-STAT pathway as well as the PI3 kinase pathways using an oral JAK1 selective inhibitor (INCB039110) and an oral PI3 kinase delta inhibitor (INCB040093). In this phase I trial, 17 patients with relapsed HL were enrolled. The majority had received multiple previous therapies and 82% had previously undergone an autologous stem cell transplant. All patients had failed brentuximab vedotin. An initial cohort of six patients received the PI3 kinase delta inhibitor alone and an overall response rate in these patients of 50% was seen. A subsequent cohort of nine patients received the combination of both the JAK1 and PI3 kinase delta inhibitor and a response rate of 67% was seen including two complete responses. The combination is being tested in a larger phase II trial, but these preliminary results appear promising.
3.6. Lenalidomide
Immunomodulatory drugs (IMiDs) including lenalidomide have multiple possible mechanisms of action, which accounts for their significant activity in lymphoproliferative disorders. The mechanisms of action include directly inducing cell death in malignant B cells, but there is also a profound effect on the tumor microenvironment. Lenalidomide has additional immunomodulatory and anti-angiogenesis properties and may specifically modulate the cytokine environment within the tumor and alter the immune cell infiltrate. Furthermore, lenalidomide may regulate recruitment of macrophages to the tumor microenvironment.
In view of the potential benefits of using lenalidomide in lymphoid diseases, this agent has been tested in a clinical trial of patients with relapsed and classical HL [23]. In this study of 38 patients, the majority of whom had been previously treated with an autologous stem cell transplant, patients received lenalidomide 25 mg daily for 21 days monthly. The treatment was well tolerated and in 36 evaluable patients, an overall response rate of 19% was seen. This included one complete response and six partial responses. Overall, however, approximately one third of patients were judged to have had clinical benefit from treatment. Correlative studies including serum levels of cytokines were tested in this study. Of interest, plasma levels of CCL17 and CCL22 were associated with responses to treatment. It was concluded that lenalidomide has moderate activity in patients with relapsed and refractory HL.
4. Conclusions and future directions
HL has a unique biology that provides opportunities for the use of multiple new therapies. As discussed above, a variety of agents in different drug classes have activity in this disease. However, brentuximab vedotin and PD-1 targeted antibodies demonstrated an impressive single agent activity in heavily treated patients, generating excitement about the possibility of incorporating these agents in novel regimens. The future hope is to see patients benefit from initial therapy and not require treatment for relapsed disease as a greater percentage of patients are cured of their disease with initial therapy.
Acknowledgments
Financial disclosures/conflicts of interest
Anas Younes has received honoraria from Takeda, Bristol Myers Squibb, and Merck Inc. Stephen Ansell receives research funding from Seattle Genetics, Bristol Myers Squibb, and Merck Inc.
References
- 1.Ansell SM. Hodgkin lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(7):771–9. doi: 10.1002/ajh.23750. [DOI] [PubMed] [Google Scholar]
- 2.Stein H, Delsol G, Pileri SA, Weiss LM, Poppema S, Jaffe ES. Classical Hodgkin lymphoma: introduction. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008. pp. 326–9. [Google Scholar]
- 3.Montes-Moreno S. Hodgkin's lymphomas: a tumor recognized by its microenvironment. Adv Hematol. 2011;2011:142395. doi: 10.1155/2011/142395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 29(14):1812–26. doi: 10.1200/JCO.2010.32.8401. 201110. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells: a possible explanation for the characteristic T-cell infiltrate in Hodgkin's lymphoma. Am J Pathol. 1999;154:1685–91. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marri PR, Hodge LS, Maurer MJ, et al. Prognostic significance of pretreatment serum cytokines in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(24):6812–9. doi: 10.1158/1078-0432.CCR-13-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greaves P, Clear A, Owen A, et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122(16):2856–63. doi: 10.1182/blood-2013-06-508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lympho-proliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porrata LF, Ristow K, Colgan JP, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma. Haematologica. 2012;97(2):262–9. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68(3):421–7. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 12.Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73(7):1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 13.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 14.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–9. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–43. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin's lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–56. doi: 10.1016/S1470-2045(13)70501-1. [DOI] [PubMed] [Google Scholar]
- 17.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013) ASH Annual Meeting. 2014 abstract 290. [Google Scholar]
- 19.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85(5):320–4. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin's lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30(18):2197–203. doi: 10.1200/JCO.2011.38.1350. [DOI] [PubMed] [Google Scholar]
- 21.Younes A, Oki Y, Bociek RG, et al. Mocetinostat for relapsed classical Hodgkin's lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011;12(13):1222–8. doi: 10.1016/S1470-2045(11)70265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younes A, Romaguera J, Fanale M, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012;30(33):4161–7. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118(19):5119–25. doi: 10.1182/blood-2011-07-362475. [DOI] [PMC free article] [PubMed] [Google Scholar]