Abstract
Diffuse large B-cell lymphomas with aberrations in MYC, BCL2 and/or BCL6 by genetic alterations or protein expression represent a group of high grade B-cell lymphomas with inferior outcomes when treated with standard RCHOP chemotherapy. As a result, intensified induction regimens have been suggested in an effort to improve outcomes. Conclusions to date have largely been drawn from retrospective data although prospective data is slowly starting to emerge. Chemoimmunotherapy refractoriness is problematic and relapse rates are high. Patients with double hit lymphoma appear to have increased risk of CNS involvement and prophylaxis is recommended. There is insufficient evidence available to date to strongly recommend for or against consolidative stem cell transplant in this population. Collaborative clinical trials will be needed to establish a preferred therapeutic regimen and an appropriate standard of care in this unique group of patients with DLBCL.
Keywords: Double hit lymphoma, DLBCL, MYC translocation, B cell lymphoma unclassifiable, MYC/BCL2 expression
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive B-cell non-Hodgkin lymphoma (NHL), accounting for 30–40% of B-cell lymphoma in the United States. At the molecular and genetic levels, DLBCL is a heterogeneous disease. Based on gene expression profiling (GEP) studies, DLBCL can be classified based on the cell of origin (COO) into two major groups; germinal center B-cell (GCB) subtype, and the activated B-cell (ABC) subtype. Approximately 15%–20% do not fit into these two categories, and therefore, are molecularly unclassifiable [1]. Different immunohistochemistry (IHC) methods are also used to classify DLBCL based on the COO. The most widely used algorithm is based on publication by Hans et al., which evaluates the expression of 3 cellular proteins: CD10, BCL6, and MUM1/IRF4 [2]. Using this algorithm, DLBCL is classified as GCB or non-GCB. In general, DLBCL of the GCB subtype is associated with a favorable outcome when treated with standard RCHOP chemotherapy regardless of how they are defined [3].
In addition to the COO, recent genetic and proteomics studies identified a prognostic role for MYC and BCL2 genetic translocations and/or protein co-expression. Earlier studies using fluorescent in situ hybridization (FISH) reported that 7% to 10% of DLBCL harbored MYC, BLC2 and/or BCL6 translocations, and were called “double hit” lymphoma (DHL), or triple hit lymphoma. In the most recent World Health Organization (WHO) revision of lymphoma classification, this category is now recognized as “high grade B-cell lymphoma (HGBL) with rearrangements of MYC and BCL2 and/or BCL6” [4]. In the 2008 WHO classification, most of these cases were included in the B cell lymphoma unclassifiable (BCLU) histologic category, which is now eliminated from the 2016 WHO update.
2. High grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements
MYC is a transcription factor which regulates the expression of several target genes involved in the cell cycle, DNA damage repair, metabolism, protein synthesis, and response to stress [5]. In humans, MYC is located on chromosome 8 (8q24) and can be activated by: mutations involving regulatory or promoter regions, chromosomal translocation, and copy number increase [6]. In normal cells, MYC activates the TP53 pathway resulting in apoptosis however cells harboring MYC translocations frequently also have TP53 inactivating mutations allowing cells to escape apoptosis. DLBCL with MYC translocations can be accompanied by BCL2 and/or BCL6 translocations, which confers aggressive clinical behavior. In most published series, MYC translocation can be detected in approximately 10% of DLBCL (range 4% to 14%), and is predominantly seen in the subset of GCB subtype (Table 1) [7–19].
Table 1.
Incidence of MYC translocations in Diffuse Large B-cell Lymphoma and Prognostic Impact.
| Study | Number of patients | % MYC translocations | Treatment | Impact on overall survival |
|---|---|---|---|---|
| Savage et al. [7] | 135 | 9% | RCHOP | Adverse |
| Obermann et al. [8] | 220 | 4% | RCHOP | Adverse |
| Barrans et al. [9] | 245 | 14% | RCHOP | Adverse |
| Visco et al. [10] | 296 | 8% | RCHOP | Adverse |
| Akyurek et al. [11] | 239 | 6% | RCHOP | Adverse |
| Green et al. [16] | 189 | 11% | RCHOP | Adverse |
| Johnson et al. [12] | 290 | 12% | RCHOP | Adverse |
| Horn et al. [13] | 407 | 9% | RCHOP | Adverse |
| Hu et al. [14] | 384 | 6%a | RCHOP | Unspecified |
| Cunningham et al. [15] | 359 (1080) | 10% | RCHOP | Adverse |
| Kojima et al. [19] | 100 | 10% | RCHOP | Adverse |
| Tzankov et al. [18] | 432 | 9% | RCHOP | Adverse |
| Copie-Bergmann et al. [17] | 574 | 8.9% | RCHOP/RACVBP | Adverse (IG onlyb) |
RACVBP = rituximab plus doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone.
Inferred from text.
Immunoglobulin partner only.
BCL2 normally serves an anti-apoptotic function. In the event of a t (14; 18), BCL2 transcription is constitutively dysregulated resulting in a survival advantage for affected B-cells. BCL2 translocations are found in 20–30% of de novo DLBCL [20] and the vast majority of cases are observed in the GCB subtype. BCL6 is expressed in normal mature germinal center B-cells and acts as a transcription repressor. When BCL6 is overexpressed, it prevents apoptosis in response to DNA damage. Its action is very closely related to p53.
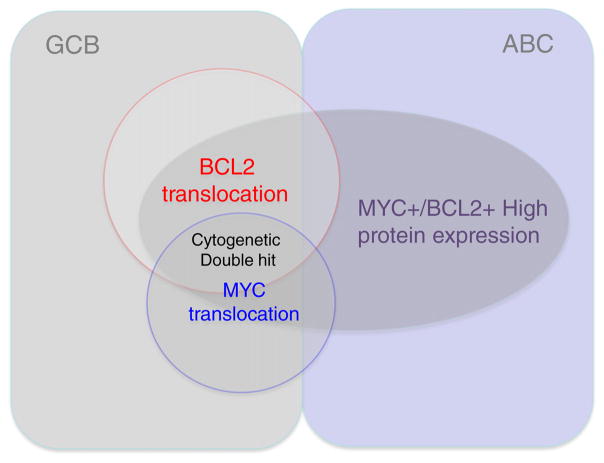
While genetic double hit lymphoma (DHL) is predominantly observed in the GCB subset of DLBCL, cases with double MYC and BCL2 protein expression are observed in both the ABC and GCB subsets. DHL accounts for a small subset of the cases with double protein expression (Fig. 1). The incidence of double protein expressing DLBCL is estimated to be 19–34%, and is associated with worse prognosis than patients who express MYC alone or no proteins (Table 2) [7–19]. The clinical course of double protein expressing lymphomas is associated with outcomes intermediate between DLBCL NOS and DHL when treated with RCHOP [16]. It has been suggested that the inferior outcomes seen with ABC subtype DLBCL are the result of MYC/BCL2 protein overexpression and resultant biology.
Fig. 1.
Relationship among cell of origin in diffuse large B-cell lymphoma in terms of MYC/ BCL2 protein expression and genetic translocations.
Table 2.
Incidence of MYC/BCL2 protein co-expression in diffuse large B-cell lymphoma patients treated with R-CHOP.
| Study | Predicts OS/PFS | % double IHC positive | Cut- point for MYC + | Predominantly ABC subtype |
|---|---|---|---|---|
| Kluk et al. [45] | Yes | Not available | 50% | Unspecified |
| Green et al. [16] | Yes | 29% | 40% | Yes |
| Johnson et al. [12] | Yes | 21% | 40% | Yes |
| Horn et al. [13] | Yes | 26% | 40% | Unspecified |
| Hu et al. [14] | Yes | 33% | 40% | Yes |
| Valera et al. [24] | Yes | 27% | 10% | Unspecified |
| Perry et al. [46] | Yes | 44% | 50% | No |
| Yan et al. [47] | Yes | 28% | 40% | Yes |
| Scott et al. [3] | Yes | 31% | 40% | Yes |
Overall survival (OS); Progression free survival (PFS); IHC (immunohistochemistry).
The definition of DHL has been evolving for several years. Initially, this entity was restricted to de novo DLBCL [21]. Subsequently, it included cases of transformed follicular lymphoma, and other rare entities. DHL is now recognized as “high grade B-cell lymphoma (HGBL) with rearrangements of MYC and BCL2 and/or BCL6” [4]. The vast majority of the cases of DLBCL with DH genetics are observed in the GCB subtype. BCL2 translocations occur only in germinal center tumors as an early event [22,23]. MYC translocations are a secondary event and are frequently associated with BCL2 or BCL6 translocations [24–26]. The aggressive clinical course and poor response to therapy seen with DHL is in direct contrast to the favorable prognosis typically associated with GCB subtype, illustrating the importance of molecular classification for prognosis and selection of appropriate therapy. Lymphoma with both MYC and BCL6 translocations (MYC/BCL6) is likely a different disease biologically in comparison to DHL with MYC/BCL2 translocations. MYC/BCL6 lymphoma is much less common and more likely to be immunoblastic, ABC subtype, and have less cytogenetic complexity [27]. These tumors are clinically distinct from their BCL2 rearranged counterparts [27]. One difference with therapeutic implications is MYC/BCL2 DHL frequently exhibits TP53 mutations while MYC/BCL6 rearranged lymphomas do not. Triple hit (TH) lymphoma is defined as B-cell lymphoma with chromosomal alterations in MYC, BCL2, and BCL6 and has a similar prognosis to DHL.
3. Double protein expressing (DE) vs. double hit lymphoma (DHL)
Two retrospective studies have compared characteristics of DE versus DHL [12,16]. In the first, 54/193 patients had DE lymphoma by IHC (MYC > 40%, BCL2 > 70%), while 11/193 were “double hit” by FISH (MYC/BCL2). Presenting characteristics did not differ; however, 91% of DHL was GCB versus 37% of DE lymphoma. DE patients exhibited a lower complete response (CR) rate with RCHOP (p = 0.004), shorter overall survival (OS) (p < 0.001) and shorter progression free survival (PFS) (p < 0.001) than DLBCL NOS independent of IPI or COO. Median OS was 13 months for DHL versus 95 months for non-translocated patients leading the authors to conclude that DE and DHL do extremely poorly and advocated for IHC being sufficient to identify DLBCL with “double hit” biology [16]. In the second, 21% of 307 patients were DE (>40% MYC, >50% BCL2) and 10 patients were “double hit” by FISH. Both were associated with advanced stage and extra-nodal disease but DHL patients were more likely to have poor performance status (PS), higher LDH, and high IPI. GCB immunophenotype was seen more commonly with DHL while DE lymphoma was more frequently ABC phenotype. Inferior OS and PFS were seen when MYC/BCL2 were co-expressed but not with MYC expression alone by IHC [12].
In an analysis of DLBCL (n = 893) treated with RCHOP, DE (34%) (MYC > 40%, BCL2 > 70%) lymphomas were more likely to be ABC subtype and have advanced stage, higher Ki67, and higher IPI. DE lymphoma had lower response rates (RR), PFS, and OS compared to non-protein expressing DLBCL [14]. Gene expression profiling across ABC and GCB subtypes identified a significantly higher frequency of BCL2 (p < 0.0001) and MYC expression (p = 0.0009) in the ABC DLBCL group compared to GCB. After excluding DE cases, ABC subtype was no longer associated with inferior outcome. This data suggests that MYC/BCL2 co-expression is a superior predictor of prognosis over COO in patients with DLBCL receiving RCHOP [14].
4. Treatment options
Patients with DHL and DE lymphoma are challenging to study as definitions have not been uniform (studies include DHL and DE as one) and incidence of true DHL is low (8–10%). The optimal treatment strategy for DHL and DE DLBCL is still poorly defined, with limited data on the value of frontline autologous transplant. Most of the data available is from retrospective studies or subgroup analyses of prospectively treated DLBCL cohorts; however some prospective data is beginning to emerge. Many patients have primary refractory disease and relapse is common contributing to poor outcomes [7,9,12,14,16,18,25, 28–31]. Survival after relapse is particularly poor as salvage therapies are ineffective in this population, highlighting the need for novel drugs and combinations for these patients.
4.1. Induction regimens
Initial reports of treatment outcome of patients with DE and DHL treated with standard RCHOP were disappointing which has led many to propose intensification of therapy (as used in MYC driven BL) as a means to improve outcomes. Treatment has been intensified using dose adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-R-EPOCH), hyper-fractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone, methotrexate, cytarabine with rituximab (R-hyperCVAD alternating with MTX/ cytarabine), and cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine, and rituximab (CODOX-M/ IVAC) but is not yet clear that regimens such as these have improved outcomes. Drawing conclusions regarding the true benefit of dose intensification of upfront therapy has been a challenge due to small patient numbers, mostly retrospective data, and lack of a proper control arm.
In a series of 54 MYC/BCL2 DHL cases (17 DLBCL, 36 BCLU) that were treated with CHOP, RCHOP, or high-dose (HD) chemotherapy (ALL type regimen [n = 2 patients]) with or without stem cell transplant (SCT)(high dose chemoradiotherapy followed by auto [n = 3 patients] or allo [n = 1 patient]), 59% died within 6 months of diagnosis and only 6 patients were alive at 5 years [25]. Poor OS was also seen in a larger series of 303 DLBCL patients treated with CHOP or RCHOP. Fourteen percent of the patients had MYC rearrangement by FISH (83% of which had concurrent BCL2 or BCL6 rearrangements [27 DHL]). MYC rearranged cases were associated with inferior 2-year OS (35% vs 61%) compared to non-MYC rearranged DLBCL [9]. In another retrospective study of DLBCL patients treated with RCHOP, the subset of patients with DHL (n = 14) had significantly worse outcomes (5-year PFS 18% vs 65%; 5-year OS 27% vs 71%) compared to DLBCL as did those with DE lymphoma (5-year PFS 32% and 5-year OS 36%) [12].
In a retrospective analysis of 69 de novo DLBCL patients treated with RCHOP, selected for high proliferation index, 28% were MYC/BCL2 (>40%/>40%) double protein expressing lymphoma and 25% co-expressed MYC/BCL6 (>40%/>25%). MYC overexpression alone or with BCL2 overexpression and/or low BCL6 expression correlated with worse prognosis independent of IPI score [32]. Another series attempted to improve survival by adding etoposide to RCHOP (RCHOEP) in young patients (age < 60 years). Forty seven percent of 106 patients were found to have DE lymphoma (MYC > 40%/BCL2 ≥ 70%). Eight (8%) patients had DHL (MYC/BCL2 or BCL6). Double protein expressing lymphoma was associated with a trend towards reduced PFS with RCHOP (n = 84) but not RCHOEP (n = 56). There were too few DHL patients to perform statistical analysis [26].
Other analyses have looked at intensification of therapy with mixed results [29,33]. In a retrospective analysis of DLBCL with abnormal MYC and BCL2 signals, 52/60 were “double hit” (MYC/BCL2) by FISH (23 DLBCL, 37 BCLU or FL) and ten patients had pre-existing follicular lymphoma (FL). The majority were treated with R-hyper-CVAD. For the “double hit” group, median OS was 18.6 months and intensification of therapy did not affect outcome [29]. In a Danish cohort of DLBCL/BCLU patients (n = 157), 11% were DHL (MYC/BCL2) which amounted to 7% of primary DLBCL cases and 21% of transformed lymphomas. The majority were treated with RCHOP (11 patients) while 3 patients received RICE/RDHAP, 1 patient received RCHOEP, and 1 underwent high dose chemotherapy followed by autologous SCT. All DHL were GCB by immunophenotype. In this analysis, presence of a “double hit” had no impact on overall response rate (ORR), relapse rate, or OS although treatments varied widely, patients included had primary, transformed or relapsed disease and observation period was limited to 1 year. ORR for DHL was 59% and no clinical characteristics were specific to DHL [33].
MD Anderson Cancer Center has published a detailed report of their institutional experience with DHL (n = 129) [31]. This report again highlights the unsatisfactory outcomes associated with RCHOP in DHL as well as the poor outcome associated with patients who do not achieve a CR to initial therapy. For patients treated with RCHOP (n = 57), CR rate was 40% while 2-year OS was 41%. Thirty-four patients were treated with R-hyper-CVAD with superior CR rate of 68% but shorter PFS and OS. Those treated with DA-R-EPOCH had better event free survival (EFS) and a trend towards improved OS. Two year EFS rates for RCHOP, DA-R-EPOCH, and R-hyperCVAD alternating with MTX/cytarabine were 25%, 67%, and 32% respectively. For patients who progressed, 3-year OS rate was 7%. For patients who underwent SCT as salvage, 3-year OS rate was 15% [31].
Preliminary results of a prospective multicenter study of DA-R-EPOCH for MYC translocated DLBCL (n = 52) have been reported. Fourteen patients were DHL (MYC/BCL2) and 24/52 overexpressed BCL2 by IHC. With a median follow-up of 14 months, PFS, time to progression (TTP), and OS were 79%, 86%, and 77% respectively. The PFS was 87% for DHL (n = 14) and 64% for MYC translocation with BCL2 overexpression (n = 24). Compared to RCHOP, DA-R-EPOCH resulted in a higher CR rate for DHL, as well as longer PFS and OS [34]. While these results are encouraging, the numbers are small and long term follow up is needed. Additional prospective trials will be needed to consider this the standard of care for DHL. Together, with a recent report of a comprehensive analysis from a recent randomized study, the poor prognostic implication of DHL remains undetermined [17].
In conclusion, the data available from several retrospective analyses and early results of one prospective trial suggest intensification of therapy may improve response rates and outcome for DHL. At this time, prospective clinical trials are preferred. If no trials are available, a patient with adequate performance status and organ function should receive DA-R-EPOCH on the basis of available data. The implications of intensification and DE lymphoma outcomes are difficult to assess as many studies lumped DE and DHL patients into the same group. With more strict definitions, future studies should be able to distinguish the two populations with greater ease.
4.2. Role of hematopoietic stem cell transplant
The role of hematopoietic stem cell transplant (SCT) consolidation in this population remains unclear with limited available data and conflicting reports regarding benefit. In the MD Anderson experience, there was no EFS or OS benefit with autologous or allogeneic SCT (n = 26) [31]. For patients achieving a CR with induction, the 2-year EFS for patients receiving consolidative autologous SCT (n = 23) was 68% compared to 53% for those who did not (n = 48)(p = 0.133). This is not enough data to draw definitive conclusions about up-front autologous SCT but suggests it should be considered in responding patients and evaluated in larger studies with extended follow-up.
In a series of 311 patients, 83 underwent SCT consolidation: 39 in first CR (28 autologous, 11 allogeneic) and 14 during first response but not CR. For patients achieving CR with front line therapy, no difference in median OS was appreciated for those who underwent SCT versus those that did not. Two year OS for patients achieving CR was >75% indicating response to induction therapy may be the best predictor of long term outcome [35]. One hundred and fifty four patients had documented progression (106 treated) and most commonly received RICE (rituximab, ifosfamide, carboplatin, etoposide) as salvage. Li and coworkers described 52 patients with DHL induced primarily with RCHOP (30 patients) or R-hyper-CVAD/MTX-cytarabine (28 patients). Eleven went on to autologous or allogeneic SCT at the discretion of the treating physician. With a median OS of 18.6 months, they concluded that neither intensification of therapy nor stem cell transplant were beneficial [29].
In a retrospective analysis, outcomes for 32 patients with DHL were reported. Twenty-five patients received R-CODOX-M/IVAC with 80% achieving at least a PR and 36% achieving a CR. Nineteen patients went on to receive a consolidative SCT (11 autologous, 8 allogeneic). Median OS was 28 months and median PFS was 11 months. Eighty two percent of patients undergoing SCT were alive at 2 years, 60% without progression [36] suggesting SCT may improve survival in those who have good disease control prior to transplant but still does not address those patients who progress on therapy or do not respond.
In a subset analysis of SWOG 9704 (RCHOPx8 vs RCHOPx6 followed by autologous SCT), Nineteen of 198 patients were DE (>40% MYC, >30% BCL2) and 3 were DHL by FISH (MYC/BCL2). Twelve of sixteen DE lymphoma patients were randomized (5 SCT, 7 no SCT). Two year PFS was 29% for no SCT and 60% for SCT. Two thirds of the DHL patients were randomized to transplant however all 3 progressed and died at a median of 6.5 months. While there was a trend towards benefit of up-front SCT in DE patients, nearly a third of MYC+ patients by IHC were never randomized secondary to progression or death further supporting the notion that RCHOP induction is insufficient [37].
Oregon Health and Science University has reported their experience with 16 patients with DHL (MYC/BCL2) and three patients with triple hit lymphoma (THL) treated with DA-R-EPOCH x6 + IT prophylaxis (14 patients), RCHOP (1 patient), or R-hyperCVAD (1 patient) followed by autologous SCT consolidation. Fifteen patients achieved CR after induction and all went on to receive high dose chemotherapy followed by hematopoietic stem cell rescue. At a median follow-up of 18 months, estimated 2-year PFS was 91% and 2-year OS was 91%. One patient relapsed 6 months post-transplant. Fifteen additional patients received DA-R-EPOCH alone. Four were primary refractory and four relapsed or died. This experience suggests autologous SCT as an upfront consolidation strategy may benefit DHL patients in first CR, however, prospective studies with more patients are necessary to confirm this benefit [38].
Additional reports from small retrospective studies reviewed the role of consolidation strategies with either autologous or allogeneic SCT, but no strong conclusions could be made due to the heterogeneity of induction regimens, patient characteristics, and selection bias [39,40].
In conclusion, there is no strong scientific evidence to indicate that consolidation with SCT is beneficial. Our recommendation is to use DA-R-EPOCH, and if patients achieve rapid CR by functional imaging (after 2–3 cycles), and remain in CR at the end of therapy, is to observe the patient without consolidative SCT. For those that are not in remission at the completion of therapy, consideration should be given to alternate chemotherapy or clinical trials. Currently available data does not support the use of autologous SCT in patients with chemotherapy refractory disease and little is known about the benefit of allogeneic SCT in this scenario.
4.3. Role of CNS prophylaxis
In two large retrospective analyses, the incidence of central nervous system (CNS) involvement in DHL is estimated to be 4–7% [31,35]. MD Anderson reports a 13% 3-year cumulative risk of CNS involvement in their experience with DHL [31]. The available evidence suggests the risk of CNS involvement is similar in DHL and BL [30]. The incidence of CNS disease in DE lymphoma has not been well quantified although at least one report suggests a higher risk of CNS relapse in DE lymphoma (MYC > 40%, BCL2 > 50%) with an incidence of 9.4% versus 2.4% in non-double expressers [41]. In addition to high IPI, leukemic presentation of DHL may also predict for CNS involvement [42].
Accordingly, it is recommended that all patients with DHL and DE lymphoma have diagnostic lumbar puncture as part of their initial staging. Prophylactic CNS therapy is recommended for all DHL patients, as it may decrease the incidence of CNS disease [43,44].
5. Conclusions and future directions
In the 2016 World Health Organization (WHO) revision of lymphoma classification, DHL is now recognized as “high grade B-cell lymphoma (HGBL) with rearrangements of MYC and BCL2 and/or BCL6” [4]. In the 2008 WHO classification, most of these cases were included in the B cell lymphoma unclassifiable (BCLU) histologic category, which is now eliminated. Most retrospective studies suggested that DHL and DE lymphoma carry a poor prognosis when treated with conventional therapy, such as RCHOP. However, recent analysis of the role of MYC and BCL2 in a large randomized study suggested that DHL may not have an inferior prognosis [17]. Similarly, a small prospective study from the NCI using DA-R-EPOCH also suggested that the majority of these patients can be cured without the need for consolidative autologous SCT [34]. There is a clear need to conduct prospective studies in patients with DHL and DE lymphoma, to determine the true prognosis of these patients. Given the rarity of DHL, such a trial will require collaborative multicenter efforts. A randomized study looking at intensified induction chemotherapy with or without a BCL2 inhibitor is planned.
For the time being, our recommendation is to treat DHL patients with DA-R-EPOCH with CNS prophylaxis. Patients who achieve a CR at interim PET and remain in CR at the end of 6 cycles of DA-R-EPOCH can be observed without consolidative SCT given the paucity of evidence for a strong OS or PFS benefit in patients achieving CR to first line therapy. Strong consideration should also be given to CNS prophylaxis in DE lymphoma as there may be an increased risk of CNS involvement.
Patients who have primary refractory disease or relapse have extremely poor outcomes with no standard treatment recommendations. Future directions should explore novel therapies aimed at targeting the underlying oncogenic process, such as MYC and BCL2 inhibitors.
6. Practice points
MYC and BCL2 double protein expressing lymphoma cases are more commonly seen in the ABC subtype, but can be GCB whereas DHL are by definition GCB subtype.
All newly diagnosed patients with DLBCL should have MYC, BCL2, and BCL6 expression assessed by IHC and FISH for MYC rand BCL2 rearrangements.
Lack of uniform IHC cutoffs for MYC, BCL2, and BCL6 protein expression complicates study comparisons and makes drawing conclusions difficult. Standardized cut-offs for positivity have since been established: MYC ≥ 40%, BCL2 ≥ 50% and BCL6 ≥ 30%.
Retrospective data that suggested a poor prognosis of “double hit” and double protein expressing lymphomas are recently challenged by prospective studies and more comprehensive data analysis from larger randomized studies.
CNS prophylaxis is recommended for all patients with DHL and should be strongly considered for patients with DE lymphoma.
Patients with “double hit” lymphoma who have primary refractory or relapsed disease have extremely poor outcomes with current standard therapies and should be considered for clinical trials when available.
7. Research agenda
Rationally designed clinical trials combining targeted agents with chemoimmunotherapy in first and subsequent lines of therapy for DHL and DE lymphomas.
Determining the role for stem cell transplant (autologous or allogeneic) in DHL and DE lymphomas.
-
Development of novel therapeutics targeting mechanisms of chemotherapy resistance including:
BET inhibitors
BCL2 inhibitors
CDK inhibitors
PD-1/PDL-1 inhibitors.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest
No conflicts to disclose.
References
- 1.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 2.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 3.Scott DW, Mottok A, Ennishi D, Wright GW, Farinha P, Ben-Neriah S, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33:2848–56. doi: 10.1200/JCO.2014.60.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–64. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 7.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–7. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 8.Obermann EC, Csato M, Dirnhofer S, Tzankov A. Aberrations of the MYC gene in unselected cases of diffuse large B-cell lymphoma are rare and unpredictable by morphological or immunohistochemical assessment. J Clin Pathol. 2009;62:754–6. doi: 10.1136/jcp.2009.065227. [DOI] [PubMed] [Google Scholar]
- 9.Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–5. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 10.Visco C, Tzankov A, Xu-Monette ZY, Miranda RN, Tai YC, Li Y, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98:255–63. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012;118:4173–83. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–9. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn H, Ziepert M, Becher C, Barth TFE, Bernd H-W, Feller AC, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–63. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/ BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–31. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–26. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 16.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–7. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 17.Copie-Bergman C, Cuilliere-Dartigues P, Baia M, Briere J, Delarue R, Canioni D, et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood. 2015;126:2466–74. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- 18.Tzankov A, Xu-Monette ZY, Gerhard M, Visco C, Dirnhofer S, Gisin N, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2014;27:958–71. doi: 10.1038/modpathol.2013.214. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M, Nishikii H, Takizawa J, Aoki S, Noguchi M, Chiba S, et al. MYC rearrangements are useful for predicting outcomes following rituximab and chemotherapy: multicenter analysis of Japanese patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:2149–54. doi: 10.3109/10428194.2013.771398. [DOI] [PubMed] [Google Scholar]
- 20.Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the T(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317:1185–9. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 21.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92:1297–301. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 22.Frick M, Dorken B, Lenz G. The molecular biology of diffuse large B-cell lymphoma. Ther Adv Hematol. 2011;2:369–79. doi: 10.1177/2040620711419001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz G. Insights into the molecular pathogenesis of activated B-cell-like diffuse large B-cell lymphoma and its therapeutic implications. Cancers. 2015;7:0812. doi: 10.3390/cancers7020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–62. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–9. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen MO, Gang AO, Poulsen TS, Knudsen H, Lauritzen AF, Nielsen SL, et al. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol. 2014;92:42–8. doi: 10.1111/ejh.12212. [DOI] [PubMed] [Google Scholar]
- 27.Pillai RK, Sathanoori M, Van Oss SB, Swerdlow SH. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol. 2013;37:323–32. doi: 10.1097/PAS.0b013e31826cebad. [DOI] [PubMed] [Google Scholar]
- 28.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma E-J, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–31. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Lin P, Fayad LE, Lennon PA, Miranda RN, Yin CC, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25:145–56. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 30.Snuderl M, Kolman OK, Chen Y-B, Hsu JJ, Ackerman AM, Dal Cin P, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327–40. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 32.Botto B, Novero D, Chiappella A, Novo M, Castellino A, Ciochetto C, et al. The prognostic value of MYC, BCL2 and BCL6 overexpression evaluated by immunohistochemistry (IHC) in de-novo diffuse large B cell lymphoma (DLBCL) treated with rituximab-CHOP. Blood. 2014;124:2964. [Google Scholar]
- 33.Pedersen MO, Gang AO, Poulsen TS, Knudsen H, Lauritzen AF, Nielsen SL, et al. Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma - a single centre’s experience. Eur J Haematol. 2012;89:63–71. doi: 10.1111/j.1600-0609.2012.01787.x. [DOI] [PubMed] [Google Scholar]
- 34.Dunleavy K, Fanale M, LaCasce A, Noy A, Caimi P, Parekh S, et al. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma. Blood. 2014;124:395. [Google Scholar]
- 35.Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–61. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 36.Sun H, Savage KJ, Karsan A, Slack GW, Gascoyne RD, Toze CL, et al. Outcome of patients with non-Hodgkin lymphomas with concurrent MYC and BCL2 rearrangements treated with CODOX-M/IVAC with rituximab followed by hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2015;15:341–8. doi: 10.1016/j.clml.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Puvvada SD, Stiff PJ, Leblanc M, Cook JR, Kahl B, Li H, et al. MYC associated and double protein lymphoma: subset analysis of SWOG S9704. Blood. 2014;124:1710. [Google Scholar]
- 38.Chen AI, Okada C, Gay N, Reiss P, Spurgeon S, Fan G, et al. Consolidative autologous stem cell transplant for double hit lymphoma. Blood. 2014;124:3993. [Google Scholar]
- 39.Savage KJ, Karsan A, Slack GW, Toze CL, Sehn LH, Abou Mourad YR, et al. Outcome of patients with double-hit lymphomas treated with CODOX-M/IVAC + R followed by hematopoietic stem cell transplantation in British Columbia. Blood. 2013;122:1788. [Google Scholar]
- 40.Goy A, Zielonka T, Pecora AL, Feldman T, Rowley SD, Donato ML, et al. Dose intensive induction followed by allogeneic stem cell transplantation more than doubles progression-free and overall survival in “double-hit” lymphoma (DHL) Blood. 2013;122:2141. [Google Scholar]
- 41.Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127:2182–8. doi: 10.1182/blood-2015-10-676700. [DOI] [PubMed] [Google Scholar]
- 42.Shuhua Y, Zhong S, Zou D, Li C, Li Z, Liu W, et al. BCL-2 and c-MYC rearrangements in leukemic phase of diffuse large B cell lymphoma predicts central nervous system involvement. Blood. 2014;124:2958. [Google Scholar]
- 43.Cheah CY, Oki Y, Westin JR, Turturro F. A clinician’s guide to double hit lymphomas. Br J Haematol. 2015;168:784–95. doi: 10.1111/bjh.13276. [DOI] [PubMed] [Google Scholar]
- 44.Recher C, Coiffier B, Haioun C, Molina TJ, Ferme C, Casasnovas O, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–67. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 45.Kluk MJ, Chapuy B, Sinha P, Roy A, Cin PD, Neuberg DS, et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7:e33813. doi: 10.1371/journal.pone.0033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry AM, Alvarado-Bernal Y, Laurini JA, Smith LM, Slack GW, Tan KL, et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165:382–91. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 47.Yan LX, Liu YH, Luo DL, Zhang F, Cheng Y, Luo XL, et al. MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B-cell lymphoma, not otherwise specified. PLoS One. 2014:9. doi: 10.1371/journal.pone.0104068. [DOI] [PMC free article] [PubMed] [Google Scholar]