Abstract
Mesenchymal stem cells (MSCs) are crucial for tissue homeostasis and regeneration. Though of prime interest, their potentially protective role on neutrophil-induced tissue damage, associated with high morbidity and mortality, has not been explored in sufficient detail. Here we report the therapeutic skill of MSCs to suppress unrestrained neutrophil activation and to attenuate severe tissue damage in a murine immune-complex mediated vasculitis model of unbalanced neutrophil activation. MSC-mediated neutrophil suppression was due to intercellular adhesion molecule 1-dependent engulfment of neutrophils by MSCs, decreasing overall neutrophil numbers. Similar to MSCs in their endogenous niche of murine and human vasculitis, therapeutically injected MSCs via upregulation of the extracellular superoxide dismutase (SOD3), reduced super-oxide anion concentrations and consequently prevented neutrophil death, neutrophil extracellular trap formation and spillage of matrix degrading neutrophil elastase, gelatinase and myeloperoxidase. SOD3-silenced MSCs did not exert tissue protective effects. Thus, MSCs hold substantial therapeutic promise to counteract tissue damage in conditions with unrestrained neutrophil activation.
Keywords: Mesenchymal stem cells, Neutrophils, Neutrophil extracellular traps, Vasculitis, Superoxide dismutase
INTRODUCTION
Neutrophils constitute the largest, evolutionary conserved fraction of circulating leukocytes. They are responsible for the first line of defense against pathogens relying on a plethora of antimicrobial mechanisms, all enacted during the process of neutrophil activation. Neutrophil activation generates high concentrations of antimicrobial reactive oxygen species (ROS) with superoxide anions ( ) as the major component [1, 2] to create an environment that is toxic for pathogens. In addition, high ROS concentrations are responsible for the release and activation of neutrophil-derived proteases[3, 4], and the formation of neutrophil extracellular traps (NETs) with expulsion of chromatin fibers from the nucleus of neutrophils as additional strategies to effectively combat and kill invading microorganisms [5]. However, when these mechanisms become overactivated either by persisting stimuli or by impeded resolution, they turn out to be highly destructive for the surrounding tissues and may cause severe damage and tissue break down as observed in neutrophil dominated diseases. Examples of such “inflammasomopathies” with enhanced release of neutrophil activating interleukin (IL)–β are pyoderma gangrenosum [6], immune complex (IC)-mediated vasculitis [7] occurring in systemic lupus erythematosus [8] as well as conditions with enhanced release of neutrophil activating danger signals as in trauma and chronic non-healing wounds [9]. Earlier studies further suggest that persistent neutrophil activation is at least in part responsible for malignant transformation observed at high frequency in chronically inflamed tissue [10]. Diseases due to uncontrolled neutrophil activation are still difficult-to-treat and based on their tissue and organ destructive nature reveal high morbidity and mortality. Hence, with the current lack of efficious treatments in therapy refractory cases, an urgent need exists to develop new therapeutic strategies.
Tissue homeostasis and regeneration critically depend on self-renewal and the precise control of inflammation by the plasticity and adaptive responsiveness of mesenchymal stem cells (MSCs) [11]. MSCs are endowed with the unique capacity to sense inflammatory cues in their environment. In the absence of danger signals, MSCs actively coordinate the resolution of inflammation, a program which most likely has evolved to protect their own DNA from deleterious ROS attacks to guarantee a reliable blue print for self-renewal and replenishment of lost somatic cells essentially required for tissue homeostasis.
MSCs exert a variety of immune-regulatory functions on cells of both adaptive and innate immunity. These include suppression of effector T cell proliferation and function with concomitant induction of their apoptosis [12, 13], enhanced generation of regulatory T cells [14, 15], inhibition of B cell activation and proliferation [16], suppression of differentiation, maturation and antigen presentation of dendritic cells [17], inhibition of proliferation and cytotoxicity of natural killer cells [18], and induction of a shift from pro-inflammatory M1 to anti-inflammatory M2 macrophages [19–21]. Though of prime relevance, the interactions between MSCs and neutrophils—while addressed in the unperturbed bone marrow stem cell niche [22], vessels [23], and in brain ischemia [24]—have not been explored in sufficient mechanistic details in tissue damage owing to unrestrained neutrophil activation under in vivo conditions of murine and human vasculitis.
In this study, we investigate the interactions between MSCs and neutrophils with particular focus on the potential protective effects of MSCs on neutrophil induced tissue damage, which would be exploitable for MSC-based therapies.
Materials and Methods
Mice and Human Samples
All animal experiments were conducted in accordance with protocols approved by the Institutional Review Board (IRB) and the Ethical Committee at the University of Ulm. For experiments using human skin samples or primary human cells, informed consent for the studies was obtained from the patients in accordance with the Declaration of Helsinki and IRB approval of University of Ulm. The details are presented in Supporting Information Experimental Procedures.
Isolation of Neutrophils
Human peripheral neutrophils (hNeu) were isolated from buffy coats (German Red Cross, Ulm, Germany, www.drk-ulm.de) using gradient centrifugation. Murine bone marrow neutrophils (mNeu) were isolated by immunomagnetic separation using the negative depletion method with mouse neutrophil isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). The details are presented in Supporting Information Experimental Procedures.
Flow Cytometry
Flow cytometry was performed on a FACS Canto II (BD Bio-sciences, San Jose, CA, http://www.bdbiosciences.com/) with FACSDiva software for data acquisition. Data were analyzed with Summit software v4.3 (Beckman Coulter, Brea, California, www.beckmancoulter.com).
Oxidative Burst Assay
Neutrophils were cultured alone or cocultured with AT-MSCs at indicated neutrophil:MSC ratios for 4 hours. Thereafter, neutrophils were activated by 100 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) at 37°C for 10 minutes. Neutrophils treated with DMSO served as non-activated controls. Cocultures were labeled with the redox sensitive dye dihydrorhodamine 123 (DHR) (Life Technologies, Carlsbad, CA, www.lifetechnologies.com) at 5 µM at 37°C for 30 minutes. The cells were put on ice to stop the reaction, washed with phosphate-buffered saline (PBS) and subjected to flow cytometric analysis. Unstained cells served as background control. The intensity of DHR dye was analyzed within the neutrophil gate.
Quantification of NET Formation
The immunostaining of extracellular chromatin fiber with anti-DNA/histone 1 antibody (Merck Millipore, Darmstadt, Germany, www.emdmillipore.com) was used to demonstrate the NET formation. The fluorescence intensity of Sytox orange nucleic acid stain (Life Technologies) was used to quantify the NET measured with Mithras LB940 microplate reader (Bert-hold Technologies, Bad Wildbad, Germany, https://www.berthold.com/), at excitation and emission wavelengths of 540 nm and 580 nm, respectively. The details are presented in Supporting Information Experimental Procedures.
Phagocytosis Assay
AT-MSCs were labeled with 2.5 µM carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies). MACS purified mNeu were labeled with 2 µM PKH26 (Sigma-Aldrich) and cultured at 37°C overnight to induce apoptosis. Thereafter, apoptotic mNeu were cocultured with AT-MSCs or macrophages at a mNeu:MSC (or macrophages) ratio of 10:1 for 24 hours in Lab-Tek II chamber slides (NUNC, Roskilde, Denmark, www.thermofisher.com/). The slides were fixed and examined with Zeiss LSM710 confocal microscope and ZEN2010 software (Carl Zeiss, Oberkochen, Germany, www.zeiss.com.) The pinhole was set to achieve 1 µm light section. In another set of experiments, the cocultures were harvested after 24 hours and analyzed by flow cytometry.
Arthus Reaction in Mouse Skin
2.5 × 105 AT-MSCs in 100 µl PBS per injection were intradermally injected to the both sides of shaved dorsal skin of mice. Mice intradermally injected with 100 µl PBS served as controls. To induce IC-mediated vasculitis, mice were challenged with the Arthus reaction [25, 26]. Briefly, the Arthus reaction was elicited by i.v. injection of 100 µl of 2% bovine serum albumin (BSA) and 1% Evans blue (Sigma-Aldrich) in PBS. Afterward 40 µl of rabbit anti-BSA antibody (Sigma-Aldrich) at the concentration of 4.8 mg/ml was injected intradermally into the dorsal skin that had been injected with AT-MSCs or PBS. Rabbit IgG served as the background control. Mice were sacrificed 4 hours after the Arthus reaction and skin specimen were harvested and digitally photographed. The areas of Evans blue spots on the skin, representing the areas of hemorrhage due to IC-mediated vasculitis, were analyzed by Image J.
Small Interfering RNA Transfection
AT-MSCs were transfected with human SOD3 small interfering RNA (siRNAs) or control siRNA (Qiagen, Hilden, Germany, https://www.qiagen.com) at a final concentration of 30 nM using Lipofectamine RNAiMAX (Life Technologies). Cells were harvested 48 hours after transfection for SOD3 mRNA and protein expression analysis, or for injection or coculture with neutrophils. The details are presented in Supporting Information Experimental Procedures.
Statistics
Two-tailed unpaired Student’s t tests or Mann–Whitney tests, as indicated in figure legends, were used to determine statistical significance.
Results
MSCs Suppress Oxidative Burst-Induced Neutrophil Death and Peroxidase/Protease Spillage
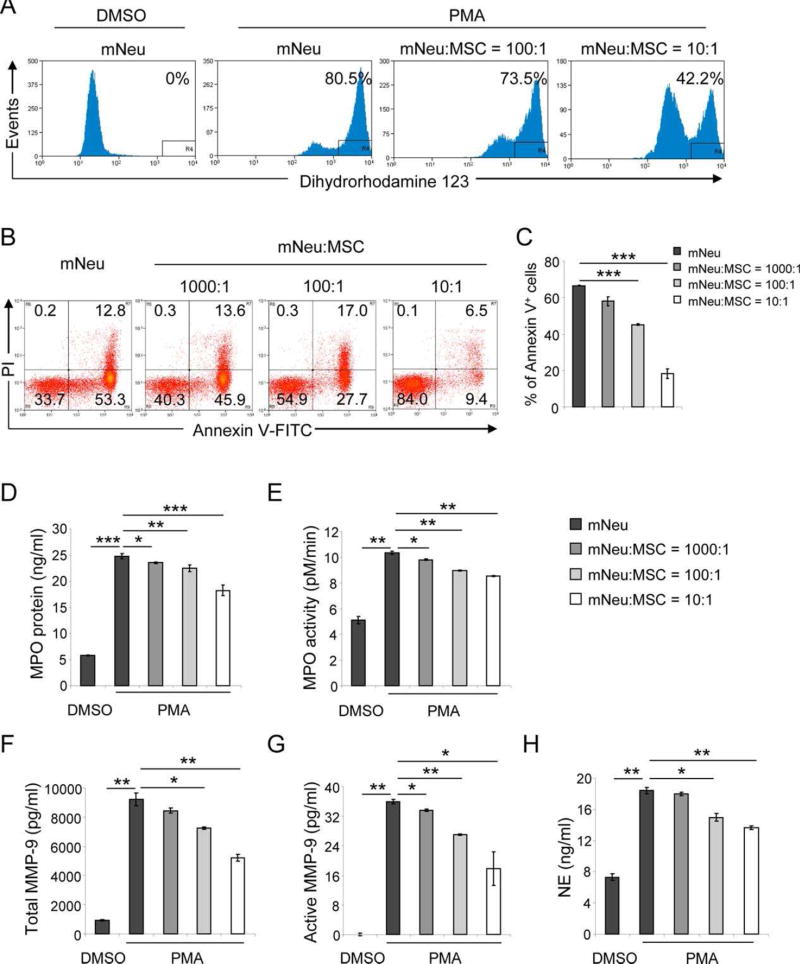
Activation of the NADPH oxidase with enhanced release of antimicrobial ROS to pathogen containing phagosomes initiates an apoptotic program in activated neutrophils which allows to prevent uncontrolled spillage of their tissue toxic cargo [27]. By contrast, unrestrained neutrophil activation with high ROS concentrations results in active degranulation and neutrophil death with the induction of leaky plasma membrane and an uncontrolled release of several proteases with specificity for vessel wall and interstitial connective tissue components eventually leading to vascular tissue break down. As unrestrained high ROS concentrations with prolonged oxidative burst are responsible for this vicious sequence of events, we first set out to explore the effect of fully characterized human adipose tissue derived mesenchymal stem cells (MSCs) (Supporting Information Fig. S1A–S1G) on the suppression of high ROS release from activated neutrophils. Following a 4 hours coculture of MSCs and untouched mNeu from C57BL/6 mice with a reproducible purity of 99% (Supporting Information Fig. S1H), PMA-induced oxidative burst was measured by flow cytometry with the ROS indicator dye DHR. The majority (80.5%) of PMA-treated mNeu revealed a DHR bright staining indicating that most of the mNeu had undergone oxidative burst, while DMSO-treated control mNeu were DHR negative. Of note, the percentage of ROS producing mNeu was reduced to 73.5% at a mNeu:MSC ratio of 100:1, and further to 42.2% at a mNeu:MSC ratio of 10:1 (Fig. 1A). This indicates that MSCs in a concentration-dependent manner could robustly reduce neutrophil oxidative burst. This was further explored in vivo in a murine model of PMA-induced dermatitis [28]. Intradermal injection of PMA induced recruitment (Supporting Information Fig. S2A) and activation of neutrophils with enhanced oxidative burst as assessed by whole body in vivo imaging with the ROS sensitive chemiluminescent probe L-012 [29] (Supporting Information Fig. S2B). By contrast, elevated ROS levels were not observed in CD18 (the common β chain of β2 integrins)-deficient mice (Supporting Information Fig. S2A, S2B), indicating that PMA-induced dermatitis is due to ROS releasing neutrophils as CD18 is essential for the emigration of neutrophils from the vessel into the skin with subsequent ROS production [30]. Importantly, similar to the in vitro data, injection of MSCs at the site of PMA-induced dermatitis significantly dampened the unrestrained oxidative burst in vivo (Supporting Information Fig. S2C, S2D).
Figure 1.
MSCs suppress oxidative burst, death, peroxidase and proteases release. 2 × 106 mNeu per well were cultured alone or cocultured with AT-MSCs at mNeu:MSC ratios of 10:1, 100:1, and 1000:1. (A): 4 hours after coculture, mNeu were activated by 100 ng/ml PMA at 37°C for 10 minutes. mNeu treated with DMSO served as nonactivated control. Cocultures were labeled with 5 µM dihydrorhodamine 123 (DHR) and APC-conjugated Gr-1 antibody at 37°C for 30 minutes, and subjected to flow cytometric analysis. DHR intensity was analyzed within the Gr-1+ gate. The depicted histograms are representative of three independent experiments. (B): Cocultures were harvested 24 hours later, and labeled with Annexin V-FITC, PI, and Gr-1-APC, and analyzed by flow cytometry. The depicted dot plots and histograms were gated on Gr-1+ population, and are representative of three independent experiments. (C): Depicted are percentages of Annexin V+ cells expressed as mean ± SEM of three independent experiments. ***, p < .001. (D–H): Four hours after coculture, mNeu were activated by 100 ng/ml PMA for 30 minutes. Supernatants from cocultures were harvested. (D) MPO concentrations were measured by ELISA. (E) The MPO activity was assessed using the TMB substrate based activity assay in a 10 minute interval. (F–G) Total and active neutrophil gelatinase (MMP-9) was evaluated based on its proteolytic activity of converting a pro-detecting enzyme to an active-detecting enzyme, which converts peptide substrate into colored product. The proMMP-9 in the supernatants was activated by p-aminophenyl mercuric acetate. (H) NE concentrations were calculated from its proteolytic activity of converting a nonfluorescent substrate to a fluorescent product. *, p < .05; **, p < .01; ***, p < .001 by Student’s t tests. These experiments were independently repeated three times with similar results. Abbreviations: MPO, myeloperoxidase; mNeu, murine bone marrow neutrophils; MSCs, mesenchymal stem cells; NE, neutrophil elastase, TMB, 3,3′,5,5′-Tetramethylbenzidine.
In case that unrestrained oxidative burst is responsible for neutrophil death and degranulation, we would expect that MSCs exert a protective role on the death rate and the release of toxic proteases from neutrophils by reducing ROS concentrations. After a 24 hours incubation period, mNeu death was analyzed by Annexin V and PI staining using flow cytometry. Out of 2 × 106 PMA-stimulated mNeu which were cultured in the absence of MSCs, 66.1% stained positive for Annexin V, while the number of dead mNeu were reduced to 59.5%, 44.7%, and 15.9%, respectively, when the same number of activated mNeu were cocultured with 2 × 103, 2 × 104, and 2 × 105 MSCs, respectively, (Fig. 1B, 1C). These data indicate that MSCs inhibit neutrophil death. Similar effects were observed in cocultures of MSC with PMA-stimulated hNeu isolated from buffy coats of healthy donors (Supporting Information Figs. S1I, S3A–Fig. S3C). These results indicate that there was no apparent species difference between human and murine cells in regard to MSC-neutrophil interactions, suggesting tractable murine models to be particularly suited to study these interactions in vivo.
Interestingly, when PMA-activated mNeu were cocultured with MSCs, the concentration and the activity of neutrophil released myeloperoxidase (MPO) was significantly reduced in supernatants (Fig. 1D, 1E). Similarly, only in the presence of MSCs, the release and the activity of neutrophil gelatinase (also known as matrix metalloproteinase-9, MMP-9) and neutrophil elastase (NE), both responsible for extracellular matrix break down, were significantly reduced (Fig. 1F–1H). Our data support the notion that MSCs are able to protect from the consequences of unrestrained oxidative burst reducing overall neutrophil death and associated spillage of their toxic cargo in vitro.
MSCs Reduce Neutrophil Extracellular Trap Formation and Engulf Neutrophils
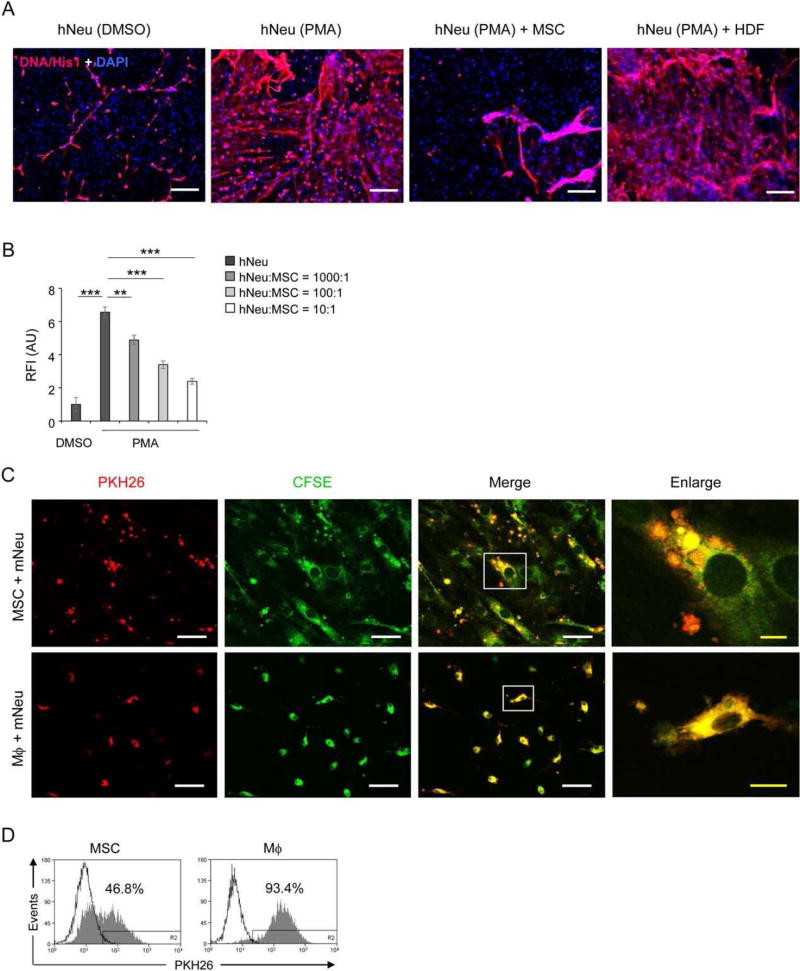
NETs have been identified as a new powerful antimicrobial weapon of neutrophils [5]. Microorganisms are trapped within the expulsed nucleic chromatin and subsequently are killed by highly concentrated, NET-entangled antimicrobial proteins as well as MPO [31] and NE [32]. Immunostaining with an antibody against DNA/histone1 complexes showed massive fiber-like NET structures around hNeu activated with PMA. In contrast, when cocultured with MSCs, significantly less NETs were observed in PMA-stimulated hNeu. For comparison, human dermal fibroblasts did not reveal any inhibitory effect on NET formation when cocultured with activated hNeu (Fig. 2A). Quantification of NET formation by Sytox orange, a noncell-permeable DNA specific dye staining extracellular NETs [33, 34], revealed a significant increase in Sytox orange fluorescence intensity of PMA-activated hNeu compared to low intensities of DMSO-treated control hNeu. Coculture of activated hNeu with increasing cell numbers of MSCs resulted in a concentration-dependent reduction of NET formation. Notably, NET formation was reduced by 63.5%, when hNeu were cocultured at a hNeu:MSC ratio of 10:1 (Fig. 2B). When MSCs were cocultured with mNeu, a similar suppressive effect on NET formation was observed (Supporting Information Fig. S3D – S3E).
Figure 2.
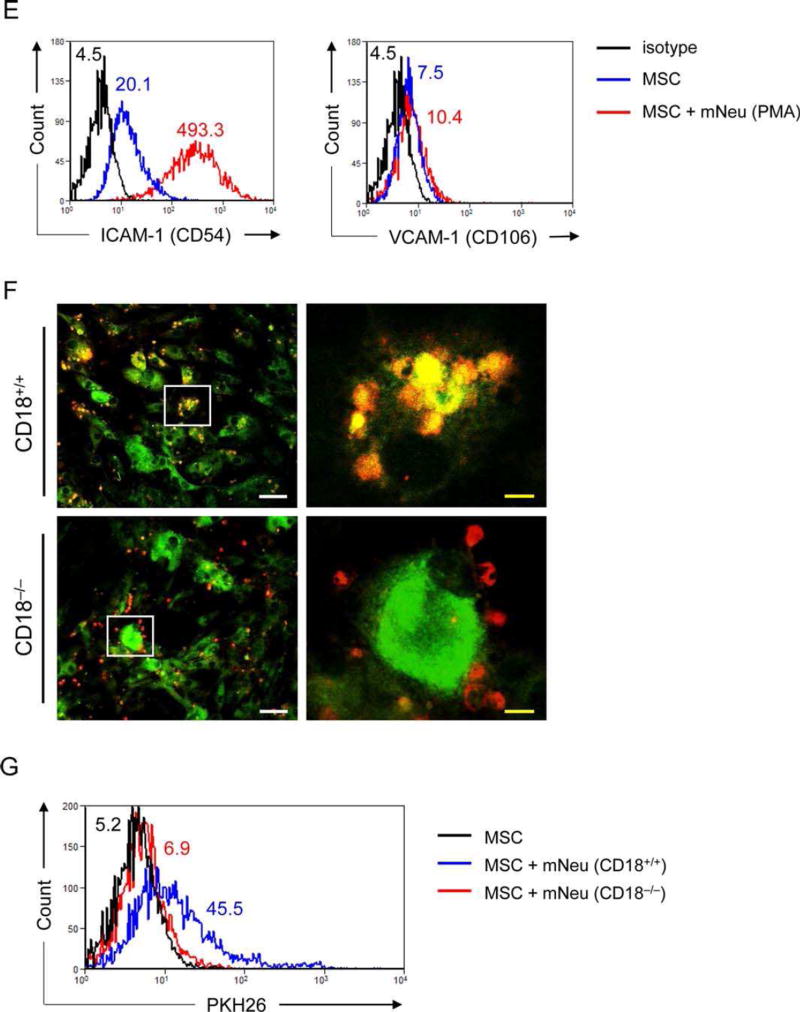
MSCs reduce NETs formation and engulf neutrophils. (A): 2 × 105 hNeu were cultured alone or cocultured with 2 × 104 AT-MSCs or HDF in wells of glass chamber slides and activated by 100 ng/ml PMA for 3 hours. Slides were fixed and labeled with anti-DNA/histone-1 followed by AF555-conjugated anti-mouse IgG (red) and counterstained with DAPI (blue). Scale bar: 200 µm. (B): 1 × 105 hNeu were cultured alone or with AT-MSCs at hNeu:MSC ratios of 10:1, 100:1 and 1000:1 and subsequently activated with 100 ng/ml PMA for 3 hours. Cocultures were labeled with 0.25 µM Sytox orange for 5 minutes at room temperature and fluorescence intensities were measured by microplate reader. Culture medium containing Sytox orange served as background fluorescent control. Fluorescence intensities were normalized on the intensity of non-activated controls. Results are expressed as mean ±SD of five-plicate measurements. This experiment was independently repeated three times with similar results. ***, p < .001. (C–D): 1×106 mNeu were labeled with 2 µM PKH26 and cultured at 37°C overnight to induce apoptosis. Thereafter, mNeu were cocultured with 1×105 of 2.5 µM CFSE labeled AT-MSCs or murine bone marrow derived macrophages for 24 hours. (C) Cocultured cells were fixed on chamber slides and photographed under confocal microscope with 1 µm light section. White scale bar: 50 µm; yellow scale bar: 10 µm. The squares in the “Merge” panel indicate the areas enlarged in the “Enlarge” panel. (D) Cocultures were analyzed by flow cytometry. AT-MSCs or macrophages cocultured with PKH26 labeled mNeu are depicted in filled grey histograms, and the counterparts cocultured with nonlabeled mNeu are shown in empty histograms. The depicted photographs and histograms are representative of three independent experiments. Mϕ, macrophages. (E): AT-MSCs were cultured alone or with PMA activated mNeu at a mNeu:MSCs ratio of 10:1. 24 hours after culture, cells were labeled with CD73-APC and ICAM-1-PE or VCAM-1-PE antibodies and subjected to flow cytometric analysis. The ICAM-1 and VCAM-1 expression were analyzed in CD73+ MSC gate. (F–G): CFSE labeled MSCs (green) were cocultured with PKH26 labeled mNeu (red) from wild-type (CD18+/+) or CD18−/− mice at a mNeu:MSC ratio of 10:1 for 24 hours. The engulfment of neutrophils by MSCs were examined by (F) confocal microscopy, white scale bar: 50 µm; yellow scale bar: 10 µm or (G) flow cytometry. Abbreviations: AU, arbitrary unit. HDF, human dermal fibroblasts; ICAM-1, intercellular adhesion molecule 1; hNeu, human peripheral neutrophils; MSCs, mesenchymal stem cells; mNeu, murine bone marrow neutrophils; NETs, neutrophil extracellular traps; RFI, relative fluorescence intensity.
Macrophage-mediated phagocytosis of activated neutrophils constitutes an effective measure to terminate or limit unrestrained neutrophil activation and to prevent spillage of tissue toxic ingredients from neutrophils. To examine a possible physical interaction between neutrophils and MSCs, mNeu were labeled with the red fluorescent dye PKH26 and cocultured with green fluorescent CFSE-labeled MSCs for 24 hours. CFSE-labeled murine bone marrow macrophages served as a control for professional phagocytes. Surprisingly, MSCs were found to engulf neutrophils in cocultures. The majority of MSCs display intracellular vesicles containing neutrophils after 24 hours of coculture as depicted in the merged and enlarged confocal images (Fig. 2C). The possibility that neutrophils simply attached to MSCs can be reliably excluded as the light section of confocal microscopy was set at 1 µm. However, compared to professional phagocytes that can degrade engulfed neutrophils in phagolysosomes indicated by a more evenly distributed yellow fluorescence, MSCs did not have such capacity and their cargos of engulfed neutrophils apparently remained within vesicles (Fig. 2C). These observations were in line with results from flow cytometric analysis. Nearly half of the MSCs (46.8%) were PKH26 positive, indicative of engulfed neutrophils, while almost all macrophages (93.4%) were positive for PKH26 and displayed elevated fluorescent intensity (Fig. 2D).
Juxtacrine cellular contacts between neutrophils and vascular cells are predominantly mediated by β2-integrin-ICAM-1 and β1-integrin-vascular cell adhesion molecule 1 (VCAM-1) interactions [30, 35]. While untreated MSCs constitutively expressed low levels of ICAM-1 and VCAM-1, the expression of ICAM-1 but not of VCAM-1 was substantially upregulated on MSCs in coculture with activated mNeu (Fig. 2E). Moreover, the engulfment of neutrophils by MSCs was significantly reduced when MSCs were cocultured with CD18−/− mNeu lacking β2 integrins (Fig. 2F, 2G). These data indicate that MSCs, in addition to reducing ROS release suppressing of NET formation, adopted an independent strategy of ICAM-1-mediated neutrophil engulfment to prevent further tissue damage.
MSCs Reduce Vessel Destruction and Hemorrhage in Immune Complex-Mediated Vasculitis
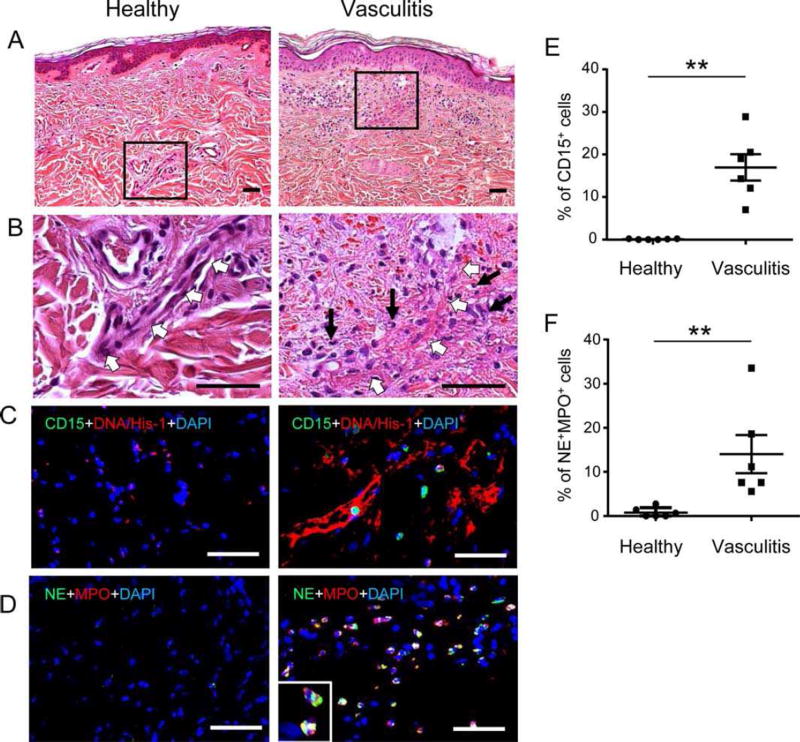
We next explored neutrophil overactivation and subsequent tissue damage in human IC-mediated vasculitis. IC-mediated vasculitis is a disease initiated by the deposition of antigen-antibody complexes in dermal blood vessels, which subsequently lead to complement activation, neutrophil recruitment and activation. The large amount of ROS and proteases released from activated neutrophils damage the endothelial lining of the vessel wall and result in edema and hemorrhage [25, 26]. In skin tissue, sections derived from six patients suffering from IC-mediated vasculitis, damaged blood vessels contained fibrin deposit and infiltrated neutrophils in the vessel wall (Fig. 3A, 3B, 3E) as well as extravasated red blood cells within the interstitial connective tissue of the skin. Importantly, NET structures (Fig. 3C) and the expression of NE and MPO (Fig. 3D–3F) were detected in skin sections derived from vasculitis patients, whereas none of these components were discernable in normal skin (Fig. 3C–3F).
Figure 3.
Activated neutrophils mediated tissue damage in vasculitis patients. (A–D): Representative microphotographs of paraffin-embedded skin sections from six healthy donor (left panel) and six vasculitis patients (right panel) with H&E staining (A–B), immunostaining of human neutrophil marker CD15 (green) and neutrophil extracellular traps marker DNA/Histone-1 (red) (C), and immunostaining of NE (green) and MPO (red) (D). In immunostainings nuclei were counterstained with DAPI (blue). The squares in (A) indicate the enlarged areas in (B). White arrows indicate blood vessels, black arrows indicate neutrophils in (B). Scale bar: 50 µm. (E–F): The percentage of CD15+ neutrophils and NE+ MPO+ neutrophils was calculated from each skin section of 6 different healthy donors and 6 patients with vasculitis and expressed as mean ± SEM of CD15+ neutrophils (E) and NE+ MPO+ neutrophils (F). n = 6; **, p< .01 by Mann–Whitney tests. Abbreviations: MPO, myeloperoxidase; NE, neutrophil elastase.
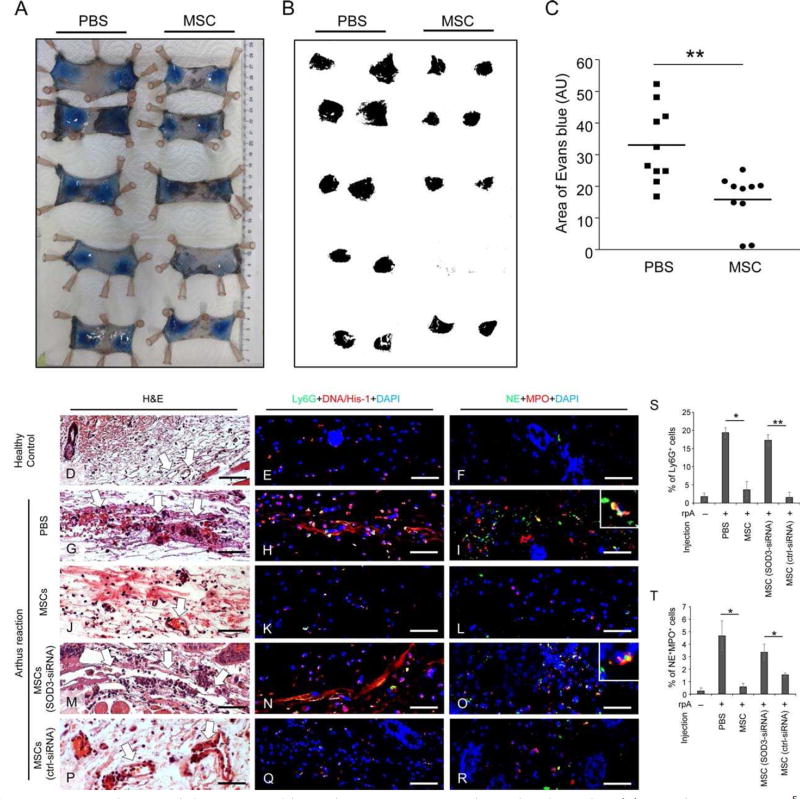
To further explore the effect of MSCs on preventing the observed vessel damage by activated neutrophils, we employed a mouse model of IC-mediated vasculitis, in which mice were challenged with an Arthus reaction. For this purpose i.v. injection of a solution containing 2% BSA and 1% Evans blue dye was performed and an anti-BSA antibody was intradermally injected to the skin area that previously had received either MSCs or PBS injection. After 4 hours, mice were sacrificed and skin specimen were harvested and digitally photographed to quantify the extent of Evans blue-marked vascular leakage (Fig. 4A). Such areas, indicative of the extent of vessel damage due to BSA/anti-BSA complex formation, were analyzed by Image J software (Fig. 4B). Intra-dermal injection of MSCs resulted in significantly reduced areas of Evans blue staining by 52.1% compared to PBS control (15.8 vs. 33.0 AU, n = 10) (Fig. 4C). Moreover, the dermis of the mice subjected to Arthus reaction with PBS injection showed severe damage of vessels surrounded by red blood cells and neutrophils (Fig. 4G, 4S) with NETs (Fig. 4H), NE and MPO in the tissue (Fig. 4I, 4T), which very much resemble the features of human vasculitis. In contrast, intradermal injection of MSCs protected vessel structure (Fig. 4J, 4S) with significantly reduced NETs (Fig. 4K) and reduced numbers of NE+ MPO+ neutrophils in the dermis (Fig. 4L, 4T), which were similar to skin specimen from healthy control mice (Fig. 4D-4F).
Figure 4.
MSCs reduce vessel destruction and hemorrhage in immune complex-mediated vasculitis. (A): 100 µl PBS or 2.5 × 105 AT-MSCs were intradermally injected to both sides of shaved dorsal skin of mice. The Arthus reaction was elicited by i.v. injection of 100 µl PBS solution containing 2% BSA and 1% Evans blue. Afterward anti-BSA antibody was intradermally injected to the area that had been injected with AT-MSCs or PBS. Four hours later, mice were sacrificed and skin specimen were harvested and digitally photographed. (B– C): The areas of Evans blue spots on skin were analyzed by Image J. n = 10; **, p < .01 by Mann-Whitney tests. (D–T): Mice were intra-dermally injected with PBS or nontransfected MSCs or SOD3-siRNA transfected MSCs or control-siRNA transfected MSCs and subjected to Arthus reaction. Mice received rabbit IgG served as healthy controls. Representative pictures of paraffin-embedded skin sections from 4 mice of each group with H&E staining (D, G, J, M, P), immunostaining of murine neutrophil marker Ly6G (green) and NETs marker DNA/Histone-1 (red) (E, H, K, N, Q), and immunostaining of NE (green) and MPO (red) (F, I, L, O, R) are shown. Nuclei were counterstained with DAPI (blue) in immunostainings. White arrows in H&E staining indicate blood vessels. Scale bar: 50 µm. (S–T) The depicted are mean ± SEM of Ly6G+ cells (S) and NE+ MPO+ cells (T) counted from each section of 4 mice. n = 4; *, p < .05; **, p < .01 by Mann-Whitney tests. Abbreviations: AU, arbitrary unit; MSCs, mesenchymal stem cells; MPO, myeloperoxidase; NE, neutrophil elastase.
MSCs Protect Tissue Damage by Enhanced SOD3 Release upon Neutrophil Activation
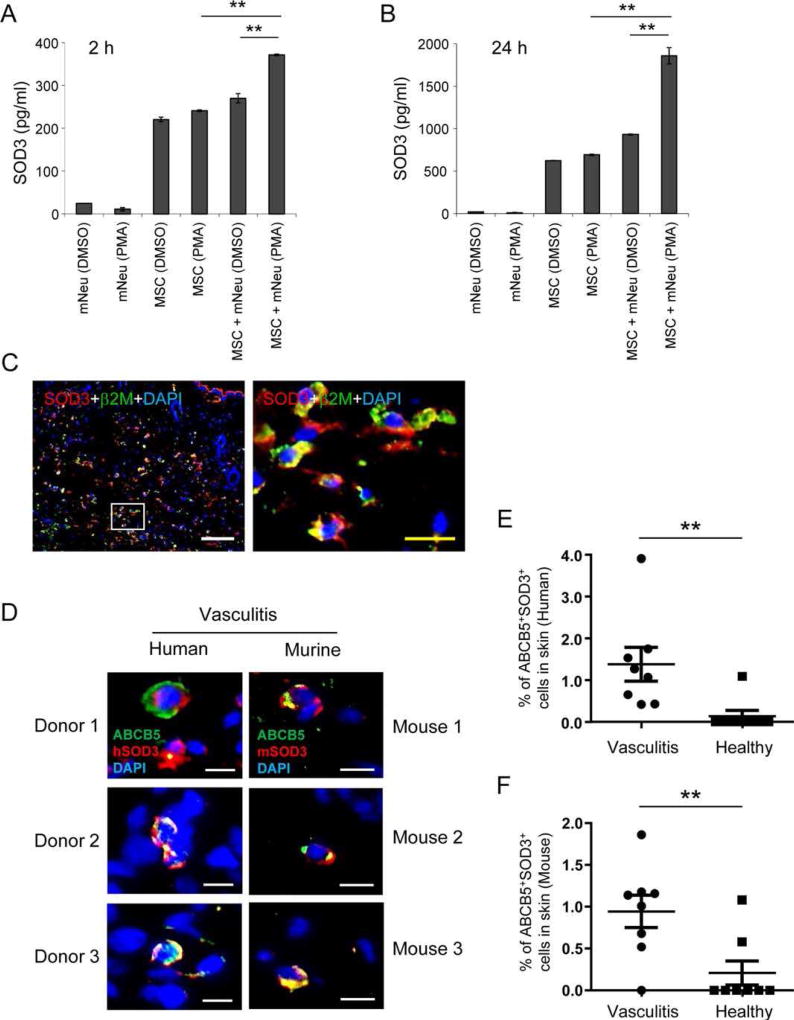
To search for the protective, possibly anti-oxidant mechanism of MSCs, the expression of anti-oxidant genes NRF2, SOD1, SOD2, and SOD3 was quantified by qPCR in MSCs that had been cocultured with PMA-activated mNeu or treated with same concentration of PMA alone. No significant differences in specific mRNA levels for these genes were found in the first 24 hours (Supporting Information Fig. S4A–S4D). However, using an SOD3 ELISA we corroborate and extend previous findings [36, 37] that MSCs constitutively release SOD3, a soluble extracellular form of superoxide dismutase, which can detoxify the superoxide anion radical in their proximate microenvironment [36]. Interestingly and previously unreported, we found that the release of SOD3 was markedly increased when MSCs were cocultured with PMA-activated mNeu as early as 2 hours of coculture (Fig. 5A), and at a significantly higher extent after 24 hours of coculture (Fig. 5B). The induction of SOD3 release could be due to ROS released by activated neutrophils, since both hydrogen peroxide (H2O2) and hypoxanthine/xanthine oxidase generated superoxide anion ( ) induced SOD3 release from MSCs in vitro (Supporting Information Fig. S5A , S5B). Moreover, MSCs were found to release SOD3 in vivo following intradermal injection into the skin of mice suffering from IC-mediated vasculitis (Fig. 5C). These data indicate that MSCs adaptively respond to the oxidative microenvironment by actively releasing SOD3. To investigate whether SOD3 is also upregulated in their endogenous niche in human vasculitis and a murine model of vasculitis, we took advantage of the only single marker ABCB5, a transporter protein at the cell membrane which specifically allows to detect a newly identified dermal MSC subpopulation within the skin in situ [38]. We found that, as is the case with adipose tissue derived MSCs, ABCB5+ MSCs exerted similar suppressive effects on ROS release (Supporting Information Fig. S6A) and NET formation (Supporting Information Fig. S6B,S6C) from activated hNeu, both key features of MSC-based prevention and treatment of IC-mediated vasculitis. Of note, immunostaining for ABCB5 and SOD3 revealed a significantly higher frequency of double positive MSCs in human and murine IC-mediated vasculitis (Fig. 5D) compared to healthy controls (Fig. 5E, 5F). These data indicate that MSCs mount an adaptive antioxidant SOD3 response in their endogenous niche to protect themselves from the threat of neutrophil oxidative burst. However, opposed to the tissue protecting effect of therapeutically injected MSCs at high numbers, endogenous MSCs at physiological low numbers of ~0.2% of total dermal cells are unable to protect from vasculitis and tissue break down.
Figure 5.
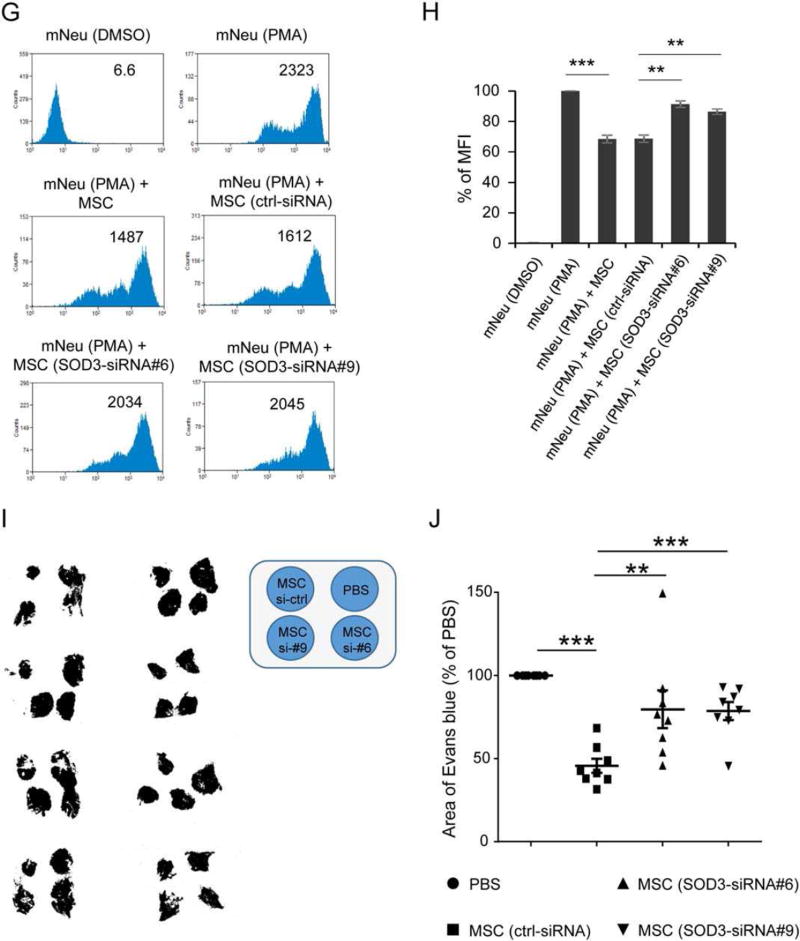
Induced SOD3 from MSCs is responsible for neutrophil suppressive effects. (A–B): 2 × 105 AT-MSCs were cultured alone or with 2 × 106 mNeu activated by 100 ng/ml PMA. mNeu treated with DMSO served as nonactivated controls. AT-MSCs treated with 100 ng/ml PMA served as control for PMA effects. Supernatants were harvested 2 hours (A) and 24 hours (B) after onset of coculture, and SOD3 concentrations in supernatants were measured by ELISA. This experiment was independently performed three times with similar results. (C): 2.5 × 105 AT-MSCs were intradermally injected to shaved dorsal skin of mice. The Arthus reaction was elicited by i.v. injection of 100 µl of 2% BSA in PBS and intradermal injection of anti-BSA antibody to the area that had been injected with AT-MSCs. 4 hours later, mice were sacrificed and skin specimen were processed for cryosections. Sections were immunostained with human specific beta-2 microglobulin (β2M, green, indicative of MSCs) and SOD3 (red), and nuclei were counterstained with DAPI (blue). The square in the left panel indicates the area enlarged in the right panel. White scale bar: 100 µm; yellow scale bar: 20 µm. (D–F): Endogenous ABCB5+ dermal MSCs express SOD3 in situ. Depicted in (D) are representative microphotographs of paraffin-embedded skin sections from human vasculitis patients with immunostaining of human ABCB5 (green) and human SOD3 (red) (left panel), and mice subjected to IC-mediated vasculitis with immunostaining of ABCB5 (green) and murine SOD3 (red) (right panel), respectively. Scale bar: 10 µm. The percentage of ABCB5+ SOD3+ cells in total dermal cells in each section are expressed as mean ± SEM in human (E) and murine (F) samples, respectively. n = 8; **, p < .01 by Student’s t tests. (G–H): 2 × 106 mNeu were cultured alone or with non-transfected MSCs or control-siRNA transfected MSCs, or SOD3-silenced MSCs at a mNeu:MSC ratio of 10:1, and subjected to PMA-induced oxidative burst assay with reactive oxygen species indicator dihydrorhodamine 123. The depicted histograms in (G) are representative of three independent experiments. The mean fluorescence intensity (MFI) of each sample in (G) was normalized to MFI of PMA activated neutrophils as percentages in the same experiment. The chart depicted in (H) is mean ± SEM of percentage of MFI derived from three experiments. **, p < .01; ***, p < .001 by Student’s t tests. (I–J): 100 µl PBS or 2.5 × 105 control-siRNA transfected MSCs or SOD3-silenced MSCs were intradermally injected into dorsal skin of mice. The Arthus reaction was elicited at the areas that had been injected with MSCs or PBS. Four hours later, mice were sacrificed and skin specimen were harvested and digitally photographed. The areas of Evans blue on skin specimen with MSCs treatment were normalized to Evans blue stained areas after PBS injection on the same mouse and expressed as percentages. n = 8; **, p < .01; ***, p < .001 by Mann–Whitney tests. Abbreviations: MSCs, mesenchymal stem cells; mNeu, murine bone marrow neutrophils; SOD3, superoxide dismutase 3.
To investigate whether SOD3 is responsible for the ROS-reducing and tissue protecting effects of injected MSCs at high numbers, SOD3 mRNA, but not SOD2 or SOD1 mRNAs, was silenced in MSCs by two independent SOD3 siRNAs selected from four tested ones (Supporting Information Fig. S5C–S5F). The capacity of SOD3-silenced MSCs to suppress the PMA-induced oxidative burst of mNeu in vitro was substantially reduced compared to control siRNA-transfected MSCs (Fig. 5G, 5H). Of note, SOD3-silenced MSCs significantly lost the capacity to reduce hemorrhage in IC-mediated vasculitis mouse model compared to control-siRNA transfected MSCs (Fig. 5I, 5J). In support of these findings, immunostaining of skin specimen derived from mice subjected to IC-mediated vasculitis and intradermal injection of SOD3-silenced MSCs showed severe damage of vessels surrounded by Ly6G+ neutrophils (Fig. 4M, 4S) with increased NETs structures (Fig. 4N), enhanced MPO and NE expression (Fig. 4O, 4T) in the affected tissue. In contrast, results with control-siRNA-transfected MSCs (Fig. 4P-4R) or non-transfected MSCs (Fig. 4J-4L) revealed a tissue-protective outcome, indicative for SOD3 being responsible for the suppressive effect of MSCs on neutrophil-mediated tissue damage.
DISCUSSION
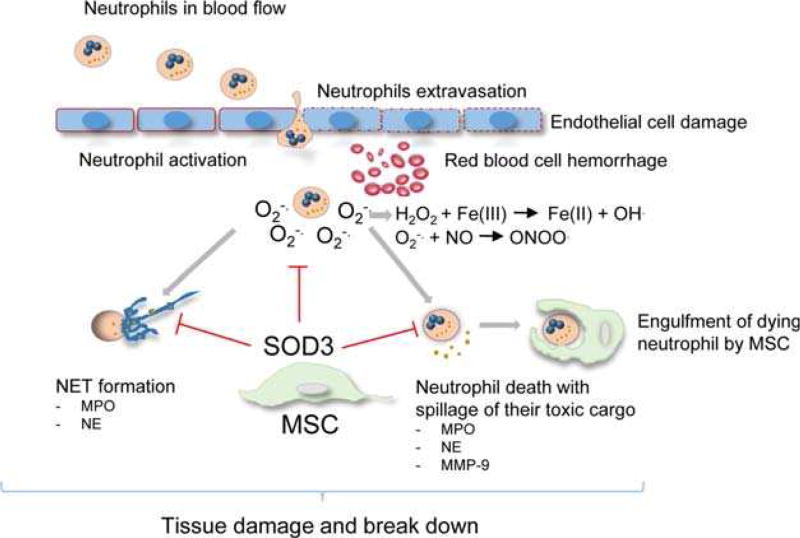
We here characterized versatile skills of MSCs to adaptively respond to changes in their microenvironment and to employ a previously unreported combination of tissue-protective strategies to combat overactivated neutrophils and their toxic content. Based on the adaptive induction of antioxidant enzymes, particularly SOD3, MSCs are able to efficiently dampen the oxidative burst outcome of neutrophils, to suppress their release of destructive enzymes such as peroxidases and proteases and to inhibit NET formation. In addition, we uncovered the previously unreported ICAM-1-dependent increase in uptake and engulfment of apoptotic neutrophils by MSCs to avoid spillage of their toxic cargo. Together, with this multitalented capacity of protective functions, MSCs take a major part in the regulation of tissue homeostasis/inflammation in order to balance the outcome of pathogen defense and niche protection. Our in vitro and in vivo data with particular emphasis on skin tissue not only provide unprecedented insights into self-regulatory mechanisms in innate immunity but also hold substantial promise to develop novel MSC-based strategies for the treatment of disorders with unlimited neutrophil activation, associated with tissue damage. The here identified mechanisms which in their combination allow MSCs to effectively suppress unrestrained neutrophil activation by complementary environmentally adaptive responses (Fig. 6) can be dissected as follows:
Figure 6.
Scheme of adaptive MSC responses to unrestrained neutrophil activation. Immune complexes inside and outside the vessel lead to unrestrained neutrophil activation with enhanced generation and release of which can stimulate NET formation with expulsion of chromatin decorated with granules and highly concentrated MPO, NE and MMP-9, neutrophil death with spillage of their toxic cargo leading to tissue damage and tissue break down. Further damage to macromolecules occur by and derivatives thereof like H2O2, ONNO. and hydroxyl radicals (HO·). MSCs are able to mount an adaptive response to unrestrained neutrophil activation, with the release of detoxifying extracellular SOD3 and ICAM-1/CD18 dependent engulfment of dying neutrophils thus preventing the spillage of their toxic cargo. By employing independent strategies MSCs effectively protect from tissue damage due to unrestrained neutrophil activation. Abbreviations: ICAM-1, intercellular adhesion molecule 1; H2O2, hydrogen peroxide; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase; MSCs, mesenchymal stem cells; NE, neutrophil elastase; NET, neutrophil extracellular trap; ONNO., peroxynitrate; SOD3, superoxide dismutase 3.
First, PMA-induced neutrophil activation with enhanced generation of ROS was alleviated in vitro in cocultures of neutrophils and MSCs as well as following MSC injection in the PMA-induced neutrophilic dermatitis in vivo. Since neither enhanced ROS-release nor neutrophil recruitment occurred in CD18-deficient mice, overactvation of neutrophils was responsible for the elevated -liberation. Second, neutrophil degranulation with the release of MPO, MMP-9 and elastase was suppressed in the presence of MSCs in vitro and in a murine IC-mediated vasculitis model in vivo. Here, the expulsion of nuclear chromatin as NETs was significantly suppressed following injection of MSCs. Since the histone components of NETs confer significant cytotoxicity [32], this self-destructing activity of NETs can equally be reduced by MSCs. Third, MSCs were found to engulf overactivated neutrophils and thus effectively suppress further tissue damage due to prevention of ROS-release, NET formation and the spillage of tissue-toxic proteases. Of note, the β2-integrin ligand, ICAM-1, which is upregulated on MSCs during inflammation [39], was identified to be indispensable for neutrophil engulfment. Fourth, MSCs were herein shown to mount an adaptive response upon exposure to high concentrations and derivatives thereof. In particular, the adaptive upregulation of SOD3 via its -dismutating activity may effectively reduce the highly toxic high levels, and hence prevents the production of peroxynitrite as well as hydroxyl radicals.
The contention that SOD3 represents an effective strategy of MSCs to reduce unrestrained neutrophil activation was clearly supported by the fact that ablation of SOD3 in MSCs by RNAi virtually abrogated the tissue-preserving functions of MSCs as shown in the murine IC-mediated vasculitis model. These findings are of clinical relevance, as they convincingly show that MSCs make use of different complementing strategies to reduce overall tissue damage. The notion that the used murine model is particularly suited to recapitulate human vasculitis with unrestrained neutrophil activation is further supported by the finding that—similar to our observations in the murine model—unlimited NET formation, enhanced release of MPO and NE and severe tissue damage also occurred in human IC-mediated vasculitis. Our data thus provide better mechanistic insights into the spectrum of human disorders with neutrophil overactivation that are currently difficult-to-treat and that reveal high morbidity and mortality. The disease entities with unrestrained neutrophil activation include microscopic polyangiitis, lupus erythematosus (both characterized by IC-mediated vasculitis with severe kidney, skin and lung damage), pyoderma gangrenosum, or genetic deficiencies of the IL-1- or IL-36-receptor antagonist with severe skin, bone and joint destruction [2, 4]. Neutrophils from lupus patients often suffering from IC-mediated vasculitis are found to have enhanced capacity to form NETs, which is associated with increased anti-dsDNA antibodies in sera of lupus patients [40]. Affected skin and kidneys from lupus patients are infiltrated by netting neutrophils, which cause endothelial cell damage, a critical step in the pathogenesis of lupus and other neutrophil overactivation syndromes [41].
Excessive release of proteases from neutrophils, such as NE and MMP-9, leads to excessive extracellular matrix (ECM) degradation and tissue damage and plays a role in several degenerative and inflammatory diseases [42, 43]. Our finding that MSCs rescue neutrophils from cell death and thus prevent NETosis and/or spillage of their toxic proteolytic cargo either decorated on expulsed chromatin or simply released to their surrounding is of major importance to limit and resolve unrestrained inflammation in the interest of organ preservation. A recent study has shown that in patients with severe carotid stenosis, statin treatment reduces neutrophil infiltration and activity with reduced serum levels of MMP-9, MPO and NE, and in consequence correlates with reduced cardiovascular risk [44]. Similarly, in rodents, the elastase inhibitor reduces NE activity and subsequently reduces inflammation and fibrosis after murine myocarditis [45], reduces liver damage due to ischemia and reperfusion injury [46], and diminishes neurologic symptoms after spinal cord injury as well [47].
As opposed to previously published data that human MSCs of different tissue origin constitutively release low level SOD3 [36, 37, 48, 49], we here advance our understanding that MSCs-upon exposure to elevated levels originating from neutrophil oxidative burst-mount an adaptive response with increased SOD3 expression in their endogenous niche in murine and human vasculitis in vivo. Stimulated by this unprecedented observation that MSCs of the endogenous niche mount an antioxidant response with SOD3 overexpression, which most likely preserves the integrity of endogenous MSCs but is not able to suppress overall tissue damage in neutrophil induced vasculitis, we have used therapeutic injections of MSCs. In support of this notion, we provide causal in vivo evidence that NET formation and the release of highly toxic ROS and proteases from neutrophils are effectively counteracted by enhanced SOD3 release from MSCs thus preserving tissue integrity. These data imply that the oxidative burst of neutrophils, in fact, cause NET formation and the release of the tissue toxic cargo from neutrophils. Independent of the SOD3 defense, neutrophils are actively engulfed by MSCs as an additional tissue protecting strategy. Hence, MSCs have evolved multilayered strategies to counteract and protect from overactivated neutrophils in the interest of tissue integrity.
SOD3 represents the only antioxidant enzyme that scavenges in the extracellular compartment. SOD3 additionally competes with nitric oxide and H2O2 for , thereby reducing the production of peroxynitrite and hydroxyl radical, respectively. Peroxynitrite and hydroxyl radicals constitute the most prevailing reactive radicals and major effectors of tissue injury [50]. SOD3 released from MSCs exerts protective effects on cardiomyocytes [49], cerebellar neurons [37] and lung remodelling [48]. Besides its antioxidant effect, SOD3 has the capacity to activate the Ras-MAPK pathway to induce proliferation and metabolic functions of injured tissue [51], and to diminish apoptosis through phosphorylation of Erk1/2, Akt and FoxO3a [52]. By its ability to regulate expression of adhesion molecules and cytokines [53], SOD3 is able to reduce inflammatory cell migration as well.
As to the question of how elevated levels result in an adaptive MSC response of increased SOD3 release, several possibilities may exist. First, oxidized extracellular DNA and oxidized cell-free GC-rich ribosomal DNA–available in excess at the site of neutrophil activation–can trigger enhanced SOD3-release via the TLR9- and NFκB-dependent signaling pathways [54, 55]. Second, enhanced -dismutation with increased H2O2 generation also may stimulate SOD3-release in a NFκB dependent manner [56]. Third, H2O2 stimulates SODs through p38 MAPK [57] and PI3K/Akt signaling [56]. Fourth, SOD3 released from MSCs can be induced by inflammatory cytokines such as TNF-α and IFN-γ [36] occurring in neutrophil-initiated inflammation. Other antioxidant response genes like NRF2, KEAP1, SOD1, BRCA1, and BCL2 involved in self-defense are also reported to be upregulated in MSCs in oxidative stress-induced conditions [54]. Though no change in the specific mRNA levels for NRF2 and other above mentioned antioxidant enzymes were detected in MSCs cocultured with activated neutrophils, we observed the translocation of NRF2 from the cytoplasm to the nucleus, indicative of NRF2 activation, in MSCs 24 hours after coculturing with activated neutrophils (Supporting Information Fig. S4E). It is tempting to speculate that activation of NRF2 upregulates SOD3 expression for the long term response, since SOD3 but not SOD1 or SOD2 is reported to be regulated through NRF2 [58]. Therefore, together with our data of SOD3-silenced MSCs, enhanced SOD3 release from MSCs represents a major tissue protective strategy counteracting microenvironmental oxidative stress during unbalanced inflammation.
We, however, cannot exclude that in addition to SOD3 other mechanisms may contribute to counteract tissue damage and injury due to unrestrained neutrophil activation. In fact, we and others have earlier shown that MSCs dampen inflammation and in consequence reduce scar formation in tissue repair via the release of tumor necrosis factor-inducible gene 6 protein (TSG-6) [21, 59]. MSCs-derived TSG-6 has been shown to bind to IL-8 directly and inhibit neutrophil migration [60]. At least, extravasation, activation and infiltration of neutrophils into the joint synovium were increased in a murine TSG-6 deficient model of rheumatoid arthritis, also displaying an increase in plasmin, MPO and NE release from neutrophils [61].
Conclusion
In summary, we identified a multilayered strategy of mesenchymal stem cells to suppress tissue damage due to overactivated neutrophils. Protective strategies included ICAM-1 mediated engulfment of neutrophils and an adaptive release of SOD3 dampening enhanced ROS level and in consequence suppress NET formation, neutrophil death and excessive spillage of proteases. Our data provide insight into MSC-mediated protective functions in the context of innate immunity and thus holds substantial promise to therapeutically counteract states of neutrophil overactivation.
Supplementary Material
Significance Statement.
We have identified a multilayered strategy of mesenchymal stem cells to suppress tissue damage mediated by overactivated neutrophils via intercellular adhesion molecule 1 mediated engulfment of neutrophils and an adaptive release of superoxide dismutase 3 dampening enhanced reactive oxygen species level and in consequence suppress neutrophil extracellular trap formation, neutrophil death, and excessive spillage of tissue damaging proteases.
Acknowledgments
We thank Carmen Hauser (The Institute for Laser Technology in Medicine and Measurement Technique, University of Ulm) for technical support of confocal microscopy, and Alexander Magnutzki (Institute of Physiological Chemistry, University of Ulm) for technical support for in vivo imaging system. This study was supported in part by research grants from the Baden-Württemberg Stiftung (P-BWS-ASII/15), the European Commission (CASCADE HEALTH-FP7-223236) and the German Research Foundation (SFB1149) to K.S.-K., the Baustein Program from the Medical Faculty, University of Ulm (LSBN.0100) to D.J., and from the Excellence Cluster Cardio-pulmonary System (ECCPS) to M.S. and K.T.P.
Footnotes
Author Contributions
D.J.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing; J.M.: Collection and/or assembly of data; Y.Q.: Collection and/ or assembly of data; A.K.: Collection and/or assembly of data; J.C.V: Collection and/or assembly of data; M.S.: Collection and/or assembly of data, Data analysis and interpretation; A.S. and S.V.B.: Conception and design, Data analysis and interpretation; M.W.: Administrative support, Final approval of manuscript; A.M.K. and C.G.: Provision of study material or patients, Final approval of manuscript; N.Y.F. and M.H.F.: Data analysis and interpretation, Final approval of manuscript; K.T.P.: Conception and design, Financial support, Final approval of manuscript; K.S.-K.: Conception and design, Financial support, Data analysis and interpretation, Manuscript writing.
Disclosure of Potential Conflicts of Interest
M.H.F. and N.Y.F. are inventors or co-inventor of US and international patents assigned to Brigham and Women’s Hospital and/or Boston Children’s Hospital, Boston, MA, and licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG. and participates in corporate sponsored research collaborations with Rheacell GmbH & Co. KG.
References
- 1.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 4.Amulic B, Cazalet C, Hayes GL, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PR. Neutrophilic dermatoses: A review of current treatment options. Am J Clin Dermatol. 2009;10:301–312. doi: 10.2165/11310730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Coxon A, Cullere X, Knight S, et al. Fc gamma RIII mediates neutrophil recruitment to immune complexes. A mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 8.Kahlenberg JM, Carmona-Rivera C, Smith CK, et al. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel JC, Roy S, Wilgus TA. Neutrophil activity in chronic venous leg ulcers-a target for therapy. Wound Repair Regen. 2013;21:339–351. doi: 10.1111/wrr.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 11.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 15.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 16.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 17.Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 18.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 19.Dayan V, Yannarelli G, Billia F, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macro-phages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 20.Jiang D, Qi Y, Walker NG, et al. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials. 2013;34:2501–2515. doi: 10.1016/j.biomaterials.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Qi Y, Jiang D, Sindrilaru A, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine Full-thickness skin Wounds. J Invest Dermatol. 2014;134:526–537. doi: 10.1038/jid.2013.328. [DOI] [PubMed] [Google Scholar]
- 22.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 23.Teo GS, Yang Z, Carman CV, et al. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells. 2015;33:265–277. doi: 10.1002/stem.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung TN, Kim JH, Choi BY, et al. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl Med. 2015;4:178–185. doi: 10.5966/sctm.2014-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feld M, Goerge T, Hillgruber C, et al. alpha-1-Antitrypsin and IFN-gamma reduce the severity of IC-mediated vasculitis by regulation of leukocyte recruitment in vivo. J Invest Dermatol. 2012;132:2286–2295. doi: 10.1038/jid.2012.137. [DOI] [PubMed] [Google Scholar]
- 26.Sindrilaru A, Seeliger S, Ehrchen JM, et al. Site of blood vessel damage and relevance of CD18 in a murine model of immune complex-mediated vasculitis. J Invest Dermatol. 2007;127:447–454. doi: 10.1038/sj.jid.5700563. [DOI] [PubMed] [Google Scholar]
- 27.Lundqvist-Gustafsson H, Bengtsson T. Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J Leukoc Biol. 1999;65:196–204. doi: 10.1002/jlb.65.2.196. [DOI] [PubMed] [Google Scholar]
- 28.Fretland DJ, Gokhale R, Mathur L, et al. Dermal inflammation in primates, mice, and guinea pigs: attenuation by second-generation leukotriene B4 receptor antagonist, SC-53228. Inflammation. 1995;19:333–346. doi: 10.1007/BF01534391. [DOI] [PubMed] [Google Scholar]
- 29.Kielland A, Blom T, Nandakumar KS, et al. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47:760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Peters T, Sindrilaru A, Hinz B, et al. Wound-healing defect of CD18(−/−) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. Embo J. 2005;24:3400–3410. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prokopowicz Z, Marcinkiewicz J, Katz DR, et al. Neutrophil myeloperoxidase: Soldier and statesman. Arch Immunol Ther Exp (Warsz) 2012;60:43–54. doi: 10.1007/s00005-011-0156-8. [DOI] [PubMed] [Google Scholar]
- 32.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruns S, Kniemeyer O, Hasenberg M, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neo-nates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams MA, Solomkin JS. Integrin-mediated signaling in human neutrophil functioning. J Leukoc Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]
- 36.Kemp K, Gray E, Mallam E, et al. Inflammatory cytokine induced regulation of super-oxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev. 2010;6:548–559. doi: 10.1007/s12015-010-9178-6. [DOI] [PubMed] [Google Scholar]
- 37.Kemp K, Hares K, Mallam E, et al. Mesenchymal stem cell-secreted superoxide dis-mutase promotes cerebellar neuronal survival. J Neurochem. 2010;114:1569–1580. doi: 10.1111/j.1471-4159.2009.06553.x. [DOI] [PubMed] [Google Scholar]
- 38.Schatton T, Yang J, Kleffel S, et al. ABCB5 identifies immunoregulatory dermal Cells. Cell Rep. 2015;12:1564–1574. doi: 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus Erythematosus. J Clin Cell Immunol. 2013;4 doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin TC, Li CY, Tsai CS, et al. Neutrophil-mediated secretion and activation of matrix metalloproteinase-9 during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2005;100:1554–1560. doi: 10.1213/01.ANE.0000154307.92060.84. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Takahashi S, Mizumoto T, et al. Neutrophil elastase and cancer. Surg Oncol. 2006;15:217–222. doi: 10.1016/j.suronc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Lenglet S, Quercioli A, Fabre M, et al. Statin treatment is associated with reduction in serum levels of receptor activator of NF-kappaB ligand and neutrophil activation in patients with severe carotid stenosis. Mediators Inflamm. 2014;2014:720987. doi: 10.1155/2014/720987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Zaidi SH, Liu P, et al. A serine elastase inhibitor reduces inflammation and fibrosis and preserves cardiac function after experimentally-induced murine myocarditis. Nat Med. 1998;4:1383–1391. doi: 10.1038/3973. [DOI] [PubMed] [Google Scholar]
- 46.Uchida Y, Freitas MC, Zhao D, et al. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2009;15:939–947. doi: 10.1002/lt.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tonai T, Shiba K, Taketani Y, et al. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 48.Chow K, Fessel JP, Kaoriihida S, et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeSantiago J, Bare DJ, Banach K. Ischemia/reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev. 2013;22:2497–2507. doi: 10.1089/scd.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nozik-Grayck E, Suliman HB, Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol. 2005;37:2466–2471. doi: 10.1016/j.biocel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Laurila JP, Castellone MD, Curcio A, et al. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol Ther. 2009;17:448–454. doi: 10.1038/mt.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laatikainen LE, Incoronato M, Castellone MD, et al. SOD3 decreases ischemic injury derived apoptosis through phosphorylation of Erk1/2, Akt, and FoxO3a. PLoS One. 2011;6:e24456. doi: 10.1371/journal.pone.0024456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurila JP, Laatikainen LE, Castellone MD, et al. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loseva P, Kostyuk S, Malinovskaya E, et al. Extracellular DNA oxidation stimulates activation of NRF2 and reduces the production of ROS in human mesenchymal stem cells. Expert Opin Biol Ther. 2012;12(Suppl 1):S85–S97. doi: 10.1517/14712598.2012.688948. [DOI] [PubMed] [Google Scholar]
- 55.Kostjuk S, Loseva P, Chvartatskaya O, et al. Extracellular GC-rich DNA activates TLR9- and NF-kB-dependent signaling pathways in human adipose-derived mesenchymal stem cells (haMSCs) Expert Opin Biol Ther. 2012;12(Suppl 1):S99–111. doi: 10.1517/14712598.2012.690028. [DOI] [PubMed] [Google Scholar]
- 56.Rojo AI, Salinas M, Martin D, et al. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutierrez-Uzquiza A, Arechederra M, Bragado P, et al. p38alpha mediates cell survival in response to oxidative stress via induction of antioxidant genes: Effect on the p70S6K pathway. J Biol Chem. 2012;287:2632–2642. doi: 10.1074/jbc.M111.323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh B, Bhat HK. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33:2601–2610. doi: 10.1093/carcin/bgs300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Jiang L, Li H, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134:2648–2657. doi: 10.1038/jid.2014.169. [DOI] [PubMed] [Google Scholar]
- 60.Dyer DP, Thomson JM, Hermant A, et al. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J Immunol. 2014;192:2177–2185. doi: 10.4049/jimmunol.1300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szanto S, Bardos T, Gal I, et al. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.