Abstract
Background:
Although the number of colorectal liver metastases (CLM) is decreasingly considered as a contraindication to surgery, patients with 10 CLM or more are often denied liver surgery. This study aimed to evaluate the outcome after liver surgery and to identify prognostic factors of survival in such patients.
Methods:
The study population consisted of a multicentre cohort of patients with CLM (N=12 406) operated on, with intention to resect, from January 2005–June 2013 and whose data were prospectively collected in the LiverMetSurvey registry.
Results:
Overall, the group ⩾10 CLM (N=529, 4.3%) experienced a 5-year overall survival (OS) of 30%. A macroscopically complete (R0/R1) resection (72.8% of patients) was associated with a 3- and 5-year OS of 61% and 39% vs 29% and 5% for R2/no resection patients (P<0.0001). At multivariate analysis, R0/R1 resection emerged as the strongest favourable factor of OS (HR 0.35 (0.26–0.48)). Other independent favourable factors were as follows: maximal tumour size <40 mm (HR 0.67 (0.49–0.92)); age <60 years (HR 0.66 (0.50–0.88)); preoperative MRI (HR 0.65 (0.47–0.89)); and adjuvant chemotherapy (HR 0.73 (0.55–0.98)). The model showed that 5-year OS rates of 30% was possible provided R0/R1 resection associated with at least an additional favourable factor.
Conclusions:
Liver resection might provide long-term survival in patients with ⩾10 CLM staged with preoperative MRI, provided R0/R1 resection followed by adjuvant therapy. A validation of these results in another cohort is needed.
Keywords: colorectal liver metastases, number of liver metastases, liver resection, unresectability, onco-surgical approach
In the past surgical series of colorectal liver metastases (CLM) published (Wilson and Adson, 1976; Ekberg et al, 1986) in the 1970s–1980s, the presence of more than three CLM was considered as a contraindication for resection. Since then, CLM management has considerably evolved (Adam et al, 2012). Innovations in surgical techniques have pushed the boundaries of resectability while the introduction of efficient cytotoxic agents in association with targeted therapies (Cutsem et al, 2009; Kabbinavar et al, 2009) have markedly increased tumour response rate and chance for surgery in patients with unresectable disease at presentation (Adam et al, 2004a, 2009). As a consequence, the oncological dogma of ‘no more than three CLM’ has been progressively challenged. However, the cut-off number for which surgery does not provide any benefit is still unknown and there is currently very few data regarding the oncological results of liver resection performed in patients exhibiting a high number of CLM. We only found studies that focused on outcome of patients exhibiting more than four to eight CLM (Ferrero et al, 2004; Elias et al, 2005; Tamandl et al, 2007; Zakaria et al, 2007; Rees et al, 2008; Viganò et al, 2015) with too limited series to draw practical conclusions (Smith and McCall, 2009). As the majority of patients with a larger number of CLM are exclusively treated by chemotherapy, results of surgery are lacking.
On the other hand, chemotherapy has significantly increased its efficacy in recent years, but long-term survival still remains anecdotal (Van Cutsem et al, 2011; Cremolini et al, 2015).
This prompts us to investigate the outcome of patients operated on for a large number of CLM. As there is no established cut-off defining what a ‘large’ number of CLM is, we choose the cut-off of 10 CLM by analogy with the EORTC randomised trial in which 10 CLM was the limit for RFA indication. Beyond such cut-off, medical oncologists and surgeons usually consider that there is no more place for surgery.
The use of a large multicentric cohort of patients operated on for CLM allows us to obtain a critical number of patients operated on for 10 CLM or more, required to achieve this analysis.
Our objectives were the following ones: (i) to report the results of patients operated on for 10 CLM or more by comparing to that observed in patients with fewer CLM; and (ii) to identify within this group of patients, the factors associated with a real survival benefit.
Materials and methods
Study population
LiverMetSurvey registry
We used the multicentric cohort of patients operated on for CLM between January 2005 and June 2013, and whose data were prospectively registered in the LiverMetSurvey international registry. LiverMetSurvey (http://www.livermetsurvey.org/) is an international database that prospectively collects clinical and pathological data of patients undergoing surgery for CLM (Adam et al, 2010; Andres et al, 2012; Viganò et al, 2012). This register currently involves 485 centres across a total of 59 countries. Data are prospectively entered by using an online questionnaire, which includes demographic and pathologic variables as well as informations concerning the type, duration and effects of preoperative treatment, the surgical procedure, the timing, location and treatment of recurrence, as well as the post-operative and long-term outcome. Data are regularly updated by each centre, and a quality control of the data is performed by a data manager who sends twice a year to each contributing centre a personalised information, concerning the items to complete or to update for each patient of the centre cohort.
Selection of the study population
Our initial study population consists of all consecutive patients registered in the LiverMetSurvey during the study period with available number of CLM on imaging studies at diagnosis (CT or MRI) of the liver disease. The resulting cohort consists on a total of 12 406 patients. Of them, we specifically focused on patients exhibiting 10 CLM or more (N=529).
All patients included in this study were operated on for CLM with an intention to resect on a curative intent. Some of them were not resected due to intraoperative discovery of extrahepatic disease or to more extended liver disease contraindicating radical surgery. In the remaining patients, resection was classified as R0, R1 or R2 as defined below.
Definition of the type of resection
R0 resection was defined by a macroscopically complete removal of the totality of hepatic metastases with ⩾1 mm-free margins. R1 resection referred to macroscopically complete removal of lesions with at least one positive margin (<1 mm) or the use of local ablation (radiofrequency and microwave ablation). R2 resection referred to a macroscopically incomplete resection.
Study design
This study was divided into three steps.
Step 1: comparisons of the group of patients with ⩾10 CLM with two other groups: 1–3 CLM and 4–9 CLM.
Step 2: identification of the factors affecting overall survival (OS) in the group ⩾10 CLM.
Step 3: calculation of survival probabilities in patients with ⩾10 CLM according to a predictive model.
Statistical analysis
Comparisons
Comparisons between categorical data and continuous data were done by using the χ2-test and ANOVA test, respectively.
Survival analysis
Continuous variables were transformed into categorical data by using the roughly rounded values of the mean as cut-off for more readability. The survival probabilities were calculated according to Kaplan–Meier method and survival plots were compared with the log rank test. For OS, time was calculated from the date of CLM diagnosis to the date of last news. For ‘primary’ DFS, time was calculated from the time of resection to the date of first relapse or death. Incomplete resection was considered as a relapse at time 0. In case of multistep procedures (i.e., two-stage hepatic resection), the DFS was calculated from the date of last resection. ‘Secondary’ DFS was calculated for patients who underwent R0/R1 liver resection. Time for secondary DFS was the period between the first hepatectomy and the date of the last relapse that could not be treated curative intent. Relapse was defined as the occurrence of new metastatic localisation.
Multiple imputations
To avoid biased estimates (Janssen et al, 2010), missing data were imputed (N=10). Variables with a proportion of missing values >30% were not selected for imputation and not considered for analysis. Imputations were generated by using the predictive mean-matching method. Then, the plausibility of imputed data was checked.
Cox proportional hazards model
Variables with P–values <0.15 at univariate analysis were entered into a Cox proportional hazard model for multivariate analysis. Continuous variables were transformed into binary variables by using their mean value. This makes scoring system easier to use. The final selection of variables retained in the final model used the minimal Akaike Information Criterion approach. The proportional hazards assumption for each covariate and for the entire model was checked by using Schoenfeld’s residuals.
Then, the coefficients obtained for each data set were combined to estimate the final regression coefficient according to Rubin’s rule. (Rubin, 1987).
Prediction of survival probabilities
For each data set, we calculated the survival probabilities predicted by the final Cox model according to the different combination of factors. We then obtained the average survival probabilities for each situation across data sets.
The analysis was done using the statistical programming language R, version 3.1.1, the ggplot2, rms and mice packages.
Results
Characteristics of patients with 10 CLM or more compared to groups: 1–3 CLM and 4–9 CLM
As expected, the three groups (1–3 CLM, 4–9 CLM and ⩾10 CLM) exhibited major differences with regard to characteristics of patients, of primary tumour features, of metastatic disease and perioperative management. Comparisons are detailed in Table 1. Briefly, the number of CLM was associated with younger patients and lower proportion of rectal cancers. It also correlates with an increased maximal tumour size, an increased proportion of bilobar distribution, of synchronous CLM, of initial non-resectability and of preoperative chemotherapy. The proportion of R2 resection/no resection was the highest in the ⩾10 CLM group (27.2% vs 14.7% and 5% in the 4–9 CLM group and 1–3 CLM group, respectively; P<0.0001).
Table 1. Baseline characteristics of each group.
|
1–3 CLM |
4–9 CLM |
⩾10 CLM |
|||||
|---|---|---|---|---|---|---|---|
|
N=9643 |
N=2234 |
N=529 |
P | ||||
| Variables | No. | % | No. | % | No. | % | |
| Sex: male | 5932 | 61.5 | 1400 | 62.7 | 315 | 59.5 | 0.36 |
| Mean age (±s.d.), years | 63.3 (±11) |
60.1 (±11) |
58.5 (±10) |
<0.0001 | |||
| Primary tumour | |||||||
| Location: rectum | 3092 | 32.8 | 737 | 33.8 | 133 | 25.8 | 0.002 |
| Stage T3–T4 | 7358 | 87.8 | 1741 | 89.7 | 396 | 90.0 | 0.03 |
| Stage N positive | 5253 | 63.1 | 1328 | 69.6 | 319 | 74.4 | <0.0001 |
| CLM characteristics | |||||||
| Mean number of CLM (±s.d.) | 1.6 (±0.7) | 5.3 (±1.4) | 13.4 (±4) | <0.0001 | |||
| Mean maximum tumour size (±s.d.), mm | 37.6 (±28) | 38.9 (±28) | 44.5 (±35) | <0.0001 | |||
| Mean CEA level, ng ml−1 | 119.4 (±80) | 123.4 (±79) | 137.1 (±77) | <0.0001 | |||
| Distribution: bilobar | 2141 | 22.4 | 1736 | 78.6 | 497 | 94.3 | <0.0001 |
| Initially resectable | 7774 | 88.2 | 1283 | 62.6 | 163 | 32.1 | <0.0001 |
| Time of occurrence: synchronous | 4650 | 48.6 | 1571 | 71.1 | 458 | 87.1 | <0.0001 |
| Concomittant extrahepatic disease | 916 | 9.7 | 238 | 10.9 | 70 | 13.5 | 0.008 |
| Perioperative management | |||||||
| Preop. MRI | 3497 | 39.7 | 951 | 45.9 | 213 | 44.6 | 0.04 |
| Preop. chemotherapy | 3391 | 37.1 | 1329 | 61.9 | 353 | 68.4 | <0.0001 |
| Preop. targeted therapy | 1277 | 38.6 | 629 | 47.9 | 208 | 59.3 | <0.0001 |
| Progression while on chemotherapya | 261 | 7.7 | 83 | 6.2 | 13 | 3.7 | 0.002 |
| Portal vein embolisation | 561 | 6.1 | 424 | 19.5 | 201 | 39.1 | <0.0001 |
| Postop. chemotherapy | 3824 | 54.2 | 963 | 56.4 | 191 | 51.3 | 0.12 |
| Postop. targeted therapy | 752 | 20.3 | 309 | 32.7 | 83 | 43.9 | <0.0001 |
| Early outcomes | |||||||
| R0/R1 liver resection | 8742 | 95.0 | 1790 | 85.3 | 346 | 72.8 | <0.0001 |
| 90-day mortality | 200 | 2.1 | 76 | 3.4 | 25 | 4.8 | 0.0001 |
| Grade III–IV morbidityb | 1389 | 16.8 | 363 | 19.0 | 81 | 18.7 | 0.05 |
Abbreviations: CEA=carcinoembryonic antigen; CLM=colorectal liver metastases; MRI=magnetic resonance imaging; Postop.=postoperative; Preop.=preoperative.
Calculations were made based on the number of patients who received preoperaive chemotherapy.
Dindo–Clavien classification.
Perioperative complications and mortality
The 90-day mortality observed in the ⩾10 CLM group was significantly higher (4.8%). Severe morbidity (grade III–IV) was comparable to that of the 3–9 CLM group but higher than the group of 1–3 CLM. There was a significant increase in the risk of 90-day mortality with the age, for each subgroup of CLM number (Supplementary File 1).
Overall survival and disease-free survival
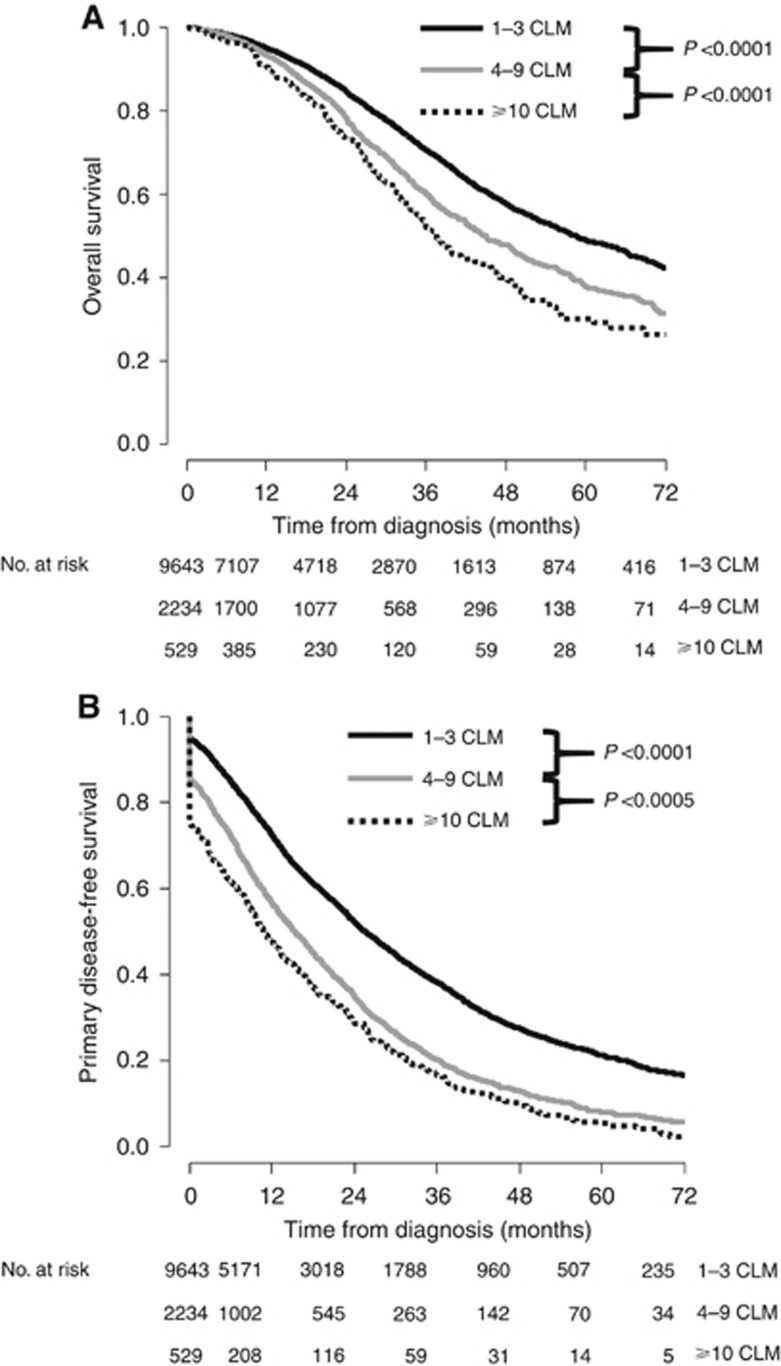
The median follow-up from diagnosis was 29.4 months. As expected, we observed a significant decrease in OS when comparing 1–3 CLM group vs 4–9 CLM group and 4–9 CLM group vs ⩾10 CLM group (Figure 1A). Accordingly, the 3- and 5-year OS rates were 70 and 49% in the 1–3 CLM group, 60 and 39% in the 4–9 CLM group and 52 and 30% in the ⩾10 CLM group. Similar impact was observed for primary DFS (Figure 1B). Indeed, 3-year primary DFS was 38%, 20% and 16% in patients with 1–3 CLM, 4–9 CLM and ⩾10 CLM, respectively (P<0.0001).
Figure 1.
Survival according to the number of CLM. (A) Kaplan–Meier overall survival curves according to the number of CLM. (B) Kaplan–Meier primary disease-free survival according to the number of CLM.
Similar findings were observed when OS probabilities were calculated from the date of resection. The Kaplan–Meier OS curves are given in Supplementary File 2.
Patients with ⩾10 CLM
Proportion
Overall, the group ⩾10 CLM accounted for 4.3% of the entire study population. We observed a significant increase in the proportion of patients operated over the study period. The group ⩾10 CLM represented 3.8% of the cohort during the first part of the study period (January 2005–December 2008) vs 4.8% (P=0.03) in the most recent period (January 2009—June 2013).
Resectability
Not surprisingly, the majority of patients (68.4%) with 10 CLM or more received preoperative chemotherapy, and resection was undertaken after a control of the disease (response or stabilisation) in 96.3% of them.
The resectability (R0/R1) rate was 72.8%. Among resected patients, the proportion of R0 resection and R1 resection (including use of local ablation) were 43.1% and 56.9%, respectively.
Overall, 27.2% of patients with ⩾10 CLM underwent either (i) R2 resection: failure of planned two-stage hepatectomy (N=62, 48.1%) or (ii) no resection at all because of intraoperative findings of unresectable CLM, and/or extrahepatic disease (lymph node, carcinomatosis (N=67, 51.9%)).
Identification of the prognostic factors for OS in patients with ⩾10 CLM
Univariate analysis
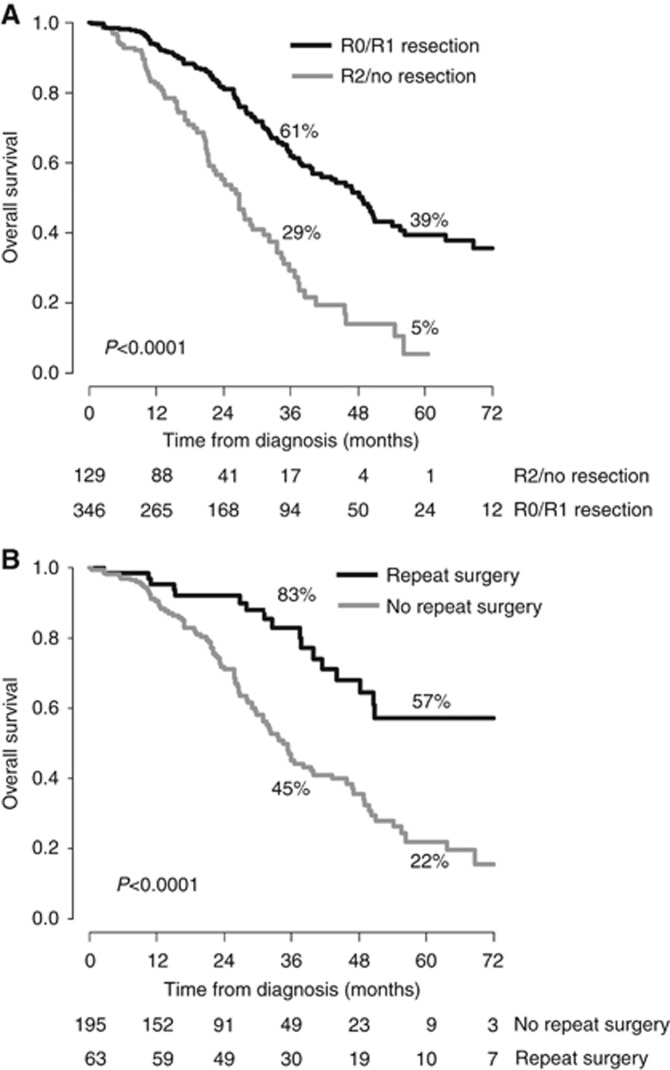
We did not observe any difference in terms of OS between R0 patients and R1/combined RFA patients (3- and 5-year OS rates of 73 and 45% vs 60 and 44% P=0.72). Patients with R2 resection achieved similar outcomes than those without any resection (3- and 5-year OS rates of 29 and 6% vs 28 and 0% for R2 resection and no resection group; P=0.77). However, the 3- and 5-year OS rates were much higher for combined R0/R1 resection groups compared to R2/no resection groups (61 and 39% for R0/R1 resection group vs 29 and 5% in the group with R2/no resection at 3 and 5 years, respectively, P<0.0001; Figure 2A). Then, we decided to perform the analysis by combining R0/R1 resection and R2/no resection groups.
Figure 2.
Survival according to the quality of resection and the possibility of repeat resection. (A) Kaplan–Meier overall survival curves according to the R0/R1 resection possibility. (B) Kaplan–Meier overall survival curves of patients who relapsed after R1/R0 resection according to the use of repeat surgery.
Results of univariate analysis in original set and imputed data are provided in Table 2. Others factors with a P-value <0.15 were as follows: age ⩾60 years; maximal tumour size ⩾40 mm; a preoperative MRI; preoperative chemotherapy; and adjuvant chemotherapy. Of note, the number of CLM in the group of patients with ⩾10 CLM had no influence on outcome (HR: 1.0 (0.97–1.03); P=0.92).
Table 2. Univariate analysis for overall survival in patients with ⩾10 CLM (original data set).
| Variables | 3-year OS (%) | 5-year OS (%) | MS (months) | P |
|---|---|---|---|---|
| Patient | ||||
| Sex | ||||
| Female | 46 | 32 | 34 | 0.22 |
| Male | 56 | 29 | 40 | |
| Age (years) | ||||
| <60 | 58 | 36 | 46 | 0.001 |
| ⩾60 | 44 | 21 | 35 | |
| Primary tumour | ||||
| Location | ||||
| Colon | 53 | 31 | 38 | 0.35 |
| Rectum | 48 | 25 | 34 | |
| Stage T | ||||
| T0–T2 | 63 | 26 | 54 | 0.16 |
| T3–T4 | 50 | 29 | 36 | |
| Lymph node | ||||
| N0 | 57 | 35 | 44 | 0.44 |
| N+ | 51 | 27 | 37 | |
| CLM characteristics | ||||
| Maximum tumour size | ||||
| <40 mm | 60 | 34 | 42 | 0.005 |
| ⩾40 mm | 43 | 26 | 32 | |
| CEA | ||||
| <100 ng ml−1 | 56 | 29 | 42 | 0.02 |
| ⩾100 ng ml−1 | 37 | 16 | 32 | |
| CLM distribution | ||||
| Unilobar | 53 | 30 | 38 | 0.67 |
| Bilobar | 43 | 43 | 34 | |
| Initial resectability | ||||
| Unresectable | 51 | 30 | 37 | 0.98 |
| Resectable | 52 | 30 | 37 | |
| CLM occurrence | ||||
| Metachronous | 48 | 36 | 34 | 0.22 |
| Synchronous | 53 | 30 | 38 | |
| Extrahep. disease | ||||
| N | 52 | 31 | 37 | 0.09 |
| Y | 47 | 22 | 34 | |
| Management | ||||
| Preop. MRI | ||||
| N | 44 | 27 | 33 | 0.0009 |
| Y | 66 | 38 | 47 | |
| Preop. chemotherapy | ||||
| N | 43 | 27 | 31 | 0.01 |
| Y | 57 | 31 | 39 | |
| Preop. targeted therapy | ||||
| N | 51 | 23 | 36 | 0.12 |
| Y | 61 | 41 | 42 | |
| Response to preop. chemotherapy | ||||
| N | 54 | 28 | 38 | 0.42 |
| Y | 66 | 53 | 69 | |
| Portal vein embolisation | ||||
| N | 49 | 35 | 36 | 0.81 |
| Y | 56 | 27 | 40 | |
| Adjuvant chemotherapy | ||||
| N | 48 | 30 | 34 | 0.08 |
| Y | 55 | 31 | 40 | |
| Postop. targeted therapy | ||||
| N | 54 | 34 | 40 | 0.97 |
| Y | 58 | 27 | 42 | |
| Quality of resection | ||||
| R2/no resection | 29 | 5 | 27 | <0.0001 |
| R0/R1 | 61 | 39 | 49 |
Abbreviations: CEA=carcinoembryonic antigen; CLM= colorectal liver metastases; Extrahep.=extrahepatic; MRI=magnetic resonance imaging; MS=median survival; OS=overall survival; postop.=postoperative; preop.=preoperative.
Multivariate analysis
The five factors that remained in the Cox model in all data sets were the following: type of resection (R0/R1); age ⩾60 years; adjuvant chemotherapy; maximal tumour size ⩾40 mm; and preoperative MRI. The C-index ranged from 0.68 to 0.71 across imputed data sets.
The pooled estimates of the factors, retained in the final model, are as follows: R0/R1 resection (HR 0.35 (0.26–0.48); P<0.0001); preoperative MRI (HR 0.65 (0.47–0.89); P=0.007); adjuvant chemotherapy (HR 0.73 (0.55–0.98); P=0.04); maximal tumour size ⩾40 mm (HR 1.49 (1.09–2.03); P=0.02); and age ⩾60 years (HR 1.51 (1.13–2.00), P=0.005; Table 3).
Table 3. Pooled estimates of multivariate analysis for overall survival in patients with ⩾10 CLM.
| Variables | HR | Lower CI | Upper CI | P |
|---|---|---|---|---|
| Max tumour size ⩾40 mm | 1.49 | 1.09 | 2.03 | 0.02 |
| Age ⩾60 years | 1.51 | 1.13 | 2.00 | 0.005 |
| Preoperative MRI | 0.65 | 0.47 | 0.89 | 0.007 |
| R0/R1 resection | 0.35 | 0.26 | 0.48 | <0.0001 |
| Adjuvant chemotherapy | 0.73 | 0.55 | 0.98 | 0.04 |
Abbreviations: CI=confidence interval; CLM= colorectal liver metastases; HR=hazards ratio; MRI=magnetic resonance imaging.
Recurrence after R0/R1 liver resection
The primary DFS was similar between the group R0 resection and that with R1 resection/RFA use (31 and 8% vs 22 and 6% at 3 and 5 years; P=0.56).
Of the 346 patients who underwent R0/R1 resection, 258 (74.6%) had developed a recurrence at last follow-up. The 3- and 5-year primary DFS rates were 23% and 7%, respectively. Hepatic recurrence and extrahepatic recurrence were surgically treated in 49 (19%) and 14 patients (5.4%), respectively. The secondary DFS rates (taking into account the impact of repeat surgery) at 3 and 5 years were 42% and 31%, respectively. The OS of patients who underwent repeat surgery was significantly better than that of patients whom recurrence was managed by exclusive chemotherapy (83 and 57% vs 45 and 22% at 3 and 5 years; P<0.0001; Figure 2B).
Survival probabilities according to the distribution of prognostic factors
We calculated the average survival probabilities across imputed data sets in patients who underwent R0/R1 resection according to each cofactor association (Table 4).
Table 4. Survival probabilities according to prognostic factors combinations in patients with ⩾10 CLM who underwent R0/R1 resection.
| R0/R1 resection | Preop. MRI | Maximum tumour size <40 mm | Age <60 years | Adjuvant chemotherapy | 3-yr OS (%) | 5-yr OS (%) | MS (months) |
|---|---|---|---|---|---|---|---|
| + | + | + | + | + | 82 | 69 | NR |
| + | − | + | + | + | 74 | 57 | NR |
| + | + | − | + | + | 70 | 50 | 69 |
| + | + | + | − | + | 77 | 61 | NR |
| + | + | + | + | − | 75 | 57 | NR |
| + | + | + | − | − | 68 | 47 | 54 |
| + | + | − | + | − | 58 | 36 | 47 |
| + | + | − | − | + | 62 | 40 | 50 |
| + | − | − | + | + | 58 | 35 | 47 |
| + | − | + | − | + | 67 | 47 | 54 |
| + | − | + | + | − | 64 | 43 | 51 |
| + | + | − | − | − | 48 | 25 | 36 |
| + | − | + | − | − | 55 | 32 | 42 |
| + | − | − | + | − | 44 | 21 | 34 |
| + | − | − | − | + | 48 | 25 | 36 |
| + | − | − | − | − | 33 | 12 | 31 |
Abbreviations: MRI=magnetic resonance imaging; MS=median survival; NR, not reached; OS=overall survival; Preop.=preoperative.
Thus, patients with all additional four favourable factors, the 3- and 5-year OS rates were 82% and 69%, respectively. The OS probability at 3 and 5 years decreased to 70–77% and 50–61% in patients with three additional favourable factors, 58–68% and 35–47% in patients with two additional favourable factors, 44–55% and 21–32% in patients with one additional factor, and 33 and 12% in patients with no additional positive factors (Table 4).
Discussion
General considerations
The main finding of the current study is that a 5-year OS and median survival of at least of 21% and 34 months can be achieved in patients with ⩾10 CLM after complete treatment of macroscopic liver disease in patients with at least a single additional favourable factor: age <60 years; a maximal tumour size <40 mm; preoperative MRI; preoperative chemotherapy; and adjuvant chemotherapy.
Although 10 liver metastases or more is a common presentation at diagnosis, the low proportion of this group reported here (4.3% of the cohort) indicates that (1) most centres consider such number of CLM as a contraindication for resection and (2) patients operated are highly selected. This also translates the fact that an important number of CLM may compromise technical feasibility of complete resection. Indeed, the proportion of patients with ⩾10 CLM, finally unresected or with incomplete resection (27.2%) demonstrates how challenging it is to achieve curative resection in these patients
Preoperative chemotherapy
Resection in patients with disease progression while on chemotherapy was anecdotal (3.7%). This is in accordance with previous studies showing the importance of a disease control before considering surgery in patients with advanced liver disease (Adam et al, 2004a, 2004b; Garufi et al, 2010). Therefore, the present findings cannot be applied in patients with a progressive metastatic disease. The interpretation of our results should be made at the light of this prerequisite.
What are the conditions that may ensure an additional benefit of surgery compared to chemotherapy alone?
Interestingly, the median survival of patients with R2/no resection (27 months) was similar to that reported in contemporary trials of chemotherapies in patients with metastatic unresectable disease (Heinemann et al, 2014; Loupakis et al, 2014), whereas the survival of R0/R1 patients (median survival 49 months) was by far better and close to survival rates observed in resected patients with fewer CLM (Nordlinger et al, 2013). This shows that even in patients with very advanced disease, resection has the potential to improve patient outcome.
Interestingly, preoperative MRI emerged as a favourable prognostic factor, independently of the other ‘oncological’ parameters. Given the superiority of MRI over CT scan for detecting small nodules, especially after neoadjuvant chemotherapy (Kulemann et al, 2011), a shift in the disease staging may be advanced to explain this finding. Indeed, it is likely that some patients, initially considered as ‘resectable’ based on CT scan, may become ineligible for surgery after preoperative MRI. This results in a more favourable tumour biology or less-advanced disease in the subgroup of patients with preoperative MRI. We also may hypothesised that the quality of resection, especially in patients at high risk of very small tumour foci, such as the ones with ⩾10 CLM may be improved after MRI.
According to our multivariate model, macroscopically complete resection, alone (i.e., without additional favourable factors), is associated with a median OS of 31 months and a 12% 5-year OS. The addition of a single favourable factor to R0/R1 surgery was associated with a 5-year OS of 21–32% and a MS of 34–42 months. This suggests that surgical treatment (often complex and potentially morbid given the extent of the liver disease) should be undertaken only in patients with at least a single additional favourable factor.
Outcomes of patients with ⩾10 CLM are hampered by a high rate of relapse (74.6%). This argues in favour of routine post-operative chemotherapy, an independent factor of improved OS. Improvement in adjuvant therapy may warrant more favourable results in this subgroup of patients at high risk of recurrence and then should be further investigated. In this setting, hepatic arterial infusion may be a valid option to control microscopic residual disease as recently suggested. (Goéré et al, 2013). However, contrary to randomised studies, the design of the present study did not allow to demonstrate the favourable impact of adjuvant therapy on survival. As most of institutions would refer patients with ⩾10 CLM for adjuvant therapy, it is likely that the absence of post-operative chemotherapy is likely to be due to adverse post-operative events such as surgical complications or deterioration of the general status.
Repeat hepatectomy or extrahepatic resection for patients with recurrence is another way to improve the outcome of these patients as demonstrated by the better survival of patients submitted to this repeat surgery, compared to those submitted to a single surgical procedure. Of course, this group consists of selected patients, with favourable tumour biology. However, these good outcomes emphasise the relevance of an aggressive onco-surgical approach.
R0/R1 resection
Here we grouped R0/R1 resection. This may be surprising knowing that R0 resection must remain an oncological goal in surgery of CLM. However, in patients with ⩾10 CLM, R0 and R1 resection yield similar survival as this has been already reported in patients with a large number of CLM (de Haas et al, 2008; Folprecht et al, 2014). The proportion of R1 resection directly results of the high number of lesions, the frequent use of RFA and the necessity to preserve vascular structures.
At least 10 CLM on imaging studies obviously represents an advanced stage of the metastatic disease and it is likely that many small tumour foci cannot be detected by imaging studies. Therefore, the survival benefit offered by a macroscopically complete surgery that cannot eradicate all microscopic tumours of the liver questions in some extent the ‘only R0’ principle of oncologic surgery. These so-called ‘macroscopically complete resections’ could in fact be likened from an oncological point of view to cytoreductive surgery advocated in ovarian cancers whose prognosis correlates with tumour residual (Winter et al, 2008).
Limitations of the study
This analysis carries some limitations. The study is retrospective, multicentric and as a result, may suffer from different selection criteria and heterogeneity in patient management (preoperative imaging studies and experience in complex liver surgery) between centres. We acknowledge that patients with at least 10 CLM represents a highly selected population. For that reason, we cannot ascertain that similar results may be observed in a less selected cohort. It would also have been interesting to precisely investigate the impact of the overall tumour burden but this would imply an accurate information of all tumour foci (for example, the tumour size of all metastases at all locations). Such data are not available in the registry. Unfortunately, the impact of preoperative PET–CT, already emphasised (Wiering et al, 2005), could not be evaluated due to lacking data. However, these results offer the advantage to reflect the ‘true life’ of resection in this setting, irrespective of the level of expertise of the centre. Moreover, these results represent the largest series reported to date of patients with such a number of CLM.
In conclusion, the long-term outcome of patients with 10 CLM or more is obviously worse compared to patients exhibiting fewer lesions, but surgery after effective chemotherapy remains the only hope of prolonged survival, especially in selected patients for whom complete resection could be performed. In fact, it is likely that the ‘10 and more’ patients only represent the visible part of the iceberg. The present results show that the number of CLM should not be considered as contraindication to surgery per se and should encourage oncologists and surgeons to extend the surgical indications beyond commonly admitted boundaries.
Acknowledgments
We thank all centres contributing to LiverMetSurvey and Valérie Delvart for performing the statistical analysis of this study. There is no financial support for the study. LiverMetSurvey is supported by a grant from Sanofi-Aventis.
Author contributions
Conceptualisation and methodology: MAA and RA; formal analysis: MAA and RA; writing and manuscript preparation: MAA and RA; review and editing: FG, RL, CH, JNMI, DFM, DE, CL, TG, GP, CL, HI, JH, VL, IP and JF.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Adam R, Bhangui P, Poston G, Mirza D, Nuzzo G, Barroso E, Ijzermans J, Hubert C, Ruers T, Capussotti L, Ouellet J-F, Laurent C, Cugat E, Colombo PE, Milicevic M (2010) Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg 252: 774–787. [DOI] [PubMed] [Google Scholar]
- Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E (2012) The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 17: 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Gh??mard O, Levi F, Bismuth H (2004. a) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy. Ann Surg 240: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H (2004. b) Tumor progression while on chemotherapy. Ann Surg 240: 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27: 1829–1835. [DOI] [PubMed] [Google Scholar]
- Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, Gerstel E, Roth A, Majno PE, Mentha G (2012) A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases. Ann Surg 256: 772–779. [DOI] [PubMed] [Google Scholar]
- Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D’Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Cutsem V, Köhne CH, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417. [DOI] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R (2008) R1 resection by necessity for colorectal liver metastases. Trans Meet Am Surg Assoc 126: 269–280. [DOI] [PubMed] [Google Scholar]
- Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hägerstrand I, Ranstam J, Bengmark S (1986) Determinants of survival in liver resection for colorectal secondaries. Br J Surg 73: 727–731. [DOI] [PubMed] [Google Scholar]
- Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, Malka D, Pignon J-P, Lasser P (2005) Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 12: 900–909. [DOI] [PubMed] [Google Scholar]
- Ferrero A, Polastri R, Muratore A, Zorzi D, Capussotti L (2004) Extensive resections for colorectal liver metastases. J Hepatobiliary Pancreat Surg 11: 92–96. [DOI] [PubMed] [Google Scholar]
- Folprecht G, Gruenberger T, Bechstein W, Raab H-R, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J, Lang H, Trarbach T, Liersch T, Ockert D, Jaeger D, Steger U, Suedhoff T, Rentsch A, Köhne C-H (2014) Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol 25: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I, Cognetti F (2010) Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer 103: 1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goéré D, Benhaim L, Bonnet S, Malka D, Faron M, Elias D, Lefèvre JH, Deschamps F, Dromain C, Boige V, Dumont F, De Baere T, Ducreux M (2013) Adjuvant chemotherapy after resection of colorectal liver metastases in patients at high risk of hepatic recurrence: a comparative study between hepatic arterial infusion of oxaliplatin and modern systemic chemotherapy. Ann Surg 257: 114–120. [DOI] [PubMed] [Google Scholar]
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes H-G, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15(10): 1065–1075. [DOI] [PubMed] [Google Scholar]
- Janssen KJM, Donders ART, Harrell FE, Vergouwe Y, Chen Q, Grobbee DE, Moons KGM (2010) Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 63: 721–727. [DOI] [PubMed] [Google Scholar]
- Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O (2009) Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 27: 199–205. [DOI] [PubMed] [Google Scholar]
- Kulemann V, Schima W, Tamandl D, Kaczirek K, Gruenberger T, Wrba F, Weber M, Ba-Ssalamah A (2011) Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol 79: 1–6. [DOI] [PubMed] [Google Scholar]
- Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371: 1609–1618. [DOI] [PubMed] [Google Scholar]
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14: 1208–1215. [DOI] [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FKS, O’Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247: 125–135. [DOI] [PubMed] [Google Scholar]
- Rubin DB (1987) Multiple imputation for nonresponse in surveys. John Wiley & Sons: New York, NY, USA.
- Smith MD, McCall JL (2009) Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg 96: 1101–1113. [DOI] [PubMed] [Google Scholar]
- Tamandl D, Gruenberger B, Herberger B, Schoppmann S, Bodingbauer M, Schindl M, Puhalla H, Fleischmann E, Schima W, Jakesz R, Laengle F, Gruenberger T (2007) Selective resection of colorectal liver metastases. Eur J Surg Oncol 33: 174–182. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Köhne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- Viganò L, Capussotti L, Barroso E, Nuzzo G, Laurent C, Ijzermans JNM, Gigot J-F, Figueras J, Gruenberger T, Mirza DF, Elias D, Poston G, Letoublon C, Isoniemi H, Herrera J, Sousa FC, Pardo F, Lucidi V, Popescu I, Adam R (2012) Progression while receiving preoperative chemotherapy should not be an absolute contraindication to liver resection for colorectal metastases. Ann Surg Oncol 19: 2786–2796. [DOI] [PubMed] [Google Scholar]
- Viganò L, Capussotti L, Majno P, Toso C, Ferrero A, De Rosa G, Rubbia-Brandt L, Mentha G (2015) Liver resection in patients with eight or more colorectal liver metastases. Br J Surg 102: 92–101. [DOI] [PubMed] [Google Scholar]
- Wiering B, Krabbe PFM, Jager GJ, Oyen WJG, Ruers TJM (2005) The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases: a systematic review and metaanalysis. Cancer 104: 2658–2670. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Adson MA (1976) Surgical treatment of hepatic metastases from colorectal cancers. Arch Surg 111: 330–334. [DOI] [PubMed] [Google Scholar]
- Winter WE, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, Rubin SC, Muggia F, McGuire WP (2008) Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 26: 83–89. [DOI] [PubMed] [Google Scholar]
- Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, Nagorney DM (2007) Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 246: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.