Abstract
Chronic treatment with fibroblast growth factor 21 (FGF-21) favorably improves obesity and nonalcoholic fatty liver disease (NAFLD) outcomes; however, FGF-21 expression is paradoxically elevated in obese conditions. Here, we sought to determine the effects of obesity prevention by daily exercise (EX) vs. caloric restriction (CR) on hepatic FGF-21 in the hyperphagic, Otsuka Long-Evans Tokushima Fatty (OLETF) rat. Four-week-old male OLETF rats were randomized into groups (n = 7–8 per group) of ad libitum fed, sedentary (OLETF-SED), voluntary wheel running exercise (OLETF-EX), or CR (OLETF-CR; 70% of SED) until 40 weeks of age. Nonhyperphagic, Long-Evans Tokushima Otsuka (LETO-SED) rats served as controls. Both daily EX and CR prevented obesity and NAFLD development observed in the OLETF-SED animals. This was associated with significantly (p < 0.01) lower serum FGF-21 (~80% lower) and hepatic FGF-21 mRNA expression (~65% lower) in the OLETF-EX and OLETF-CR rats compared with the OLETF-SED rats. However, hepatic FGF-21 protein content was reduced to the greatest extent in the OLETF-EX animals (50% of OLETF-SED) and did not differ between the OLETF-SED and OLETF-CR rats. Hepatic FGF-21 signaling mediators — hepatic FGF-21 receptor 2 (FGFR2, mRNA expression), hepatic FGF-21 receptor substrate 2 (FRS2, protein content), and co-receptor β-Klotho (protein content) — were all elevated (60%–100%, ~40%, and +30%–50%, respectively) in the OLETF-EX and OLETF-CR animals compared with the OLETF-SED animals. Daily exercise and caloric restriction modulate hepatic FGF-21 and its primary signaling mediators in the hyperphagic OLETF rat. Enhanced metabolic action of FGF-21 may partially explain the benefits of exercise and caloric restriction on NAFLD outcomes.
Keywords: FGF-21, OLETF rats, FGFR2, β-klotho, physical activity, obesity
Introduction
It is now estimated that 34% of American adults are obese (Flegal et al. 2010), and these numbers are expected to continue to grow. Obesity is associated with a multitude of deleterious conditions such as type 2 diabetes, coronary heart disease (Abbasi et al. 2002), and nonalcoholic fatty liver disease (NAFLD) (Wiegand et al. 2010). Obesity is also considered an insulin-resistant state (Abbasi et al. 2002) and, more recently, described as a state of fibroblast growth factor 21 (FGF-21) resistance (Fisher et al. 2010).
The FGF family currently has 22 identified members (Long and Kharitonenkov 2011). Three members of this family (FGF-19, FGF-21, and FGF-23) are classified as the FGF-19 subfamily (Uebanso et al. 2011) and may have the capacity to function as hormones (Dushay et al. 2010). The specific role of FGF-21 in modulating obesity and obesity-associated metabolic disorders is under intense investigation. FGF-21 administration has been shown to regulate many important metabolic processes such as ketogenesis, gluco-neogenesis, and lipid metabolism (Inagaki et al. 2007; Potthoff et al. 2009; Xu et al. 2009a; Fisher et al. 2011), including increasing glucose clearance into adipose tissue in ob/ob mice (Kharitonenkov et al. 2005) and lowering circulating lipids (Xu et al. 2009a). Furthermore, overexpression of FGF-21 has been shown to increase hepatic β-oxidation (Potthoff et al. 2009).
It has been shown previously that FGF-21 mRNA expression is increased in white adipose tissue of mice with obesity (Fisher et al. 2010). However, hepatic FGF-21 mRNA expression in the liver is 100 times higher than that observed in adipose tissue (Mraz et al. 2009), and hepatic expression is further elevated with obesity (Fisher et al. 2010). These data suggest that the liver is the primary source of circulating FGF-21. In addition, although FGF-21 is elevated in obese conditions (Dushay et al. 2010; Fisher et al. 2010; Mashili et al. 2011), its effectiveness to lower serum lipids is attenuated in obese animals compared with lean ones (Fisher et al. 2010), suggesting a state of FGF-21 resistance. Collectively, obesity-associated conditions such as NAFLD could have a significant impact on hepatic expression and circulating levels of FGF-21 and perhaps its biological activity (Dushay et al. 2010; Tyynismaa et al. 2011).
Our group has previously utilized the Otsuka Long-Evans Tokushima Fatty (OLETF) rat to examine the relationship between obesity and NAFLD. OLETFs rats lack the cholecysto-kinin-1 receptor in the brain, causing hyperphagia and subsequent NAFLD, insulin resistance, obesity, and type 2 diabetes (Moran and Bi 2006; Rector et al. 2008; Rector et al. 2011). Furthermore, the prevention of excess weight gain and adiposity by either access to voluntary running wheels for daily exercise or food restriction prohibits the development and progression of NAFLD (Rector et al. 2008; Rector et al. 2011). Although existing data demonstrated elevated FGF-21 in obese conditions, the effects of exercise and caloric restriction on FGF-21 in an overeating model of obesity has not previously been examined. Therefore, the purpose of this study was to test the hypothesis that hepatic FGF-21 is elevated in obese, sedentary OLETF rats and that prevention of weight gain by either daily wheel running exercise or daily caloric restriction would attenuate these changes and alter the hepatic expression of FGF-21 signaling mediators.
Methods
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. Male OLETF rats at 4 weeks of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). OLETF rats were randomly assigned to one of three groups. Two groups remained sedentary but differed in that one group was fed ad libitum (OLETF-SED; n = 7) and the other (OLETF-CR; n = 8) was fed 70% of that consumed by the OLETF-SED. The third OLETF group (OLETF-EX; n = 8) was kept in cages that contained voluntary running wheels. A Sigma Sport BC 606 bicycle computer (Cherry Creek Cyclery, Foster Falls, Virginia) was used to measure running activity. Nonhyperphagic, control strain, Long-Evans Tokushima Otsuka (LETO) rats were kept in the sedentary cage conditions for the duration of the study (LETO-SED; n = 8). All animals were individually caged throughout the entire experiment. Cages were in temperature-controlled animal quarters (21 °C) with a 0600–1800 light – 1800–0600 dark cycle. The OLETF-SED, OLETF-EX, and LETO-SED animals were provided standard rodent chow (Formulab 5008; Purina Mills, St. Louis, Missouri) for ad libitum feeding in new cages at the beginning of each week. Standard rodent chow also was provided to the OLETF-CR animals daily based on ~70% of the satiated OLETF-SED rat food, which is also the amount of chow consumed by the LETO control animals. Body mass was measured weekly throughout the investigation. OLETF-EX animals were given free access to voluntary running wheels for 24 h·day−1, and the daily running activity was obtained between 0800 and 1000 each morning. OLETF-EX and LETO-SED rats also had ad libitum access to food and water until the designated time of death. At 40 weeks of age, rats were anesthetized with pentobarbital sodium (100 mg·kg−1) and then exsanguinated by removal of the heart 5 h after locking of the wheels; sedentary rats (OLETF-SED, OLETF-CR, and LETO-SED) were killed at the same time. All animals were fasted for 5 h before death. Immediately postmortem, the liver from each animal was quickly sectioned and either put into formalin fixative or snap frozen in liquid nitrogen and stored for later analysis.
A portion of the animal characteristics and liver triglyceride data presented in the current report has been previous published (Rector et al. 2011).
Serum assays and fat pad mass
Epididymal fat pads were removed from exsanguinated animals and weighed. For serum analyses, venipuncture blood samples were collected from the lateral tail vein after a 12 h overnight fast and, in the case of the OLETF-EX rats, a 12 h wheel lock. This 12 h overnight fast occurred at 38–39 weeks of age. After centrifugation at 3000g at 4 °C, serum samples were stored at –80 °C until further analyses. Glucose and insulin measurements were performed by glucose oxidase kit (Thermo Electron, Louisville, Colorado) and ELISA (Milli-pore, Billerica, Massachusetts), respectively. Serum FGF-21 concentration was determined by a commercially available ELISA (R&D Systems, Minneapolis, Minnesota). Serum triglycerides (TGs) were measured by a commercially available kit (Sigma, St. Louis, Missouri).
Liver homogenization and Western blots
Liver was prepared as previously described for Western blot analyses (Rector et al. 2008). Western blots were performed to determine FGF-21 (Abcam, Cambridge, Massachusetts), FGF-21 receptor substrate 2 (FRS2) (R&D Systems, Minneapolis, Minnesota), phospho-FRS2 (Cell Signaling, Danvers, Massachusetts), and β-Klotho protein (Lifespan BioSciences, Seattle, Washington) content in the liver utilizing the procedure previously described (Rector et al. 2008). To control for equal protein loading and transfer, the membranes were stained with 0.1% amido-black (Sigma-Aldrich, St. Louis, Missouri) as previously described (Rector et al. 2008). The total protein staining for each lane was quantified, and these values were used to correct for any differences in protein loading or transfer of all band densities. β-Klotho mRNA, which is typically reported in murine studies, was not examined in the current report due to the unpublished gene sequence in rats.
Intrahepatic lipid content and histological staining
Intrahepatic TG content and hematoxylin and eosin (H&E) staining were determined as previously described by our group (Rector et al. 2008). For assessment of hepatic steatosis, histopathological criteria proposed by Kleiner et al. (2005) were adopted in five to six animals per group and three views per animal. Hepatic steatosis was graded as follows: <5%, score 0; 5%–33%, score 1; >33%–66%, score 2; or >66%, score 3.
FGF-21 and FGFR2 mRNA expression
FGF-21 (ABI) and FGF-21 receptor 2 (FGFR2; Sigma, St. Louis, Missouri) mRNA expression was quantified by RT-PCR using the ABI 7000 Sequence Detection System and software with commercially available primers. Liver samples were pulverized in RLT buffer using the Qiagen TissueLyser system with subsequent RNA isolation from the RNeasy kit, with the optional DNaseI step (Qiagen, Valencia, California). Purity was ensured and concentration was determined with a spectrophotometer. Reverse transcription was performed by combining RNA with the reverse transcription reaction mixture (Nuclease-Free Water, ImProm-II 5× Reaction Buffer, MgCl2, dNTP mix, and ImProm-II Reverse Transcriptase), and cDNA was synthesized. The reaction mixture (Nuclease-Free Water, FGF-21, FGFR2 primers and (or) probes, and either TAQman Master Mix (for FGF-21; Applied Biosystems, Carlsbad, California) or ABsolute Blue SYBR green (for FGFR2; Thermo Scientific, Waltham, Massachusetts)) was loaded onto a 96-well microplate, along with the cDNA sample (50 ng), and was placed into the ABI 7000 Sequence Detection System for polymerization. After polymerization, results were quantified using the DdCT method relative to 18S. Comparison of the differences in raw CT values did not differ (P = 0.4) among groups, indicating that 18S mRNA was an appropriate normalizer.
Statistics
Each outcome measure was examined in seven or eight animals per group, with the exception of steatosis score and serum TGs, which we examined in five or six animals per group. For each outcome measure, a one-way ANOVA was performed (SPSS, version 19.0; SPSS, Chicago, Illinois). Significant main effects (P < 0.05) were followed up with Fisher LSD post hoc comparisons. Pearson correlations also were performed to examine the potential relationships between hepatic and serum TGs with FGF-21. Values are reported as means ± standard errors (SE), and a P value of <0.05 denotes a statistically significant difference.
Results
Animal characteristics
Among the eight OLETF-EX rats, the average daily running distance throughout the 36-week intervention was 5.9 km·day−1 (range between 3.6 and 7.9 km·day−1), and the average during the last three weeks of the study was 3.6 km·day−1 (range between 2.5 and 5.9 km·day−1). By design, absolute food consumption of the OLETF-CR animals was ~70% of that of the OLETF-SED rats and equal to the amount consumed by LETO-SED animals, and ad libitum food consumption was similar between OLETF-SED and OLETF-EX animals. Caloric restriction and daily wheel running resulted in significantly lower body mass in the OLETF-CR and OLETF-EX rats compared with the OLETF-SED rats (70% of OLETF-SED values; P < 0.001), which were equal to LETO-SED values (Table 1). Voluntary running and caloric restriction suppressed epididymal fat pad mass compared with that of the OLETF-SED animals (P < 0.01) to the level of the LETO-SED animals (Table 1). As previously reported (Rector et al. 2011), OLETF-EX and OLETF-CR rats had significantly lower hepatic TG and steatosis scores compared with OLETF-SED rats (Table 1), and serum TG levels also were significantly elevated in the OLETF-SED animals compared with the other groups (P < 0.01; Table 1). In addition, fasting glucose was significantly elevated in OLETF-SED rats compared with the other groups, with LETO-SED and OLETF-EX concentrations also being significantly lower than OLETF-CR concentrations (Table 1). OLETF-EX and LETO-SED animals also exhibited significantly lower fasting serum insulin compared with OLETF-CR animals (Table 1), with differences between OLETF-SED and LETO-SED being due to the development of frank type 2 diabetes and a loss of normal pancreatic insulin secretion in the 40-week-old OLETF-SED rats, as we have previously reported (Rector et al. 2010).
Table 1.
Animal, serum, and liver characteristics.
| Variable | LETO-SED | OLETF-SED | OLETF-EX | OLETF-CR |
|---|---|---|---|---|
| Body mass (g) | 542.6±16.08a | 647.6±30.9b | 520.1±22.3a | 503.4±12.0a |
| Epididymal fat mass (g) | 10.86±0.77a | 16.75±2.05b | 6.24±0.94a | 8.16±0.83a |
| Heart mass/body mass (mg·g−1) | 2.54±0.06a | 2.42±0.12a | 3.19±0.11b | 2.62±0.06a |
| Serum TG (mg·dL−1) | 42.8±4.4a | 237.4±45.8b | 51.5±7.3a | 66.3±4.0a |
| Serum glucose (mg·dL−1) | 98.5±5.9a | 169.0±9.4b | 104.7±4.6a | 121.3±5.4c |
| Serum insulin (nmol·g−1) | 1.93±0.28a | 3.13±0.97a,b | 1.48±0.46a | 3.90±0.49c |
| Liver TG (nmol·g−1) | 1.3±0.2a | 6.2±0.9b | 1.5±0.1a | 2.3±0.3c |
| Steatosis score | 0.2±0.1a | 2.9±0.1b | 0.7±0.1a | 0.5±0.2a |
Note: Values are means ± standard error (SE); n = 7–8 and n = 5–6 for steatosis score and serum TG, respectively. Values followed by different letters are significantly different (p < 0.05). TG, triglycerides.
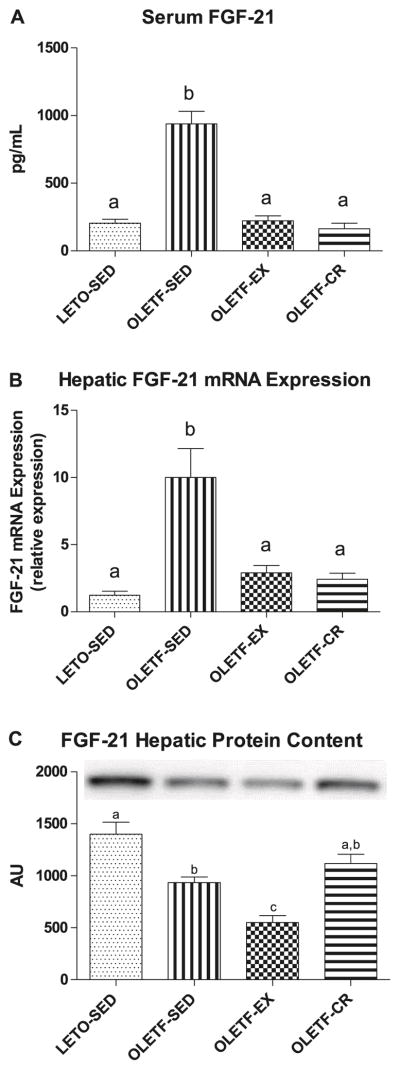
Serum and hepatic FGF-21
Hepatic mRNA expression of FGF-21 and serum FGF-21 levels were significantly (p = 0.001) higher in OLETF-SED rats than in LETO-SED, OLETF-EX, and OLETF-CR rats, with no differences among LETO-SED, OLETF-EX, or OLETF-CR animals (p = 1.000; Fig. 1). However, hepatic protein content of FGF-21 was significantly higher in LETO-SED rats than in OLETF-SED (p = 0.004) and OLETF-EX (P < 0.001; Fig. 1) rats and was significantly lower in OLETF-EX than in OLETF-SED (p = 0.022) and OLETF-CR (p = 0.001) rats.
Fig. 1.
The effects of daily exercise or caloric restriction on (A) FGF-21 serum concentration, (B) hepatic FGF-21 mRNA expression, and (C) hepatic FGF-21 protein content. Different letters above the bars indicate that the values (means ± standard error (SE); n = 6–8) are significantly different (P < 0.05). AU, arbitrary units.
FGF-21 signaling mediators
An examination of hepatic FGF-21 signaling mediators revealed a significant reduction in β-Klotho protein content in the OLETF-SED rats compared with the LETO-SED rats (p < 0.05; Fig. 2) but no differences between these two sedentary groups for FGF receptor 2 (FGFR2) mRNA or FGF receptor substrate (FRS2) mRNA expression (Fig. 2). However, daily exercise (trend, p = 0.1) and caloric restriction (p < 0.05) increased β-Klotho and FRS-2 protein content compared with OLETF-SED. In addition, OLETF-EX and OLETF-CR rats had significantly higher hepatic FGFR2 mRNA expression than the OLETF-SED rats (p < 0.05). Hepatic pFRS2 protein content was undetectable by Western blot analysis.
Fig. 2.

Effects of daily exercise or caloric restriction on (A) β-Klotho protein content, (B) FGFR2 mRNA expression, and (C) FRS2 protein content. Different letters above the bars indicate that the values (means ± standard error (SE); n = 6–8) are significantly different (P < 0.05). AU, arbitrary units.
Correlations
For correlation analyses, all four groups of animals were combined. As expected, body mass was significantly associated with hepatic FGF-21 mRNA and serum levels (r = 0.728, p < 0.001, and r = 0.455, p = 0.015, respectively; Fig. 3). Highlighting the relationship between a high lipid environment and elevated FGF-21, there were significant correlations between serum TGs vs. hepatic FGF-21 mRNA (r = 0.734, p < 0.001), serum TGs vs. serum FGF-21 (r = 0.907, p < 0.001), liver TGs vs. liver FGF-21 mRNA (r = 0.518, p = 0.005), and liver TGs vs. serum FGF-21 (r = 0.825, p < 0.001; Fig. 3). There also were significant correlations between hepatic FGF-21 mRNA expression and serum FGF-21 (r = 0.629, p = 0.001). Moreover, the hepatic steatosis score was significantly associated with FGF-21 hepatic mRNA expression (r = 0.681, p = 0.005) and serum FGF-21 levels (r = 0.752, p = 0.001). However, hepatic FGF-21 protein content was not significantly associated with any of the outcome variables (data not shown). In relation to FGF-21 signaling mediators, serum TGs were not related to β-Klotho, FRS2, or FGFR2 levels (data not shown), but liver TGs were negatively associated with liver β-Klotho protein content (r = –0.424, p = 0.019) but not FRS2 or FGFR2 (data not shown). Examination of daily running distance in the OLETF-EX rats throughout the 36-week intervention, in the final three weeks of the intervention, or after the last wheel running bout revealed no significant correlations with FGF-21 hepatic mRNA expression, serum FGF-21 levels, hepatic FGF-21 protein content, or any of the assessed signaling mediators (data not shown).
Fig. 3.
Correlations between (A and B) body mass, (C and D) serum triglycerides (TGs), and (E and F) liver TGs with hepatic FGF-21 mRNA and serum FGF-21. AU, arbitrary units.
Discussion
There has been intense recent investigation into the potential for FGF-21 as both a molecular target and therapeutic agent in the fight against obesity and obesity-associated chronic diseases. In the current investigation, hyperphagic, obese, sedentary OLETF rats exhibited elevated hepatic mRNA expression and circulating serum levels of FGF-21, which we demonstrate, for the first time, can be dramatically attenuated when daily exercise and caloric restriction are utilized to prevent obesity. In addition, here we report novel findings that daily-exercised and calorie-restricted OLETF rats had increased hepatic FGF-21 signaling mediators, including hepatic co-factor of FGF-21 (β-Klotho), hepatic FGF-21 receptor 2 (FGFR2), and hepatic FGF-21 receptor substrate 2 (FRS2) compared with sedentary OLETF rats.
Elevated hepatic FGF-21 mRNA expression and serum FGF-21 levels in the obese OLETF rats are in agreement with levels in previous reports (Lundåsen et al. 2007; Zhang et al. 2008; Mraz et al. 2009; Dushay et al. 2010; Fisher et al. 2010; Mai et al. 2010; Mashili et al. 2011). In addition, as hypothesized, obesity prevention through either daily exercise or caloric restriction both normalized hepatic FGF-21 mRNA expression and serum concentrations to the level of the lean, nonhyperphagic LETO control animals, highlighting the importance of obesity prevention on these variables. Furthermore, in the present study, hepatic TGs were significantly correlated with hepatic FGF-21 mRNA expression and FGF-21 serum concentration, but not the hepatic protein content of FGF-21. This is in agreement with a previous report in which liver fat was found to explain 23% of the variance in serum FGF-21 concentrations (Tyynismaa et al. 2011) and individuals with NAFLD had six times greater hepatic FGF-21 mRNA expression than normal-weight controls (Dushay et al. 2010). These findings collectively suggest that transcription of FGF-21 may be upregulated in the liver in response to hepatic lipid accumulation seen in the obese condition. However, it remains to be determined if the elevation in hepatic FGF-21 expression is in response to the hepatic accumulation of fat or perhaps other circulating factors such as FFAs.
FGFs are known to signal through four fibroblast growth factor receptors (FGFR1–FGFR4); however, although the liver contains all four receptors (Fisher et al. 2011), FGFR2 is considered to be the most highly expressed in hepatic tissue (Fisher et al. 2010). For FGF-21 to initiate signaling through the FGFR, FGF-21 also must bind to the co-receptor β-Klotho (Kurosu et al. 2007; Ogawa et al. 2007; Yie et al. 2009; Fisher et al. 2011), forming an FGFR–β-Klotho complex, which results in the phosphorylation of the FGF receptor substrate (pFRS2) and the activation of downstream signaling (Xu et al. 2009b). In the present study, we chose to measure β-Klotho, FGFR2, and FRS2/pFRS2 to assess differences in the content and (or) expression of FGF-21 signaling mediators between the four groups of rats. Contrary to previous reports in murine models of obesity (Fisher et al. 2010; Hale et al. 2012), we found that hepatic β-Klotho protein content was reduced in the obese OLETF-SED rats compared with the lean animals and that hepatic TGs were negatively associated with β-Klotho protein content, indicating the potential blunting in the initial step in the hepatic FGF-21 signaling cascade under conditions of fatty liver disease. We observed no differences in FGFR2 mRNA expression or FRS2 protein content between OLETF-SED and LETO-SED rats, with the FGFR2 findings being consistent with other lean vs. obese comparisons (Fisher et al. 2010); however, both daily exercise and caloric restriction resulted in elevated hepatic FGFR2 mRNA expression and FRS2 protein content. Unfortunately, pFRS2 was below the detectable limits by Western blot analysis. Collectively, these findings support the concept that the signaling machinery for FGF-21 is downregulated in the liver of an obese rodent model of NAFLD; however, it appears that obesity prevention with exercise and caloric restriction preserves and (or) upregulates the quantity of these signaling mediators, perhaps to enhance FGF-21 sensitivity in the liver. The notion of obesity prevention being a key component in the upregulation in these signaling mediators is further supported by the lack of correlation between amount of daily physical activity performed in the OLETF-EX rats and these signaling parameters.
It is difficult to pinpoint the exact contributions that hepatic FGF-21 mRNA expression has on circulating FGF-21 levels (Zhang et al. 2008; Berglund et al. 2010). This is especially challenging as multiple tissues, including liver (Dushay et al. 2010; Fisher et al. 2010), adipose (Fisher et al. 2010), pancreas (Johnson et al. 2009), and possibly muscle (Izumiya et al. 2008), express FGF-21 and could contribute to circulating levels. Because FGF-21 has been shown to act in both a paracrine and endocrine fashion (Fisher et al. 2011), FGF-21 may target both local hepatocytes or may contribute to the FGF-21 serum concentrations. In the current report, hepatic FGF-21 protein content was not associated with hepatic FGF-21 mRNA expression. With the observed elevations in hepatic FGF-21 mRNA expression but reduced hepatic FGF-21 protein levels and elevated circulating levels seen in the obese sedentary OLETF rats, perhaps under obese conditions, signals are being sensed by the liver to release FGF-21 into the circulation to target other metabolically active tissues such as adipose tissue or muscle. This is also indirectly supported by the observation that the LETO-SED animals exhibited the lowest hepatic mRNA expression and low circulating levels and yet had the highest hepatic protein content of FGF-21, suggesting an enhanced role for FGF-21 in hepatic metabolism and a reduced need for FGF-21 to function in an endocrine fashion. This is currently speculation, and the exact molecular signals potentially being sensed are unknown but warrant future investigation.
FGF-21 has been shown to have marked effects on carbohydrate and lipid metabolism (Inagaki et al. 2007; Potthoff et al. 2009; Xu et al. 2009b; Fisher et al. 2011). We have previously reported that exercise upregulated hepatic mitochondrial fatty acid β-oxidation in OLETF rats compared with sedentary animals (Rector et al. 2008). In addition, both exercise and caloric restriction prevents the development of NAFLD in OLETF rats (Rector et al. 2011). In the current report, we found that hepatic mRNA and protein expression of FGF-21 were significantly lower in the OLETF-EX rats than in the OLETF-SED animals. Intriguingly, these findings are opposed to previous reports in which overexpression of FGF-21 has been shown to induce increases in hepatic β-oxidation (Potthoff et al. 2009). Moreover, it has previously been reported that acute exhaustive exercise increases hepatic FGF-21 mRNA expression in nonobese mice (Berglund et al. 2010) and in mice fed a high fat diet (Berglund et al. 2011), both of which appear to be dependent on hepatic glucagon receptor signaling. However, although we did not observe elevated hepatic FGF-21 mRNA expression with chronic wheel running exercise compared with OLETF-SED rats, with the observed upregulation in the signaling mediators, there exists the potential for the ability of chronic exercise to act as an FGF-21 sensitizer, perhaps through a hepatic glucagon receptor signaling mediated pathway. Furthermore, with the observed differences in hepatic FGF-21 protein content between OLETF-EX and OLETF-CR, we can only speculate that perhaps less translation of the FGF-21 protein is needed after daily exercise to accomplish the same biological function compared with the CR animals. These discrepancies and the exact role of FGF-21 in regulating hepatic lipid metabolism in an obese model of fatty liver disease warrant further investigation.
In summary, elevated FGF-21 hepatic mRNA expression and serum concentrations were observed in hyperphagic, obese, sedentary OLETF rats compared with lean controls, and these elevations were associated with hepatic TG accumulation. In addition, daily exercise and caloric restriction modulated hepatic FGF-21 and its primary signaling mediators in the hyperphagic OLETF rat. Enhanced metabolic action of FGF-21 may partially explain the benefits of exercise and caloric restriction on NAFLD outcomes.
Acknowledgments
This work was supported by NIH grants HL-36088 (M.H.L.), T32 AR 048523-07 (J.A.F.), and VHA-CDA2 IK2BX001299-01 (R.S.R.). The OLETF and LETO rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors thank Suzie Ridenhour, Craig Meers, and Meghan Ruebel for excellent technical assistance to this work and Whitney Collins and Aaron Bunker for help with animal husbandry. This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, Missouri.
Contributor Information
Justin A. Fletcher, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65201, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65201, USA
Grace M. Meers, Department of Internal Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65201, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65201, USA
M. Harold Laughlin, Department of Biomedical Sciences, University of Missouri, Columbia, MO 65201, USA.
Jamal A. Ibdah, Department of Internal Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65201, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65201, USA
John P. Thyfault, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65201, USA; Department of Internal Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65201, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65201, USA
R. Scott Rector, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65201, USA; Department of Internal Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65201, USA; Harry S. Truman Memorial Veterans Medical Center, Columbia, MO 65201, USA.
References
- Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40(5):937–943. doi: 10.1016/S0735-1097(02)02051-X. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Kang L, Lee-Young RS, Hasenour CM, Lustig DG, Lynes SE, et al. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARα and FGF21 transcripts in vivo. Am J Physiol Endocrinol Metab. 2010;299(4):E607–E614. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berglund ED, Lustig DG, Baheza RA, Hasenour CM, Lee-Young RS, Donahue EP, et al. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes. 2011;60(11):2720–2729. doi: 10.2337/db11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152(8):2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153(1):69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582(27):3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137(5):1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Kharitonenkov A. Hormone-like fibroblast growth factors and metabolic regulation. Biochim Biophys Acta. 2011;1812(7):791–795. doi: 10.1016/j.bbadis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARα is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360(2):437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Mai K, Bobbert T, Groth C, Assmann A, Meinus S, Kraatz J, et al. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab. 2010;299(1):E126–E130. doi: 10.1152/ajpendo.00020.2010. [DOI] [PubMed] [Google Scholar]
- Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27(3):286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48(5):360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104(18):7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G874–G883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynismaa H, Raivio T, Hakkarainen A, Ortega-Alonso A, Lundbom N, Kaprio J, et al. Liver fat but not other adiposity measures influence circulating FGF21 levels in healthy young adult twins. J Clin Endocrinol Metab. 2011;96(2):E351–E355. doi: 10.1210/jc.2010-1326. [DOI] [PubMed] [Google Scholar]
- Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H, et al. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PLoS ONE. 2011;6(8):e22976. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand S, Keller KM, Robl M, L’Allemand D, Reinehr T, Widhalm K, Holl RW. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes. 2010;34(10):1468–1474. doi: 10.1038/ijo.2010.106. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009a;58(1):250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg RA, Veniant MM. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models — association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009b;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- Yie J, Hecht R, Patel J, Stevens J, Wang W, Hawkins N, et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 2009;583(1):19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]