Abstract
Unstable cytokeratins are associated with tumor transformation in the development of human hepatocellular carcinoma. We previously demonstrated that the cytokeratin 18 was modulated and that a histone H3-specific modification occured, among members of the histone family, during the development of human hepatocellular carcinoma. Evidence suggested that the modification of histone H3 was highly correlated with the modulation of cytokeratin 18 and probably plays an important role in tumorigenesis of hepatocytes. Aberrant expression of histone deacetylase leading to imbalance between acetylation and deacetylation of histones may exhibit regulatory roles in tumor transformation. Recently we found that overexpression of histone deacetylase-1 and hypoacetylation of histone H3 were associated with hepatocellular carcinoma. The underlying roles of histone H3 modulation are discussed in this mini-review article.
Keywords: Cytokeratin, hepatocellular carcinoma, histone H3, histone deacetylase 1, review
Hepatocellular carcinoma (HCC) is a cancer with high incidence and mortality in Taiwan. HCC cells exhibit different morphological features in divergent shapes and having pleomorphic nuclei from normal liver cells. Microtubules (MTs), intermediate filaments (IFs), and microfilaments (MFs) are major components of the cytoskeleton that are essential to maintain the integrity of eukaryotic cells (1). Proper cross-linking structures among these cytoskeletal proteins are crucial for intracellular architectures and normal cellular morphology. Plectin exhibits the binding sites accessible to IFs, MTs and MFs rendering the interaction with a variety of cytoskeletal components to maintain the integrity of the cytoskeletal network (2). The rope-like IFs have a mean diameter of 10 nm and mainly play a role in maintaining the shape and mechanical integrity of a cell (3). In human hepatocytes, a cytokeratin (CK) pair composed of CK8 (type II, 52 kD) and CK18 (type I, 45 kD) renders IFs (4). CKs are required for maintaining the integrity of hepatocytes (5). Altered expression of keratin genes were associated with chronic hepatitis, increased hepatocyte fragility and decreased bile secretion (6).
In eukaryotic nucleus, the organization and packaging of DNA are achieved by addition of histone proteins to form chromatin. Post-translational modifications of histones, such as acetylation, phosphorylation, methylation and ADP-ribosylation, frequently alter the structure of chromatin (7). Epigenetic modifications of histone, especially acetylation status, altering the chromatin structure are involved in gene expression and further intervened in the pathogenesis of cancer. Two types of enzymes, including histone acetyltransferases (HATs) and histone deacetylases (HDACs), affect the acetylation status of histones. Recently it was reported that alterations in the activities of HDACs, as well as aberrant acetylation of histone, lead to diseases including cancer (8). HDACs catalyze histone deacetylation and repress transcription of specific genes, such as p21, resulting in cell-cycle activation and cell proliferation (9).
The investigation of histones in our laboratory originated in a study of CK18, in which we found that CK18 was co-immunoprecipitated with histone H3 in human HCC, but not in normal liver. We speculated that both histone H3 and CK18 were modulated in HCC, which was shown in the co-immunoprecipitation of our experiments. Our opinion could be important for exploring the tumor transformation in the development of human HCC. However, by reviewing the literature to date, we found that the relation between histone modification and cytoskeleton modulation was not investigated. In this review, we display our series of studies proposing towards CK18-associated histone 3 modulations in HCC.
Disorganization of CK18 Triggered by Plectin Deficiency in Human HCC
We investigated the CK molecules of liver cells and asserted the modulation of CK18 during tumor transformation in HCC tissue and hepatoma cell lines in our previous studies (10,11). We also found that deficiency of plectin (a cross-linking protein in cytoskeleton) in liver cells triggered the modulation of CK18. The disorganization of CK18 and altered levels of CK18 expression is associated with plectin knock-down (12,13) and degradation (14,15) in liver cells. In consequence, liver cells showed cytoskeletal augmentation and pleomorphic changes. Furthermore, we discovered plectin-deficient human hepatic cells exhibited higher cell motility in association with increased focal adhesion kinase activity that was comparable to the properties of invasive HCC (16). Recently, we reported that higher cell motility and collective cell migration are associated with deficiency in plectin and increased expression of E-cadherin in hepatoma cells (17). Plectin may be involved in the regulation of cell motility that is related to the invasiveness of HCC. We also suggest that hepatoma cells with plectin deficiency and high level of E-cadherin expression are more sensitive to sorafenib treatment (17).
Histone H3 Co-immunoprecipitated with CK18 in Human HCC
In addition to the modulation of CK18, a group of proteins with a molecular weight range between 12.4-18.4 kDa (mainly 14 kDa) were also found in HCC. These low molecular-weight proteins were designated as HCC CK in our previous reports due to their specific association with CK18 in immunoprecipitation experiments (10,11). It was, at first, thought that HCC CK was derived from CK18 in HCC. However, the following evidence demonstrating different origins of HCC CK and CK18 were found in our studies. Firstly, use of an anti-CK18 antibody failed to detect HCC CK on western blot assays; in contrast, HCC CK was detected by the use of an anti-histone antibody, indicating discrepancy in antigenicity. Secondly, N-terminal sequence mapping of HCC CK revealed high homology to that of histone rather than CK18. However, not all histones were found in the HCC CK fraction isolated from human HCC. Western blotting assay only detects histone H3 in HCC while the signals of H1, H2A, H2B, and H4 were not present (18).
Therefore, we proposed a hypothesis that the structure of chromosomes and the organization of nuclei were unstable and more fragile in HCC, leading to histone proteins accessible to extraction procedures, and in consequence the histone H3 was co-immunoprecipitated with CK18. The hypothesis was verified by series of experiments using anti-CK18 and anti-histone H3 antibody for immunoprecipitation in human liver and HCC tissues, respectively. The data suggested that histone H3 and CK18 was co-immunoprecipitated and simultaneously exist in HCC cells, while this phenomenon was not observed in normal liver cells (19). It is widely accepted that expression of CK18 affects nuclear organization then further impacts the integrity and stability of HCC cell nuclei. Reorganization of cytoskeleton perturbing the function of nuclear matrix was the suggested mechanism since Barboro et al. reported that dramatic changes in the expression of both the nuclear matrix and IF proteins occur during transformation of rat hepatocyte nodules (20). Our latest study also showed that nuclear pleomorphism of hepatoma cells is correlated with the cytoplasmic disorganization of cytoskeleton caused by plectin deficiency; irregular nuclear shape resembled pleomorphic nuclei in cancer cells were observed in the plectin-knockdown liver cells (21).
Hypoacetylation of Histone H3 in Human HCC
The roles of histone modification, especially acetylation and deacetylation, in the development of cancer have been recently investigated. HDACs exhibit important roles in histone modification and chromatin remodeling. Deacetylation of histones activates gene expression involved in the molecular pathway of HCC tumorigenesis was reported by Buurman et al. (22). Abnormal expression of HDACs causes aberrant level of histone acetylation and in consequence may lead to tumor transformation in human HCC. Our evidence showed that aberrant acetylation was associated with the modulation of histone H3 in human HCC. We found in human HCC tissues, the level of histone H3 and HDAC1 was increased whereas the content of acetylated histone H3 (AH3) was reduced in comparison to those in normal liver tissues (23). Therefore, we suggested that hypoacetylated modification of histone H3 is derived from enhanced expression of HDAC1 in human HCC. In a separate report by Farooq et al., altered expression of HDAC1 in HCC was found to regulate the critical factors related to the processes of liver carcinogenesis (24). In our studies, data supported that hypoacetylation of histone H3 accompanied with enhanced expression of HDAC1 was involved in the development of human HCC (23). The mechanism of HDACs involved in tumor transformation of HCC is still unclear. Aberrant regulation of cell cycle and apoptosis were the proposed mechanisms. Buurman et al. found that in liver cells, microRNA-449 specifically binds to c-MET mRNA, can promote apoptosis and inactivates cell proliferation. It has been found that enhanced expression of HDAC1-3 was associated with reduced level of microRNA-449. Consequently, proliferation-inhibiting effect mediated by microRNA-449 was suppressed (22). Sun et al. also reported that microRNA-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Low microRNA-34a expression and high HDAC1 expression in HCC are closely related to the occurrence and development of HCC (25). The correlation of the HDAC expression with tumor progression was reported but the completion was still controversial. Rikimaru et al. advocated that in human HCC, increased expression of HDAC1 was associated with higher invasiveness, lower differentiation, and poor prognosis (26). In contrast, Zhao et al. claimed that expression of HDAC1 was significantly higher in HCC tissues than that in adjacent tissues, but there was no statistical difference between HDAC1 with tumor stage (27).
Applications of HDAC Inhibitor for the Therapy of HCC
Recently, HDAC inhibitors are being applied under the rationale of cancer therapy. Many investigations applying HDAC inhibitors for treating HCC are undergoing and many articles have been published worldwide. A novel hydroxamic acid-derived HDAC inhibitor, LBH589, suppresses proliferation and metastasis of HCC by mediating inhibitory effect on gankyrin/stat3/akt pathway (28). The pan-deacetylase inhibitor, panobinostat, affects angiogenesis in HCC models by regulating connective tissue growth factor expression (29). Vaproic acid exhibits HDAC4-inhibitory activity arresting HCC cell growth via increasing acetylation of histone H4 and suppressing notch signaling (30). Droxinostat, a HDAC inhibitor, was found to suppress HDAC3 expression and induce acetylation of histones H3 and H4. This compound can inhibit cell proliferation and induce apoptosis in HCC cell lines by activating mitochondrial apoptotic pathways (31). A novel histone deacetylase inhibitor, MPT0G009 was reported to be a potential new candidate drug for HCC therapy. The research team found that MPT0G009 can induce cell apoptosis and synergistic anticancer activity with tumor necrosis factor-related apoptosis-inducing ligand against HCC (32). Considering the immunotherapy, suberoylanilide hydroxamic acid (SAHA, a HDAC inhibitor) regulates the expression of microRNA-17-92 cluster then epigenetically elevates the level of major histocompatibility complex class I-related chain molecules A (MICA) and maintains complex component 7 (MCM7) in hepatoma. The outcome is to enhance the sensitivity of HCC to natural killer cell-mediated lysis (33). Combined pharmaceutical treatment has also become a popular issue in cancer therapy. Sorafenib inhibits SAHA-induced NF-ĸB activity that regulates the expression of oncogenic proteins. Combined with sorafenib, SAHA exhibits enhanced therapeutic efficacy against HCC in vitro and in vivo through the mechanism suppressing ERK/NF-ĸB signaling pathway (34). A phase-I dose-escalation study of the mTOR inhibitor sirolimus and the HDAC inhibitor vorinostat in patients with advanced malignancy was reported. The authors reported that this therapy is a safe and efficacious for several cancer types including HCC (35). This evidence supports that HDAC inhibitors reveal emerging applications for treating advanced HCC.
Conclusion
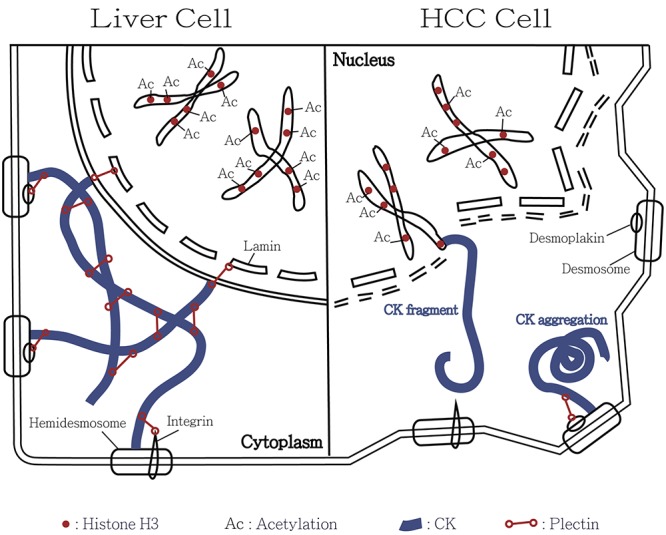
In human HCC, our investigational data suggested discrepancy in immunospecificity of CK18 between HCC and normal liver tissues. We further specified the human HCC-associated changes in histone H3. A great amount of evidence indicates that expression of histone H3 was highly related to the modulation of CK18 and likely plays an important role in tumorigenesis of human HCC. Furthermore, we confirmed that CK18 modulation and nuclear pleomorphism in HCC was correlated with the plectin deficiency. Our studies verified the previous observation that overexpression of HDAC1 in HCC catalyzes histone deacetylation and results in hypo-acetylated histone H3. Therefore, activation of HDAC1 is likely involved in tumor transformation of human HCC. In addition to surgical resection and trans-arterial chemoembolization, application of HDAC inhibitors provides an option for the treatment of human HCC. Figure 1 summarizes our concept regarding the CK18-associated histone 3 modulation involved in the tumor transformation of human HCC.
Figure 1. Illustrative model for the involvement of cytokeratin-associated histone 3 modulation in the tumor transformation of hepatocellular carcinoma (HCC). In normal liver cells, cytokeratin is maintained by the cytokeratin-connected plectin. The filamentous meshwork attaches to the lamin, integrin and desmoplakin forming stable cell architecture and nuclear membrane. Normally acetylation of histone H3 is under equilibrium. Size and shape of liver cells are consistent. However, deficiency in plectin and cytokeratin meshwork results in collapse of HCC cells. In consequence, the cytokeratin filaments may aggregate or become short fragments. Overexpression of histone deacetylase-1 results in hypoacetylation of histone H3 in HCC. It leads to unstable cell membrane, nuclear envelope and chromosome and ultimately to cell transformation. We hypothesize that some cytokeratin fragments connect to histone H3 in HCC cells. This model explains co-immunoprecipitation of histone H3 and CK18 in human HCC.
Acknowledgements
The Authors greatly appreciate Miss Chen, You-Yin and Miss Cheng, Jia-Min for their skillful laboratory assistance.
References
- 1.Huber F, Boire A, Lopez MP, Koenderink GH. Cytoskeletal crosstalk: when three different personalities team up. Curr Opin Cell Biol. 2015;32:39–47. doi: 10.1016/j.ceb.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Castanon MJ, Walko G, Winter L, Wiche G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol. 2013;140:33–53. doi: 10.1007/s00418-013-1102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman RD, Cleland MM, Murthy SN, Mahammad S, Kuczmarski ER. Inroads into the structure and function of intermediate filament networks. J Struct Biol. 2012;177:14–23. doi: 10.1016/j.jsb.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omary MB, Ku NO, Toivola DM. Keratins: guardians of the liver. Hepatology. 2002;35:251–257. doi: 10.1053/jhep.2002.31165. [DOI] [PubMed] [Google Scholar]
- 5.Loranger A, Duclos S, Grenier A, Price J, Wilson-Heiner M, Baribault H, Marceau N. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pathol. 1997;151:1673–1683. [PMC free article] [PubMed] [Google Scholar]
- 6.Omary MB, Ku NO. Intermediate filament proteins of the liver: emerging disease association and functions. Hepatology. 1997;25:1043–1048. doi: 10.1002/hep.510250537. [DOI] [PubMed] [Google Scholar]
- 7.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 8.Timmermann S, Lehrmann H, Polesskaya A, Harel-Bellan A. Histone acetylation and disease. Cell Mol Life Sci. 2001;58:728–736. doi: 10.1007/PL00000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archer SY, Hodin RA. Histone acetylation and cancer. Curr Opin Genet Dev. 1999;9:171–174. doi: 10.1016/s0959-437x(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 10.Su B, Pei RJ, Yeh KT, Hsu YH, Lai YS. Could the cytokeratin molecule be modulated during tumor transformation in hepatocellular carcinoma. Pathobiology. 1994;62:155–159. doi: 10.1159/000163897. [DOI] [PubMed] [Google Scholar]
- 11.Liu YH, Pei RJ, Yeh CC, Lee KY, Yeh KT, Hsu YH, Ho CC, Lai YS. The alteration of cytokeratin 18 molecule and its mRNA expression during tumor transformation in hepatoma. Res Commun Mol Pathol Pharmacol. 1997;96:243–253. [PubMed] [Google Scholar]
- 12.Cheng CC, Liu YH, Ho CC, Chao WT, Pei RJ, Hsu YH, Yeh KT, Ho LC, Tsai MC, Lai YS. The influence of plectin deficiency on stability of cytokeratin18 in hepatocellular carcinoma. J Mol Histol. 2008;39:209–216. doi: 10.1007/s10735-007-9155-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu YH, Cheng CC, Ho CC, Chao WT, Pei RJ, Hsu YH, Ho LC, Shiu BH, Lai YS. Plectin deficiency on cytoskeletal disorganization and transformation of human liver cells in vitro. Med Mol Morphol. 2011;44:21–26. doi: 10.1007/s00795-010-0499-y. [DOI] [PubMed] [Google Scholar]
- 14.Liu YH, Cheng CC, Ho CC, Chao WT, Pei RJ, Hsu YH, Yeh KT, Ho LC, Tsai MC, Lai YS. Degradation of plectin with modulation of cytokeratin 18 in human liver cells during staurosporine-induced apoptosis. In Vivo. 2008;22:543–548. [PubMed] [Google Scholar]
- 15.Liu YH, Ho CC, Cheng CC, Chao WT, Pei RJ, Hsu YH, Lai YS. Cytokeratin 18-mediated disorganization of intermediate filaments is induced by degradation of plectin in human liver cells. Biochem Biophys Res Commun. 2011;407:575–580. doi: 10.1016/j.bbrc.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CC, Lai YC, Lai YS, Hsu YH, Chao WT, Sia KC, Tseng YH, Liu YH. Transient knockdown-mediated deficiency in plectin alters hepatocellular motility in association with activated FAK and Rac1-GTPase. Cancer Cell Int. 2015;15:29–35. doi: 10.1186/s12935-015-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng CC, Chao WT, Liao CC, Tseng YH, Lai YC, Lai YS, Hsu YH, Liu YH. Plectin deficiency in liver cancer cells promotes cell migration and sensitivity to sorafenib treatment. Cell Adh Migr. 2017 doi: 10.1080/19336918.2017.1288789. doi: 10.1080/19336918.2017.1288789.[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YH, Pei RJ, Cheng CC, Ho HC, Ho CC, Yeh KT, Lee KY, Lai YS. HCC CKs are altered histones during tumor transformation in hepatoma. Res Commun Mol Pathol Pharmacol. 2002;112:27–38. [PubMed] [Google Scholar]
- 19.Ho CC, Cheng CC, Liu YH, Pei RJ, Hsu YH, Yeh KT, Ho LC, Tsai MC, Lai YS. Possible relation between histone 3 and cytokeratin 18 in human hepatocellular carcinoma. In Vivo. 2008;22:457–462. [PubMed] [Google Scholar]
- 20.Barboro P, Alberti I, Sanna P, Parodi S, Balbi C, Allera C, Patrone E. Changes in the cytoskeletal and nuclear matrix proteins in rat hepatocyte neoplastic nodules in their relation to the process of transformation. Exp Cell Res. 1996;225:315–327. doi: 10.1006/excr.1996.0182. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CC, Lai YC, Lai YS, Chao WT, Tseng YH, Hsu YH, Chen YY, Liu YH. Cell Pleomorphism and Cytoskeleton Disorganization in Human Liver Cancer. In Vivo. 2016;30:549–555. [PubMed] [Google Scholar]
- 22.Buurman R, Gurlevik E, Schaffer V, Eilers M, Sandbothe M, Kreipe H, Wilkens L, Schlegelberger B, Kuhnel F, Skawran B. Histone deacetylases activate hepatocyte growth factor signaling by repressing microRNA-449 in hepatocellular carcinoma cells. Gastroenterology. 2012;143:811–820. doi: 10.1053/j.gastro.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Cheng CC, Liu YH, Lai YC, Hsu YH, Chao WT, Lai YS. Hypoacetylation in association with histone 3 modulation in human hepatocellular carcinoma. In Vivo. 2015;29:237–242. [PubMed] [Google Scholar]
- 24.Farooq M, Hozzein WN, Elsayed EA, Taha NA, Wadaan MA. Identification of histone deacetylase 1 protein complexes in liver cancer cells. Asian Pac J Cancer Prev. 2013;14:915–921. doi: 10.7314/apjcp.2013.14.2.915. [DOI] [PubMed] [Google Scholar]
- 25.Sun TY, Xie HJ, Li Z, Kong LF, Gou XN, Li DJ, Shi YJ, Ding YZ. miR-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Am J Transl Res. 2017;9:103–114. [PMC free article] [PubMed] [Google Scholar]
- 26.Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, Maehara Y. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology. 2007;72:69–74. doi: 10.1159/000111106. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, He D, Jiao M, Kong L, Shao C, Chen J, Fang Z, Ma X, Chen H, Li L, Luo S, Zheng N, Chen Y, Wang Q, Fang S. Overexpression of histone deacetylase and amyloid precursor protein in hepatocellular carcinoma. Technol Cancer Res Treat. 2016 doi: 10.1177/1533034616661664. pii: 1533034616661664. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Wang J, Zheng T, Song R, Liang Y, Bhatta N, Yin D, Pan S, Liu J, Jiang H, Liu L. LBH589 Inhibits proliferation and metastasis of hepatocellular carcinoma via inhibition of gankyrin/STAT3/Akt pathway. Mol Cancer. 2013;12:114–126. doi: 10.1186/1476-4598-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gahr S, Mayr C, Kiesslich T, Illig R, Neureiter D, Alinger B, Ganslmayer M, Wissniowski T, Fazio PD, Montalbano R, Ficker JH, Ocker M, Quint K. The pan-deacetylase inhibitor panobinostat affects angiogenesis in hepatocellular carcinoma models via modulation of CTGF expression. Int J Oncol. 2015;47:963–970. doi: 10.3892/ijo.2015.3087. [DOI] [PubMed] [Google Scholar]
- 30.Sun G, Mackey LV, Coy DH, Yu CY, Sun L. The Histone Deacetylase Inhibitor Vaproic Acid Induces Cell Growth Arrest in Hepatocellular Carcinoma Cells via Suppressing Notch Signaling. J Cancer. 2015;6:996–1004. doi: 10.7150/jca.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Li G, Wang X, Wang L, Zhao R, Wang J, Kong Y, Ding J, Li J, Zhang L. Droxinostat, a Histone Deacetylase Inhibitor, Induces Apoptosis in Hepatocellular Carcinoma Cell Lines via Activation of the Mitochondrial Pathway and Downregulation of FLIP. Transl Oncol. 2016;9:70–78. doi: 10.1016/j.tranon.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen MC, Huang HH, Lai CY, Lin YJ, Liou JP, Lai MJ, Li YH, Teng CM, Yang CR. Novel histone deacetylase inhibitor MPT0G009 induces cell apoptosis and synergistic anticancer activity with tumor necrosis factor-related apoptosis-inducing ligand against human hepatocellular carcinoma. Oncotarget. 2016;7:402–417. doi: 10.18632/oncotarget.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, Tian Z, Zhang C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer. 2015;112:112–121. doi: 10.1038/bjc.2014.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu FT, Liu YC, Chiang IT, Liu RS, Wang HE, Lin WJ, Hwang JJ. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappaB signaling. Int J Oncol. 2014;45:177–188. doi: 10.3892/ijo.2014.2423. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Garrido-Laguna I, Naing A, Fu S, Falchook GS, Piha-Paul SA, Wheler JJ, Hong DS, Tsimberidou AM, Subbiah V, Zinner RG, Kaseb AO, Patel S, Fanale MA, Velez-Bravo VM, Meric-Bernstam F, Kurzrock R, Janku F. Phase I dose-escalation study of the mTOR inhibitor sirolimus and the HDAC inhibitor vorinostat in patients with advanced malignancy. Oncotarget. 2016;7:67521–67531. doi: 10.18632/oncotarget.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]


