Abstract
Protein synthesis is a key process in all living organisms. In eukaryotes, Initiation Factor 2 (eIF2) plays an important role in translation initiation as it selects and delivers the initiator tRNA to the small ribosomal subunit. Under stress conditions, phosphorylation of the α-subunit of eIF2 downregulates cellular protein synthesis. However, translation of certain mRNAs continues via the eIF2D dependent non-canonical initiation pathway. The molecular mechanism of this process remains elusive. In addition, eIF2D plays a role in translation re-initiation and ribosome recycling. Currently, there has been no structural information of eIF2D. We have now determined the crystal structure of the C-terminal domains of eIF2D at 1.4 Å resolution. One domain has the fold similar to that of eIF1, which is crucial for the scanning and initiation codon selection. The second domain has a known SWIB/MDM2 fold, which was not observed before in other translation initiation factors. Our structure reveals atomic details of inter-domain interactions in the C-terminal part of eIF2D and sheds light on the possible role of these domains in eIF2D during translation.
Keywords: Re-initiation, eIF2, Ribosome, Initiation of protein synthesis
Graphical Abstract
Translation of mRNAs into proteins is a fundamental process that is critical to all life forms and is carried out by the ribosome. Though the general architecture of the ribosome and the mechanism of translation are remarkably similar in both the eukaryotes and the prokaryotes, the mechanisms of translation initiation vary; eukaryotic initiation is much more complex than the prokaryotic initiation. Consequently, many human diseases are associated with dysfunctional proteins involved in initiation [1–3]. Prokaryotic initiation involves the recognition of the Shine-Dalgarno sequence in the mRNA by a complementary sequence in the 16S rRNA, which places the start codon in the Psite of the 30S subunit in a process that is facilitated by IF3. This is followed by IF2-promoted binding of the initiator tRNA to the start codon and the association of the 50S subunit with the 30S subunit [4]. In the canonical eukaryotic initiation pathway, the 43S pre-initiation complex (43S PIC), comprised of the 40S ribosomal subunit, initiation factors eIF1, eIF1A, eIF3, eIF2, eIF5, and the methionine initiator tRNA, associates with the mRNA via the eIF4F complex bound to the 5′ cap and scans the mRNA to locate the start codon [5–7]. The initiation factor eIF2, which is comprised of α, β and γ subunits, forms a 1ternary complex with the Met-initiator tRNA and GTP and is critical in delivering the initiator tRNA to the ribosome. The recognition of the start codon by the initiator tRNA results in the formation of the 48S PIC followed by GTP hydrolysis. The concomitant release of phosphate, promoted by dissociation of eIF1, results in the release of initiation factors and the association of the 60S subunit assisted by eIF5B [6]. In certain viruses, translation initiation occurs through an internal ribosome entry site (IRES) located on the viral RNA and requires IRES-specific initiation factors [8].
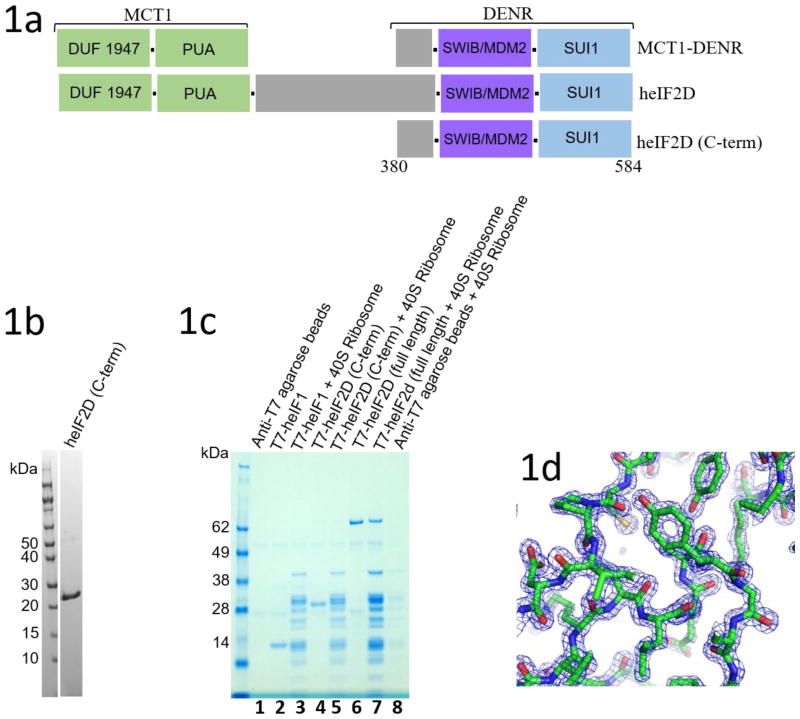
Under conditions of stress, the alpha subunit of eIF2 gets phosphorylated, resulting in shutdown of the canonical initiation pathway. However, certain mRNAs can initiate translation via non-canonical mechanisms. One such mechanism is mediated by the density regulated protein and multiple copies in T-cell lymphoma-1 (DENR-MCT-1) protein complex or eIF2D (Ligatin in Ref 9). The molecular mechanism of non-canonical (eIF2D- or DENR-MCT-1 - dependent) translation initiation is not well understood [9–11]. eIF2D forms a complex with mRNA, tRNA, and the 40S ribosomal subunit and has been shown to stabilize both initiator and non-initiator tRNA with or without the aminoacyl moiety [9, 10]. It has homologs with similar domain organization in almost all eukaryotes [9, 10]. One of the N-terminal domains is a predicted RNA binding domain (PUA domain) and the other is of unknown functions. Together, they are homologous to MCT-1 (Figure 1a). The C-terminal SUI1 domain is homologous to the translation initiation factor eIF1, which plays a vital role in scanning and the selection of initiation codon [6, 7]. Together with the SWIB domain, this part of eIF2D is homologous to DENR (Figure 1a). DENR forms a heterodimer with MCT-1 that predictably functions similarly to eIF2D in most biochemical assays [9, 10]. In addition, the DENR-MCT-1 complex and eIF2D have been implicated in translation re-initiation on mRNAs when the AUG is directly placed in the P-site of the 40S ribosomal subunit and in ribosome recycling [9, 12, 13]. Though the structure of MCT-1 alone has previously been determined by X-ray crystallography [14], no structural information is available about DENR and eIF2D, and about their interaction with the ribosome. In this study, we determined the crystal structure of the C-terminal domains of human eIF2D, heIF2D (C-term) at 1.4 Å resolution.
Figure 1.
(a) Domain architecture of eIF2D, MCT1 and DENR proteins. The C-terminal domain of human eIF2D, heIF2D (C-term), was analyzed in this work. (b) SDS-PAGE gel of purified heIF2D (C-term). (c) T7 tagged heIF1, heIF2D (C-term) and heIF2D (full length) bind to eukaryotic 40S ribosome. Lanes 1–8 have anti-T7 tag antibody immobilized on agarose beads. (d) Representative electron density map (2Fo-Fc) corresponding to a section of the built model at 2.0 σ contour level. Figure 1(e) was made using Pymol [25].
The C-terminal region of heIF2D (heIF2D (C-term)) comprised of residues D380 to K584, that include the SWIB/MDM2 and SUI1 domains, was crystallized and the structure was determined using single-wavelength anomalous diffraction (SAD). DNA fragment with the codon optimized sequence of heIF2D (C-term) corresponding to amino acid residues 380–584 was cloned in to pET28a+ vector between NdeI and XhoI sites, with an additional TEV-protease cleavage on the N-terminus. The protein was expressed in BL21 DE3 Star cells in LB media for 20 hours at 18° C after induction using 250 μM IPTG. The cells were centrifuged and lysed in MF buffer (500 mM NaCl, 50 mM HEPES pH 7.0, 5% glycerol, 20 mM imidazole, 2 mM β-ME), followed by affinity purification with Ni-NTA beads. The protein was eluted in the same buffer with 250 mM imidazole followed by overnight incubation with 6XHis-tagged TEV protease at 4° C while dialyzing against MF buffer with 20 mM imidazole. TEV protease and the cleaved His-tag were removed using Ni-NTA beads and the untagged eIF2D (C-term) was purified on Superdex75 column (GE Healthcare) equilibrated in the buffer A (100 mM KCl, 10 mM HEPES pH 7.0, 2mM β-ME). The protein was concentrated to 20 mg/ml and aliquots were flash frozen in liquid nitrogen for storage at −80° C. Seleno-Methione (Se-Met) protein was expressed in B834(DE3) cells (Novagen) in minimal media supplemented by Se-Met just before induction with IPTG. The growth and purification protocol was similar to the native protein. The protein was concentrated to 20 mg/ml and 2 μl of native protein was mixed with 2 μl of the reservoir buffer (3.5 M Sodium Formate, 100 mM Sodium acetate pH 4.5) and crystallized at 20° C by sitting drop vapor diffusion method within a week, while the Seleno-Methione (Se-Met) protein crystallized under similar condition at pH 4.0. 25% glycerol was used as cryoprotectant and the diffraction data was collected at NECAT sector 24 beamlines at Advanced Photon Source, Argonne, IL. The native crystal diffracted to 1.4 Å resolution, while the Se-Met crystal diffracted to 1.8 Å resolution. The native data was scaled using HKL-2000 and the Se-Met data using SHELX. The structure was determined using phases from the SAD data and the structure obtained was used as a model for molecular replacement for the native dataset using Phaser in the CCP4 software. Models were built in COOT and refined using Phenix and Refmac5. To express T7-tagged proteins, DNA coding regions were cloned in a pET28a vector using the BamHI and HindIII restriction sites. T7-eIF2D and T7-eIF2D (C-term) were purified as described above. The pull down assay was performed thrice using a protocol described previously [26].
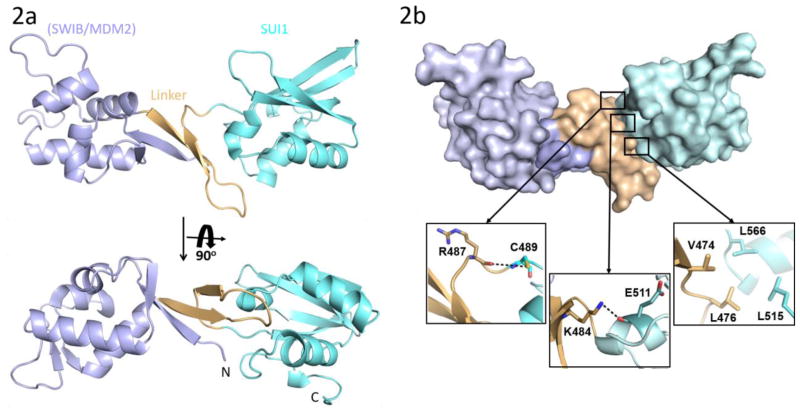
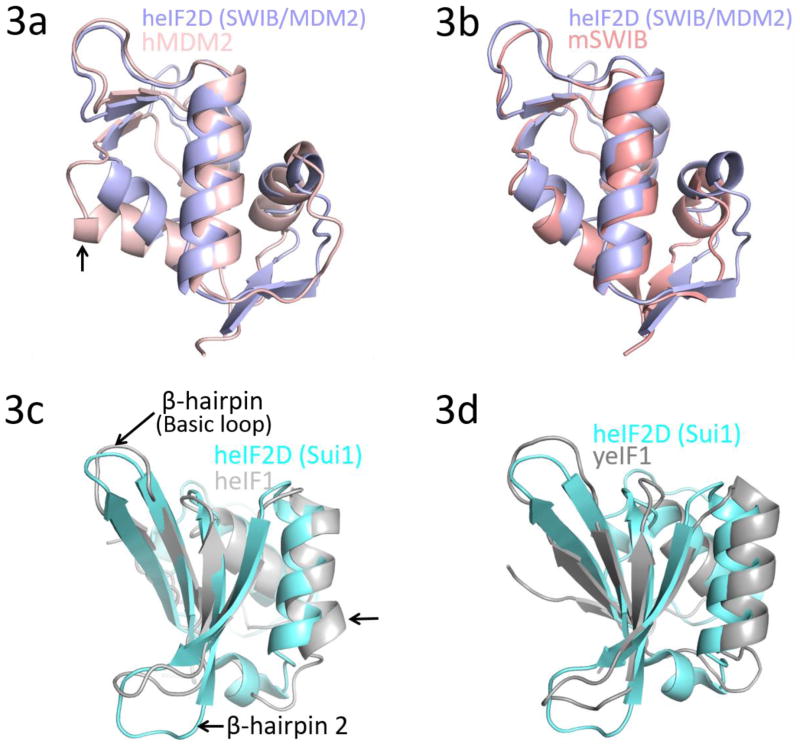
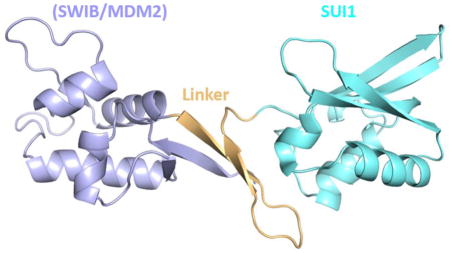
Based on the secondary structure prediction and predicted disordered regions, a number of truncated versions of heIF2D were purified and tested for crystallization [15]. The C-terminal region of heIF2D (heIF2D (C-term)), comprised of residues D380 to K584, that include the SWIB/MDM2 and SUI1 domains (Figure 1a), was crystallized, and the structure was determined using single-wavelength anomalous diffraction (SAD) [16–19] (Figures 1b, d,, 2a, b Table 1). The structure reveals that the SUI1 and SWIB/MDM2 domains are connected by a structured linker (Figure 2a). The SWIB/MDM2 domain is similar to the human MDM2 (Mouse Double Minute 2 homolog) and the SWIB domain from the mouse BAF60a protein, with an rmsd of 1.8 Å (Figure 3a, b). Most of the differences are observed in the loops, while the β-strands and the α-helices align well except for the α4 helix, which is 6.7 Å closer to the protein core in eIF2D compared to its position in MDM2 [20] (Figure 3a). The α4 helix of MDM2, along with the α2 helix, forms a binding pocket for the transactivation domain of the tumor suppressor protein p53. This interaction mainly involves hydrophobic residues and downregulates the transcription activity of p53 [20, 21]. Though the 6.7 Å shift of the α4 helix in eIF2D and amino acid substitutions modify the binding pocket, the MDM2 domain of eIF2D may function as a potential binding site for other eIFs or regulatory proteins. The superposition of SUI1 domain of eIF2D on the NMR structures of the human eIF1 (heIF1) and yeast eIF1 (yeIF1) has an rmsd of 2.2 Å [22, 23] (Figure 3c, d). The β-strands align well and the α-helices are shifted, but most of the differences are in the loops; the α2 helix of eIF2D SUI1 domain is 3 Å closer to the β4 strand when compared to heIF1 (Figure 3c). The Basic loop 498RASNKK503 (β-hairpin 1) adopts a similar conformation as in eIF1, while the β-hairpin 2 has a different orientation. Since the general architecture of the SUI1 domain of eIF2D is similar to that of eIF1, it might have similar interactions with the ribosome as eIF1. To demonstrate that eIF2D SUI1 domain binds 40S ribosomal subunit we performed a pull-down assay using T7-tagged proteins and anti-T7 antibody immobilized to agarose beads. The heIF2D (C-term) pulls down eukaryotic 40S ribosome similar to heIF2D (full length) and heIF1 (Figure 1c). Thus, the structure of the SUI1 domain presented here would likely be similar in the context of the full length protein, and the eIF2D SUI1 domain might occupy the same position on the 40S ribosomal subunit as eIF1.
Figure 2.
(a) Crystal structure of heIF2D (C-term). The SWIB/MDM2 (blue), linker (orange) and SUI1 (cyan) domains are color coded similar to Figure 1(a). (b) Space filling representation of heIF2D (C-term) showing the extensive surface contacts between the linker and the SUI1, SWIB/MDM2 domains. The hydrophobic and hydrogen bonding interactions between the Linker and the SUI1 domain are shown in the inserts. Pymol was used to calculate the surface area and to make the figure.
Table 1.
Crystallography Table
| Native | SeMet | |
|---|---|---|
| Wavelength | ||
| Resolution range | 48.83 - 1.4 (1.45 - 1.4) | 48.77 - 1.64 (1.699 - 1.64) |
| Space group | P 43 21 2 | P 43 21 2 |
| Unit cell | 50.65 50.65 183.653 90 90 90 | 50.588 50.588 183.449 90 90 90 |
| Total reflections | 198619 (16937) | 421799 (41432) |
| Unique reflections | 47737 (4637) | 30285 (2927) |
| Multiplicity | 4.2 (3.7) | 13.9 (14.0) |
| Completeness (%) | 98.74 (97.72) | 99.84 (99.15) |
| Mean I/sigma(I) | 14.72 (1.18) | 16.56 (0.95) |
| Wilson B-factor | 22.42 | 16.93 |
| R-merge | 0.04245 (0.8449) | 0.0886 (2.137) |
| R-meas | 0.04851 (0.98) | 0.09203 (2.217) |
| R-pim | 0.02293 (0.4868) | 0.02452 (0.5817) |
| CC1/2 | 0.999 (0.626) | 0.999 (0.452) |
| CC* | 1 (0.878) | 1 (0.789) |
| Reflections used in refinement | 47732 (4637) | 30260 (2927) |
| Reflections used for R-free | 2257 (211) | 2000 (194) |
| R-work | 0.1296 (0.2739) | 0.1744 (0.2762) |
| R-free | 0.1703 (0.2952) | 0.2519 (0.3425) |
| CC(work) | 0.968 (0.759) | 0.940 (0.631) |
| CC(free) | 0.968 (0.813) | 0.908 (0.580) |
| Number of non-hydrogen atoms | 1883 | 1791 |
| macromolecules | 1628 | 1566 |
| ligands | 23 | None |
| solvent | 232 | 225 |
| Protein residues | 203 | 203 |
| RMS(bonds) | 0.033 | 0.022 |
| RMS(angles) | 2.51 | 2.16 |
| Ramachandran favored (%) | 98.51 | 94.53 |
| Ramachandran allowed (%) | 1.49 | 2.49 |
| Ramachandran outliers (%) | 0 | 2.99 |
| Rotamer outliers (%) | 2.14 | 2.31 |
| Clashscore | 9.22 | 8.13 |
| Average B-factor | 32.39 | 25.07 |
| macromolecules | 30.46 | 24.05 |
| ligands | 53.63 | None |
| solvent | 43.82 | 32.11 |
Figure 3.
Superposition of the SWIB/MDM2 domain of heIF2D with (a) N-terminal domain (E25-V109) of human MDM2 (PDB: 1YCR) and (b) SWIB domain of mouse BAF60a (PDB: 1UHR). The arrow in 3(a) points toward helix α4 that shifts by 6.7 Å. Superposition of the SUI1 domain of heIF2D with (c) human eIF1 (heIF1) (PDB: 2IF1) and (d) yeast eIF1 (yeIF1) (PDB: 2OGH). The arrow in 3(c) points toward helix α2 that shifts by 3.0 Å. The structures were superposed using COOT and the figure was made in Pymol.
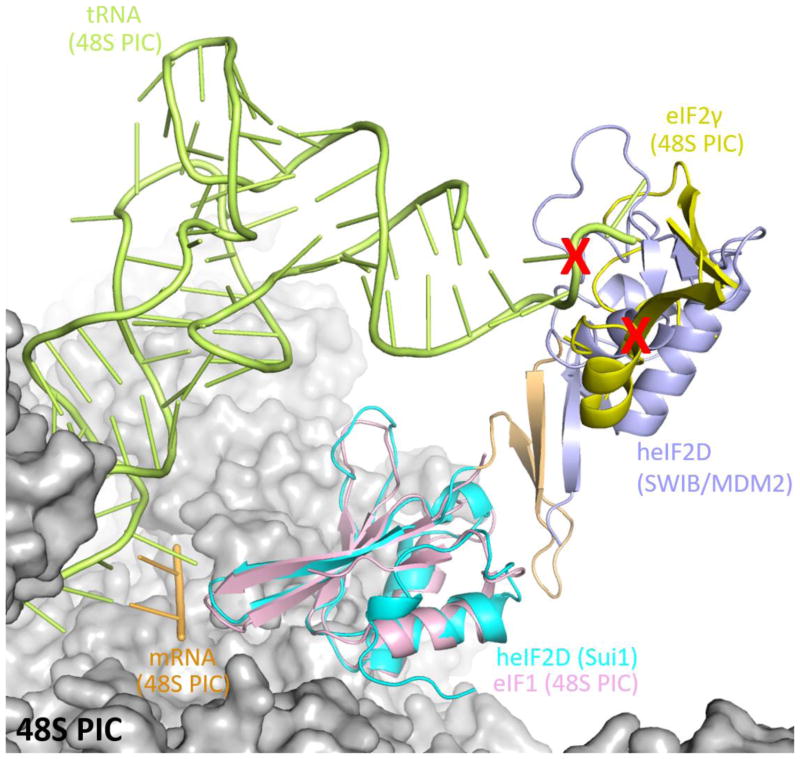
The linker connecting the two domains is made up of an anti-parallel β-sheet and loops. Two β-strands of SWIB/MDM2 form the anti-parallel β-sheet along with two β-strands from the linker, which leads to extensive interactions of the linker with this domain (Figure 2a, b). The SUI1 domain is connected to the linker by a small loop 485KGRICP490. Though the G486 residue in the loop could possibly impart flexibility to the orientation of the SUI1 domain with respect to the rest of the protein, extensive electrostatic and hydrophobic interactions between the SUI1 domain and the linker confers rigidity to the entire structure (Figure 2a, b). These electrostatic interactions involve the nitrogen of K484 from the linker and the main chain oxygen of E511 from the SUI1 domain and the main chain oxygen of R487 from the linker and the sulfur of C489 from the SUI1 domain. The side chains of V474, L476 from the linker and L515, L566 from the SUI1 domain are involved in hydrophobic interactions (Figure 2b). The large surface area of interaction between the linker and the SWIB/MDM2 and SUI1 domains, of 672 Å2 and 582 Å2, respectively, suggests that these domains along with the linker may act as a single rigid domain. In the context of the ribosome, when the SUI1 domain of heIF2D is superimposed on eIF1 of the partial 48S PIC, the SWIB/MDM2 domain clashes with the eIF2γ subunit and the initiator tRNA (PDB: 3JAQ) [24] (Figure 4). Thus, the SUI1 domain would anchor eIF2D on the ribosome, while the SWIB/MDM2 domain might prevent the simultaneous binding of eIF2 to the ribosome. It is not clear if these domains need to be rearranged to allow stabilization of the initiator tRNA on the ribosome. The structure of eIF2D on the 48S pre-initiation complex is needed to understand further how eIF2D interacts with the 40S ribosomal subunit and initiator tRNA to promote translation initiation and re-initiation.
Figure 4.
Superposition of heIF2D (C-term) on the eIF1 of the 48S pre-initiation complex (48S PIC, PDB: 3JAQ) reveals clashes (red cross) with the initiator tRNA and eIF2γ. The 48S PIC is represented as surface model, while the eIF1 (pink), initiator tRNA (green), mRNA (gold) and eIF2γ (yellow) of the 48S PIC are represented as cartoon model. Only the regions of eIF2γ that clash with eIF2D are shown here, while the rest of eIF2γ along with eIF2β and eIF2α are not shown for clarity. The domains of eIF2D are colored the same as in Figure 2(a). The superposition was done in COOT and the figure was made in Pymol.
Research Highlights.
eIF2D regulates the initiation of protein synthesis under stress condition.
The structure of the C-terminal part of eIF2D was determined at 1.4 Å resolution.
eIF2D has eIF1-like domain, crucial for scanning and the fidelity of AUG recognition.
Extensive atomic interaction between the domains imparts rigidity to the structure.
Acknowledgments
We thank the members of the Steitz lab for useful suggestions and discussions, the staff of the Advanced Photon Source beamline 24-ID and the Richards Center facility at Yale University. Special thanks to Dr. Jimin Wang for advice with crystallographic softwares and Dr. Mattthieu Gagnon for critical reading of the manuscript. This work was supported by the National Institutes of Health (NIH) grant GM022778 (to T.A.S.) and the NIH NIDDK grant P30KD034989 (to I.B.L.).
Abbreviations
- eIF
eukaryotic Initiation Factor
- RNA
ribonucleic acid
- tRNA
transfer RNA
- mRNA
messenger RNA
- rRNA
ribosomal RNA
- GTP
gunosine-5′-triphosphate
- DENR
density regulated protein and multiple
- MCT-1
multiple copies in T-cell lymphoma-1
- SWIB
SWI/SNF complex, including complex B
- MDM2
Mouse Double Minute 2 homolog
- SUI1
suppressor of initiator codon mutations 1
- NMR
Nuclear Magnetic Resonance
Footnotes
Author Contributions: A.T.V., I.B.L. designed and performed experiments, analyzed data, wrote the paper and directed research; N.N.J. performed experiments, analyzed data; S.E.D. analyzed data and wrote the paper; T.A.S. analyzed data, wrote the paper and directed research.
Accession Number: Coordinates and structure factors of heIF2D (C-term) have been deposited in the Protein Data Bank with accession number 5W2F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chu J, Cargnello M, Topisirovic I, Pelletier J. Translation Initiation Factors: Reprogramming Protein Synthesis in Cancer. Trends Cell Biol. 2016;26:918–33. doi: 10.1016/j.tcb.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Jan E, Mohr I, Walsh D. A Cap-to-Tail Guide to mRNA Translation Strategies in Virus-Infected Cells. Annu Rev Virol. 2016;3:283–307. doi: 10.1146/annurev-virology-100114-055014. [DOI] [PubMed] [Google Scholar]
- 3.Mohr I, Sonenberg N. Host translation at the nexus of infection and immunity. Cell Host Microbe. 2012;12:470–83. doi: 10.1016/j.chom.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myasnikov AG, Simonetti A, Marzi S, Klaholz BP. Structure–function insights into prokaryotic and eukaryotic translation initiation. Current opinion in structural biology. 2009;19:300–9. doi: 10.1016/j.sbi.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–27. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75:434–67. doi: 10.1128/MMBR.00008-11. first page of table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomakin IB, Steitz TA. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature. 2013;500:307–11. doi: 10.1038/nature12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plank TDM, Kieft JS. The structures of nonprotein-coding RNAs that drive internal ribosome entry site function. Wiley Interdisciplinary Reviews: RNA. 2012;3:195–212. doi: 10.1002/wrna.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skabkin MA, Skabkina OV, Dhote V, Komar AA, Hellen CU, Pestova TV. Activities of Ligatin and MCT-1/DENR in eukaryotic translation initiation and ribosomal recycling. Genes Dev. 2010;24:1787–801. doi: 10.1101/gad.1957510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitriev SE, Terenin IM, Andreev DE, Ivanov PA, Dunaevsky JE, Merrick WC, et al. GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J Biol Chem. 2010;285:26779–87. doi: 10.1074/jbc.M110.119693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcik M. Could the eIF2alpha-Independent Translation Be the Achilles Heel of Cancer? Front Oncol. 2015;5:264. doi: 10.3389/fonc.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skabkin MA, Skabkina OV, Hellen CU, Pestova TV. Reinitiation and other unconventional posttermination events during eukaryotic translation. Mol Cell. 2013;51:249–64. doi: 10.1016/j.molcel.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleich S, Strassburger K, Janiesch PC, Koledachkina T, Miller KK, Haneke K, et al. DENR-MCT-1 promotes translation re-initiation downstream of uORFs to control tissue growth. Nature. 2014;512:208–12. doi: 10.1038/nature13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempel W, Dimov S, Tong Y, Park HW, Hong BS. Crystal structure of human multiple copies in T-cell lymphoma-1 oncoprotein. Proteins: Structure, Function, and Bioinformatics. 2013;81:519–25. doi: 10.1002/prot.24198. [DOI] [PubMed] [Google Scholar]
- 15.Huang YJ, Acton TB, Montelione GT. DisMeta: a meta server for construct design and optimization. Structural Genomics: General Applications. 2014:3–16. doi: 10.1007/978-1-62703-691-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallographica Section D: Biological Crystallography. 2011;67:235–42. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. Journal of applied crystallography. 2007;40:658–74. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D: Biological Crystallography. 2010;66:213–21. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallographica Section D: Biological Crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kussie PH, Gorina S, Marechal V, Elenbaas B. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Jiang X. Mdm2 and MdmX partner to regulate p53. FEBS letters. 2012;586:1390–6. doi: 10.1016/j.febslet.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher CM, Pestova TV, Hellen CU, Wagner G. Structure and interactions of the translation initiation factor eIF1. The EMBO journal. 1999;18:2631–7. doi: 10.1093/emboj/18.9.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reibarkh M, Yamamoto Y, Singh CR, del Rio F, Fahmy A, Lee B, et al. Eukaryotic initiation factor (eIF) 1 carries two distinct eIF5-binding faces important for multifactor assembly and AUG selection. Journal of Biological Chemistry. 2008;283:1094–103. doi: 10.1074/jbc.M708155200. [DOI] [PubMed] [Google Scholar]
- 24.Llácer JL, Hussain T, Marler L, Aitken CE, Thakur A, Lorsch JR, et al. Conformational differences between open and closed states of the eukaryotic translation initiation complex. Molecular cell. 2015;59:399–412. doi: 10.1016/j.molcel.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLano WL. The PyMOL molecular graphics system. 2002 [Google Scholar]
- 26.Lomakin IB, Shirokikh NE, Yusupov MM, Hellen CU, Pestova TV. The fidelity of translation initiation: reciprocal activities of eIF1, IF3 and YciH. The EMBO journal. 2006;25:196–210. doi: 10.1038/sj.emboj.7600904. [DOI] [PMC free article] [PubMed] [Google Scholar]