Abstract
Background
Two valid and reliable estimated glomerular filtration rate (GFR) equations for the pediatric population have been developed from directly measured GFR data in the Chronic Kidney Disease in Children (CKiD) cohort: the full CKiD and bedside CKiD equations. While adult GFR estimating equations replicate relationships of measured GFR with biomarkers, it is unclear whether similar patterns exist among children and adolescents with chronic kidney disease (CKD).
Study Design
Prospective cohort study in children and adolescents.
Settings & Participants
730 participants contributed 1539 study visits.
Predictors
Measured GFR by plasma iohexol disappearance (mGFR), estimated GFR by the full CKiD equation (eGFRCKiDfull; based on serum creatinine, cystatin C, serum urea nitrogen, height, and sex), and estimated GFR by the bedside CKiD equation (eGFRCKiDbed; calculated as 41.3 × height[m]/serum creatinine [mg/dL]) were predictors of CKD-related biomarkers. Deviations of mGFR from eGFRCKiDfull and deviations of eGFRCKiDfull from eGFRCKiDbed from linear regressions (i.e., residuals) were included in bivariate analyses.
Outcomes & Measurements
CKD-related biomarkers included urine protein-creatinine ratio, blood hemoglobin, serum phosphate, bicarbonate, potassium, systolic and diastolic blood pressure z scores, and height z scores.
Results
The median age of 730 participants with CKD was 12.5 years, with median mGFR, eGFRCKiDfull, and eGFRCKiDbed of 51.8, 54.0, and 53.2 ml/min/1.73 m2, respectively. eGFRCKiDfull demonstrated as strong or stronger associations with CKD-related biomarkers than mGFR; eGFRCKiDbed associations were significantly attenuated (i.e., closer to the null). Residual information in mGFR did not substantially increase explained variability. eGFRCKiDbed estimated faster GFR decline relative to mGFR and eGFRCKiDfull.
Limitations
Simple linear summaries of biomarkers may not capture non-linear associations.
Conclusions
eGFRCKiDfull closely approximated mGFR to describe relationships with CKD-severity indicators and progression in this pediatric CKD population. eGFRCKiDbed offered similar inferences, but the associations were attenuated and rate of progression was overestimated. The eGFRCKiDfull equation from 2012 is preferred for pediatric research purposes.
Keywords: pediatric, glomerular filtration rate (GFR), estimated GFR, measured GFR, iohexol, kidney function, kidney measure, chronic kidney disease (CKD), children, adolescents, Chronic Kidney Disease in Children (CKiD), GFR estimating equation, CKD biomarker
Glomerular filtration rate (GFR) is considered to be the best assessment of kidney health and may be measured directly by the plasma disappearance of an exogenously administered agent. Because direct measurement of GFR is burdensome and invasive, equations developed to determine estimated GFR (eGFR) are part of routine clinical care and are substituted for measured GFR (mGFR) in research studies. The Chronic Kidney Disease in Children (CKiD) cohort has obtained over 2500 iohexol-based mGFRs, which have been used to develop GFR estimating equations. These include the full CKiD equation (eGFRCKiDfull) based on serum creatinine, cystatin C, serum urea nitrogen and height, introduced in a 2012 publication1; and the simple bedside equation2 (eGFRCKiDbed), which is 41.3 times the ratio of height to serum creatinine. These equations have performed extremely well internally1,2 and in diverse external special populations using non-iohexol-based methods3–17. In addition, several publications have used these equations for epidemiologic purposes, including etiologic18–21 and prospective studies22,23.
While published estimating equations are valid, with low bias and good precision, agreement metrics have been primarily comparisons between the proposed eGFR equation and mGFR in terms of bias, accuracy, precision, and correlation. Investigating potential differences between these two assessments for epidemiologic inference is another metric of agreement and recent analyses in adult populations have explored this24–29. To our knowledge, investigating potential differences in how GFR methods characterize relationships between kidney function and CKD biomarkers in children and adolescents has not yet been fully characterized.
The objective of this study was, in turn, to compare the relationships between different GFR assessment methods and indicators of CKD severity and GFR trajectory in a population of children and adolescents with diverse CKD diagnoses. Using GFR as the predictor, we investigated whether eGFR described the same relationship as mGFR with markers of CKD (e.g., proteinuria, metabolic, cardiovascular and growth variables). For this aim, we not only quantified the strength of the association, but also the variability explained by eGFR and how that was improved by including the information in mGFR that is not present in eGFR. In addition, using GFR as the outcome, we investigated whether the characterization of GFR decline over time by proteinuria level at baseline was different when using mGFR or eGFR. Comparing how mGFR, eGFRCKiDfull, and eGFRCKiDbed describe relationships of CKD severity and progression may provide guidance for pediatric CKD study populations external to CKiD and help facilitate simple and standardized assessments of GFR for research to improve reproducibility.
Methods
Study Population
As of 2016, the CKiD cohort study enrolled 891 children and adolescents aged 1-16 years from the United States and Canada, representing diverse CKD diagnoses with eGFR between 30 and 90 ml/min/1.73m2. Details of the study design have been previously described30. The iohexol-based mGFR was obtained at the first two annual visits and every other annual visit thereafter. Blood chemistry, including serum creatinine (Scr), cystatin C, serum urea nitrogen (SUN), and urine data were collected at each annual visit. The study design and conduct were approved by the internal review boards for each of the participating centers and by an external advisory committee appointed by the National Institutes of Health. Written informed consent/assent was obtained from all participants/families according to local IRB requirements.
Glomerular Filtration Rate
mGFR was calculated from a two-compartment model for visits occurring from 2005 to 2011, and from a universal equation for a one-compartment model from 2011 to 201531. Iohexol concentrations from November 2006 to March 2016 were increased by 12% as part of a multi-laboratory assay recalibration (Schwartz et al., manuscript in preparation). Equations for eGFR included 1) a full equation published in 20121, referred hereafter as eGFRCKiDfull, and defined as 39.8 × (height [m]/Scr [mg/dL])0.456 × (1.8/cystatin C [mg/dL])0.418 × (30/SUN [mg/dL])0.079 × 1.076 (if male) × (height [m]/1.4)0.179; and 2) the 2009 bedside equation2 (referred hereafter as eGFRCKiDbed) which is commonly used clinically and is simply 41.3 × height [m] / Scr [mg/dL].
Biomarkers Related to CKD Severity
Biomarkers as outcomes were urine protein-creatinine ratio (UPCR; in mg per mg of creatinine; measured as a portion of first morning void), blood hemoglobin (g/dl), serum bicarbonate (mEq/L), potassium (mEq/L), and phosphate (mg/dl)27. For the assessment of cardiovascular and growth markers, systolic blood pressure (SBP) and diastolic blood pressure (DBP) z scores (adjusted for age, sex and height32) and height z scores (adjusted for age and sex33) were included as outcomes.
Statistical Methods
The first approach of the analyses employed three separate univariate regression models for each biomarker (as the dependent variable) and mGFR, eGFRCKiDfull and eGFRCKiDbed as separate independent variables (in the log scale to provide measures of percent change) for each model. Biomarkers, with the exception of z scores, were log transformed (to achieve normality) and the slope from this model was expressed as the change in biomarker associated with a 50% lower GFR level. To account for the correlation of repeated measurements within individuals, generalized estimating equations were used to appropriately adjust standard errors for all models. To compare whether estimates by eGFRCKiDfull and eGFRCKiDbed were statistically different from mGFR, we used bootstrap methods and sampled at the individual level to preserve the within-person correlation of biomarkers and of repeated measurements. Specifically, 500 datasets were created from children and adolescents in our analytic dataset sampled with replacement.
The objective of the second approach was to quantify the putative additional information that is in mGFR but not in eGFRCKiDfull (i.e., obtaining mGFR deviations from eGFRCKiDfull based on the regression of mGFR on eGFRCKiDfull; i.e., residuals). Specifically, we expanded the model including eGFRCKiDfull in the first approach to the following:
where the error term is normally distributed with mean 0 and standard deviation σ. This model allows for a comparison of R2 values in which the null model assumes γ2 equal zero (i.e., first approach) and the test model allows γ2 to be estimated from the data. This provides an estimate of the explanatory power of information in mGFR not present in eGFRCKiDfull.
As part of the second approach, we also carried out similar analyses in which the primary predictor was eGFRCKiDbed. That is, we quantified how much more explained variability is added by including the deviations of mGFR from eGFRCKiDbed, and those of eGFRCKiDfull from eGFRCKiDbed to the third univariate model in the first approach (i.e., using only eGFRCKiDbed). The summary measures were the comparisons of the R2 values for the univariate and bivariate models. Statistical significance was determined by the same bootstrapping method described for first approach.
The third approach in the analysis used longitudinal data to determine changes in GFR over time. In addition to the overall changes in GFR over time, we assessed how each method characterized the clinically and epidemiologically meaningful interaction between initial UPCR and the effect of time. This linear mixed effects model with random intercepts and slopes used GFR as the outcome in the log scale, and the independent variables were baseline UPCR (in the log scale), time in years, and the interaction between the two. This model for the data provided by the ith individual at the jth visit (where j= 0 being the baseline CKiD visit) is formally expressed as:
where ai and bi are the random effects and eit is assumed to be normally distributed with mean 0 and standard deviation, θ, representing the deviations of the observed data from the line of the ith individual.
The population mean GFR level at entry and average percent change per year were presented for UPCR levels that represent doubling across the spectrum commonly observed in CKiD: at 0.25, 0.50, 1, 2 and 4 mg/mg.
Results
Of 891 children and adolescents enrolled, only person-visits with valid mGFR, eGFRCKiDfull and eGFRCKiDbed and complete data on 8 biomarkers were eligible to be included in our analysis (n=832 contributing 2088 person-visits). To ensure that our analytic sample was independent from (i.e., external to) the data used to develop the eGFRCKiDfull equation1, we further excluded those person-visits from our analysis (n= 102 contributing 533 person-visits). Hence, our analytic sample comprised 730 children and adolescents providing 1539 person-visits. In a comparison of the 730 children and adolescents included in our analysis and the 161 who were excluded, there were no significant differences demographically or by body size. However, GFRs were higher among those included in our analysis corresponding to recruitment in 2012-2014 at which time higher GFR was a criterion for study entry.
Table 1 describes the baseline (i.e., first observation) demographic and clinical characteristics, as well as longitudinal data available. The median mGFR, eGFRCKiDfull and eGFRCKiDbed values were 51.8, 54.0 and 53.2 ml/min/1.73m2, respectively. Median baseline UPCR was 0.35 mg/mg. Compared to the age-, sex- and height-adjusted apparently healthy population, our study sample had higher blood pressure z scores, specifically 0.24 standard deviation (SD) for SBP and 0.39 SD for DBP. The participants exhibited substantial deficits in height, specifically about 0.44 SD below the age- and gender-adjusted average.
Table 1. Baseline demographic and clinical characteristics.
| Variable | All children (n= 730) |
|---|---|
| Age, years | 12.5 [8.7 to 15.5] |
| Male sex | 62.7% (458) |
| Black race | 21.4% (156) |
| Growth and development | |
| Height, cm | 147 [127 to 163] |
| Height percentile | 33 [11 to 63] |
| Weight, kg | 42.5 [27.6 to 61.2] |
| Weight percentile | 54 [25 to 86] |
| Body surface area, m2 | 1.31 [0.99 to 1.68] |
| CKD history and kidney function | |
| Glomerular diagnosis | 29.7% (217) |
| Time since CKD onset, y | 9.2 [4.9 to 13.3] |
| Scr, mg/dL | 1.10 [0.80 to 1.61] |
| Cystatin C, mg/L | 1.37 [1.07 to 1.87] |
| SUN, mg/dL | 23 [17 to 32] |
| mGFR, ml/min/1.73 m2 | 51.8 [36.8 to 68.1] |
| eGFRCKiDfull, ml/min/1.73 m2 | 54.0 [39.0 to 67.4] |
| eGFRCKiDbed, ml/min/1.73 m2 | 53.2 [38.4 to 70.0] |
| Markers of CKD severity | |
| Urine protein, mg/mg | 0.35 [0.11 to 1.10] |
| Hemoglobin, g/dL | 12.8 [11.7 to 13.8] |
| Phosphate, mg/dL | 4.5 [4.0 to 4.9] |
| Bicarbonate, mEq/L | 24.0 [22.0 to 26.0] |
| Potassium, mEq/L | 4.3 [4.1 to 4.6] |
| Adjusted SBP z score* | 0.24 [-0.52 to 0.98] |
| Adjusted DBP z score* | 0.39 [-0.26 to 1.06] |
| Adjusted height z score** | -0.44 [-1.24 to 0.34] |
| No. of observations | |
| 1 visit | 31.9% (233) |
| 2 visits | 34.5% (252) |
| 3 visits | 26.2% (191) |
| ≥4 visits | 7.4% (54) |
Note: Baseline demographic and clinical characteristics of children enrolled in CKD in Children (CKiD) Study and contributing at least one study visit with directly measured iohexol-based GFR (mGFR); Scr-, cystatin C-, and SUN-based estimated GFR (eGFRCKiDfull); and Scr–based bedside GFR (eGFRCKiDbed). Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range]. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; SUN in mg/dL to mmol/L, ×0.357.
CKD, chronic kidney disease; Scr, serum creatinine; DBP, diastolic blood pressure; GFR, glomerular filtration rate; SUN, serum urea nitrogen;
Age-, sex-, and height-adjusted.
Age- and sex-adjusted.
Using separate models for each GFR measure, Table 2 presents the univariate relationships between markers of CKD severity and three measures of GFR: mGFR, eGFRCKiDfull, and eGFRCKiDbed. A 50% lower mGFR was associated with a 169.7% (95% confidence interval [CI], 139.4%-203.8%) higher UPCR. In contrast, a 50% lower eGFRCKiDfull was associated with a 204.0% (95% CI, 167.2%-245.9%) higher UPCR, a stronger association than mGFR. By contrast, a 50% lower eGFRCKiDbed was associated with a 155.0% (95% CI, 129.8%-182.9%) higher UPCR, a weaker association than mGFR. While both eGFRCKiDfull and eGFRCKiDbed offered the same inference of effect size (i.e., that GFR is significantly associated with proteinuria), eGFRCKiDbed was weaker than the estimate by mGFR, with borderline significance (p= 0.05), and eGFRCKiDfull was significantly stronger than the mGFR estimate (p<0.001).
Table 2. Univariate associations of markers of CKD severity and three GFR measures.
| Variable | mGFR^ | eGFRCKiDfull | eGFRCKiDbed |
|---|---|---|---|
| UPCR, mg/mg | +169.7% (+139.4% to +203.8%) | +204.0% (+167.2% to +245.9%); P < 0.001 | +155.0% (+129.8% to +182.9%); 0.05 |
| Hemoglobin, g/dL | -7.6% (-8.7% to -6.5%) | -7.9% (-9.0% to -6.7%); 0.1 | -5.7% (-6.7% to -4.7%); <0.001 |
| Phosphate, mg/dL | +9.4% (+7.6% to +11.2%) | +11.1% (+9.2% to +13.0%); <0.001 | +7.3% (+5.7% to +8.9%); <0.001 |
| Bicarbonate, mEq/L | -7.6% (-8.8% to -6.5%) | -8.1% (-9.3% to -6.9%); 0.02 | -6.0% (-7.0% to -4.9%); <0.001 |
| Potassium, mEq/L | +3.3% (+2.2% to +4.4%) | +3.3% (+2.1% to +4.6%); 0.7 | +1.9%; (+0.9% to +3.0%); <0.001 |
| Adjusted SBP z score* | +0.15 (+0.04 to +0.26) | +0.19 (+0.07 to +0.31); 0.009 | +0.13 (+0.03 to +0.24); 0.5 |
| Adjusted DBP z score* | +0.06 (-0.04 to +0.15) | +0.05 (-0.05 to +0.16); 0.6 | 0.00 (-0.10 to +0.09); <0.001 |
| Adjusted height z score** | -0.36 (-0.48 to -0.24) | -0.39 (-0.52 to -0.26); 0.09 | -0.28 (-0.39 to -0.17); 0.002 |
Note: Values are given as univariate associations (95% confidence intervals); P values for 730 children contributing 1539 observations. The measure of association was scaled to a 50% decrease in GFR.
Association and confidence interval values are based on linear regression models accounting for repeated measurements within individuals by generalized estimating equations. P values are based on bootstrap methods.
CKD, chronic kidney disease; GFR, glomerular filtration rate; mGFR, measured GFR (using iohexol); eGFRCKiDfull, GFR estimated with 2012 CKD in Children Study equation based on serum creatinine, cystatin C, and serum urea nitrogen; eGFRCKiDbed, 2009 serum creatinine-based bedside GFR;.UPCR, urinary protein-creatinine ratio
Reference.
Age-, sex-, and height-adjusted.
Age- and sex-adjusted.
Similar patterns were observed for the other 7 biomarkers. The relationships between eGFRCKiDfull and phosphate, bicarbonate, and SBP z scores were significantly stronger (i.e., away from the null) than the relationship estimated by mGFR (all p< 0.001). The relationship between eGFRCKiDfull and hemoglobin was slightly stronger than that for mGFR but not significantly different (-7.9% versus -7.6%; p= 0.1). This phenomenon was also observed for height z scores (-0.39 versus -0.36; p= 0.09). The estimates between eGFRCKiDfull and mGFR were the same for potassium (+3.3% versus +3.3%; p= 0.7) and similar for DBP z scores (+0.05 versus +0.06; p= 0.6). In contrast, for eGFRCKiDbed, the relationships were attenuated (i.e., toward the null) compared to mGFR for all outcomes, and these were significantly different for hemoglobin, phosphate, bicarbonate, potassium, DBP z scores and height z scores (all p ≤ 0.002). For SBP z score, the estimates did not differ statistically between mGFR and eGFRCKiDbed (+0.15 versus +0.13; p= 0.5). Overall, the relationships were inferentially the same across all three GFR assessments in that all estimates were significantly different from 0 (with the exception of DBP z scores), but eGFRCKiDbed consistently demonstrated weaker associations and was most often significantly different from mGFR. In contrast, eGFRCKiDfull showed stronger associations with known markers related to CKD severity than mGFR.
Figure 1 depicts a diamond graph34 comparing the R2 values of univariate (GFR only) and bivariate (GFR and deviations) models. The shaded polygon within each cell is proportional to the outcome variability explained by the predictors, scaled to 25% (i.e., an R2 of 25% represents the full area within each cell). Shown in the upper left row of the graph, eGFRCKiDfull explained 22.2% of UPCR variability, and when mGFR deviations from eGFRCKiDfull were included in a bivariate model, this R2 value increased slightly (22.4%; +0.2%). Similarly, eGFRCKiDbed in a univariate model explained 21.8% of UPCR variability; the addition of mGFR deviations in the bivariate model increased the R2 to 22.6% (+0.8%) and when eGFRCKiDfull deviations were added, the R2 was 22.5% (+0.7%). For the other 7 biomarkers as outcomes, relative to the eGFRCKiDfull, the additional explained variability offered by the mGFR deviations was minor, ranging from no difference in phosphate to 1.7% higher R2 for hemoglobin (15.1% versus 16.8%), which was the only significant difference (p= 0.01). However, for eGFRCKiDbed (depicted in the 3 rows in the upper right part of the graph), substantially higher R2 values were observed from the bivariate model including mGFR deviations as compared to the univariate model of eGFRCKiDbed, likely due to the simplicity and constraints of the eGFRCKiDbed equation. In particular, models with hemoglobin, phosphate, bicarbonate, and potassium as outcomes had significantly (p ≤ 0.003) higher R2 increases of +7.2% (10.6% versus 17.8%), +3.5% (8.1% versus 11.6%), +4.2% (9.4% versus 13.6%) and +3.8% (1.6% versus 5.4%), respectively.
Figure 1.
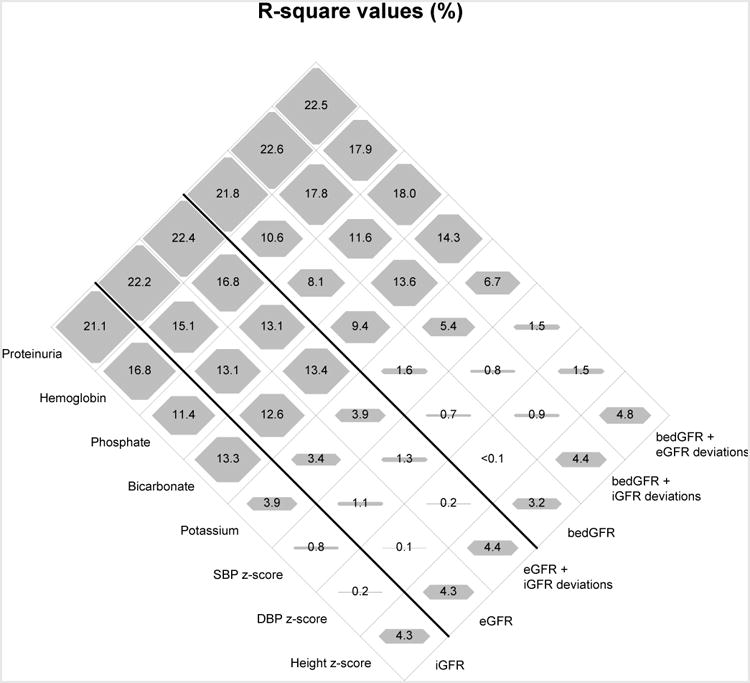
Diamond plot comparison of differences in naïve R2 values (in %) for univariate directly measured iohexol-based GFR (iGFR), serum creatinine, cystatin C and BUN-based estimated GFR (eGFR) and serum creatinine-based bedside GFR (bedGFR) and for bivariate models including iGFR and eGFR deviations (residuals) among 730 children and adolescents providing 1539 visits. R2 values are scaled to 25% (total area of each diamond).
Lastly, we compared how different GFR assessments may alter inferences related to the longitudinal changes in GFR both overall and at given values of initial UPCR level. Table 3 presents the population average mean GFR at baseline (i.e., study entry) and changes over time, for each GFR assessment (mGFR, eGFRCKiDfull, and eGFRCKiDbed) overall and for varying levels of baseline UPCR. The baseline GFR was similar by GFR assessment overall, and all methods consistently showed lower GFR among those with higher baseline UPCR. The estimated average change per year was slightly attenuated for eGFRCKiDfull compared to mGFR (-3.8% versus -4.3% per year for the overall model) and was very similar when baseline UPCR was allowed to modify change. When eGFRCKiDbed was used, a higher decline was estimated compared with mGFR or eGFRCKiDfull in the overall model (-6.4% versus -4.3%), and this was consistent across levels of UPCR when baseline UPCR was included as an independent variable. In summary, population averages of GFR at entry were similar across the three methods (within approximately 2 ml/min/1.73m2); estimates of change were similar between mGFR and eGFRCKiDfull, but eGFRCKiDbed estimated faster declines, particularly at higher levels of baseline UPCR. Although the measure of change used here is in the multiplicative scale (i.e., percent change per year), using the results in Table 3, one can easily estimate the rate of change in the arithmetic scale (e.g., for mGFR, 49.9 ml/min/1.73 m2 × (-4.3)% = -2.1 ml/min/1.73 m2 in the first year).
Table 3. Longitudinal analysis describing GFR at entry and percent change per year overall, and for varying baseline UPCR based on 3 GFR measures.
| GFR at entry, ml/min/1.73 m2 | Change per year | |||||
|---|---|---|---|---|---|---|
| mGFR | eGFRCKiDfull | eGFRCKiDbed | mGFR | eGFRCKiDfull | eGFRCKiDbed | |
| Overall | 49.9 (48.3 to 51.6) | 51.1 (49.6 to 52.6) | 50.2 (48.6 to 51.9) | -4.3% (-5.0% to -3.5%) | -3.8% (-4.5% to -3.1%) | -6.4% (-7.2% to -5.5%) |
| Baseline UPCR | ||||||
| 0.25 mg/mg (= 43rd percentile) | 52.4 (50.8 to 54.0) | 53.4 (52.0 to 54.9) | 52.8 (51.2 to 54.5) | -4.0% (-4.7% to -3.3%) | -3.4% (-4.0% to -2.7%) | -5.9% (-6.7% to -5.0%) |
| 0.50 mg/mg (= 58th percentile) | 48.0 (46.6 to 49.4) | 49.3 (48.0 to 50.6) | 48.2 (46.7 to 49.7) | -5.1% (-5.8% to -4.3%) | -4.8% (-5.5% to -4.1%) | -7.4% (-8.3% to -6.5%) |
| 1 mg/mg (= 75th percentile) | 43.9 (42.4 to 45.5) | 45.5 (44.0 to 46.9) | 43.9 (42.3 to 45.6) | -6.1% (-7.0% to -5.2%) | -6.2% (-7.1% to -5.3%) | -8.9% (-10.0% to -7.8%) |
| 2 mg/mg (= 88th percentile) | 40.2 (38.5 to 42.1) | 41.9 (40.3 to 43.7) | 40.1 (38.2 to 42.0) | -7.2% (-8.3% to -6.0%) | -7.6% (-8.8% to -6.5%) | -10.4% (-11.7% to -8.9%) |
| 4 mg/mg (= 94th percentile) | 36.9 (34.8 to 39.0) | 38.7 (36.8 to 40.7) | 36.6 (34.5 to 38.8) | -8.2% (-9.7% to -6.7%) | -9.0% (-10.4% to -7.6%) | -11.8% (-13.5% to -10.1%) |
Note: Longitudinal analysis among 730 children contributing 1539 person-visits of longitudinal data. Results are from linear mixed effects models with random intercepts and slopes. Values in parentheses are 95% confidence intervals.
GFR, glomerular filtration rate; mGFR, measured GFR (using iohexol); eGFRCKiDfull, GFR estimated with 2012 CKD in Children Study equation based on serum creatinine, cystatin C, and serum urea nitrogen; eGFRCKiDbed, 2009 serum creatinine–based bedside GFR; UPCR, urinary protein-creatinine ratio.
Discussion
This study assessed how GFR assessment methods differ in describing a diverse panel of biomarkers related to disease severity and disease trajectory in a pediatric population. We showed that the use of eGFR based on the 2012 CKiD equation (eGFRCKiDfull in this study) did not alter inferences in terms of characterizing biomarker relationships and disease progression compared to mGFR. Furthermore, the use of this equation offered associations that were consistently as strong as or stronger than that of mGFR. In quantifying relationships with biomarkers, eGFRCKiDbed tended to be biased toward the null (i.e., attenuated) and was significantly different from mGFR in 7 of the 8 biomarkers explored. In addition, eGFRCKiDbed overestimated the decline per year compared to mGFR and eGFRCKiDfull, which were similar to one another.
While inferentially similar, some relationships with biomarkers were actually stronger for eGFRCKiDfull compared to mGFR. These findings were consistent with previous reports demonstrating that eGFR offers stronger predictive power than mGFR24,27,29. This is likely due to a combination of removing non-informative variability in iohexol-based mGFR31,35,36 and to the predictive power of Scr, cystatin C and SUN for extra-renal indicators of health. The latter explanation may be more plausible, as eGFR is a summary value based on one or more variables (Scr, cystatin C and SUN) that are known to be independently associated with non-CKD comorbidities24,37. For example, in the regression in which eGFRCKiDbed and the residuals from eGFRCKiDfull were included as predictors of phosphate (Figure 1), substantial increases in R2 values were observed compared to eGFRCKiDbed alone and eGFRCKiDbed plus mGFR residuals. We hypothesize this change is predominantly due to the effect of cystatin C as part of the eGFR formula. For unique patient profiles that can influence cystatin C (e.g., thyroid disease) or serum creatinine levels (e.g., muscle wasting), univariate eGFR equations may be more appropriate38.
For eGFRCKiDbed, the associations with biomarkers were attenuated and GFR decline was overestimated. In epidemiologic studies in which strength of association (i.e., the signal) is crucial for inference, attenuation of effects is generally not desirable. Indeed, it is well documented that measurement error is often a cause of attenuation of associations39,40. Given the simplicity of the equation, it was not expected to perform as well as mGFR or the more complex eGFRCKiDfull equation. Nonetheless, despite the attenuated associations, the inferences were similar and eGFRCKiDbed has been shown to be robust in diverse external populations4,7,9,12,15. While eGFRCKiDbed is simple to use and an excellent clinical tool for efficiency, for research purposes we recommend eGFRCKiDfull rather than eGFRCKiDbed.
Our study population included several sources of heterogeneity in CKD-related characteristics beyond GFR (eg, CKD diagnosis). The full analysis was carried out separately by underlying glomerular and non-glomerular forms of CKD, and they were entirely consistent with the present analyses, in which diagnoses were pooled. This was not unexpected because the design of this analysis ensured that the three GFR methods were equally affected by any CKD-related heterogeneities within the data, thus providing a valid and fair comparison. Another source of heterogeneity was therapy use for proteinuria, anemia, blood pressure and/or growth failure in this population. Importantly, the proportions receiving therapy did not vary by GFR method used. While these results do not provide a characterization of treated or untreated relationships of biomarkers with GFR, this study design allows for valid comparisons between measured and estimated GFR methods.
Glomerular filtration rate is commonly used in epidemiologic research in three potential ways: a predictor, an outcome or a covariate. While the present analysis offers a detailed description of select scenarios, researchers should proceed carefully and consider how each GFR method will be used in analyses, and if one method may be better suited than another. For example, previous reports have documented the superiority of eGFR in predicting outcomes24,26,37, and eGFR may therefore be preferable for prognostic purposes. Measured GFR should, on the other hand, be selected for investigations of kidney function outside the range in which eGFR equations were developed, such as in populations at risk for hyperfiltration41 or in settings in which discrepancies between cystatin C–only eGFR and Scr-only eGFR require resolution38. These examples serve to illustrate that particular research questions should dictate selection of the GFR assessment with careful scientific consideration. Given that the explained variability of biomarkers and characterizations of disease progression were not greatly enhanced by direct measures of GFR in the present analyses, the use of eGFRCKiDfull in a similar pediatric population with CKD for epidemiologic research can now be recommended, especially given the costs and burdens of obtaining mGFRs in children and adolescents.
There were several limitations to this study. First, this study did not characterize nonlinear relationships of GFR with biomarkers; these relationships were summarized using a simple linear assumption. While this approach allows for valid comparison across different GFR assessments (i.e., the relationships from the three methods were equally constrained by the model), it does not account for non-linear relationships and may not best describe the relationship of the biomarker with GFR. Second, our results may not be applicable to adults, or children and adolescents with GFR outside the range presented in these analyses (1412 eGFRCKiDfull observations [92% of our population] were between 20 and 90 ml/min/1.73 m2). However, these results are consistent with numerous studies comparing mGFR with eGFR. Third, cystatin C data used in this analysis, and for the CKiD equation development, were obtained prior to the availability of International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)–approved reference materials and are based on standard nephelometric output. Despite these data not being calibrated to IFCC material, the eGFRCKiDfull equation performed very well compared to mGFR. Fourth, GFR may also be used as a restriction variable in epidemiologic research for selecting subjects who fit a particular profile, and this analysis did not address the impact of potential misclassification by GFR method. Lastly, while eGFRCKiDfull closely replicated the relationships determined by mGFR, consistency with other biomarkers (e.g., fibroblast growth factor 23) or in different populations is not assured. Nonetheless, the diversity of markers presented gives some confidence to expect a high degree of preservation of associations.
In conclusion, our analyses investigated differences in directly measured iohexol-based GFR and two eGFR equations (eGFRCKiDfull and eGFRCKiDbed) in a cohort of children and adolescents with CKD by quantifying the relationships of each method with biomarkers, as well as changes over time. While the three methods were inferentially similar for quantifying the relationships with biomarkers, the eGFRCKiDbed Scr-only equation generally had attenuated estimates of effect compared to the eGFRCKiDfull equation using Scr and cystatin C, which was comparable to mGFR. The information contained in mGFR not present in eGFRCKiDfull had minimal effects on explaining relationships with biomarkers. Compared to mGFR, eGFRCKiDfull described similar heterogeneity of longitudinal changes in GFR by UPCR levels, as well as relationships with biomarkers. In summary, our results suggest that the eGFRCKiDfull equation closely replicated mGFR for these selected relationships and should be the preferred GFR method for research studies of pediatric CKD.
Acknowledgments
Data in this manuscript were collected by the CKiD prospective cohort study with clinical coordinating centers (principal investigators) at Children's Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady, MD) and Children's Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Website can be found at www.statepi.jhsph.edu/ckid.
Support: The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116)..
Footnotes
N Section: Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled by an Editorial Board Member (Kathleen Liu, MD, PhD) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: DKN, AM; data acquisition: GJS, BAW, SLF; data analysis/interpretation: DKN, AM, GJS, BAW, SLF; statistical analysis: DKN; supervision or mentorship: AM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol JASN. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pottel H, Mottaghy FM, Zaman Z, Martens F. On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr Nephrol Berl Ger. 2010;25(5):927–934. doi: 10.1007/s00467-009-1389-1. [DOI] [PubMed] [Google Scholar]
- 4.Staples A, LeBlond R, Watkins S, Wong C, Brandt J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol Berl Ger. 2010;25(11):2321–2326. doi: 10.1007/s00467-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 5.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol CJASN. 2011;6(7):1599–1608. doi: 10.2215/CJN.10161110. [DOI] [PubMed] [Google Scholar]
- 6.Laskin BL, Nehus E, Goebel J, Khoury JC, Davies SM, Jodele S. Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18(11):1745–1752. doi: 10.1016/j.bbmt.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Chehade H, Cachat F, Jannot AS, et al. Combined serum creatinine and cystatin C Schwartz formula predicts kidney function better than the combined CKD-EPI formula in children. Am J Nephrol. 2013;38(4):300–306. doi: 10.1159/000354920. [DOI] [PubMed] [Google Scholar]
- 8.Chehade H, Cachat F, Jannot AS, et al. New combined serum creatinine and cystatin C quadratic formula for GFR assessment in children. Clin J Am Soc Nephrol CJASN. 2014;9(1):54–63. doi: 10.2215/CJN.00940113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westland R, Abraham Y, Bökenkamp A, Stoffel-Wagner B, Schreuder MF, van Wijk JAE. Precision of estimating equations for GFR in children with a solitary functioning kidney: the KIMONO study. Clin J Am Soc Nephrol CJASN. 2013;8(5):764–772. doi: 10.2215/CJN.07870812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nehus EJ, Laskin BL, Kathman TI, Bissler JJ. Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol Berl Ger. 2013;28(3):453–461. doi: 10.1007/s00467-012-2341-3. [DOI] [PubMed] [Google Scholar]
- 11.Papez KE, Barletta GM, Hsieh S, Joseph M, Morgenstern BZ. Iothalamate versus estimated GFR in a Hispanic-dominant pediatric renal transplant population. Pediatr Nephrol Berl Ger. 2013;28(12):2369–2376. doi: 10.1007/s00467-013-2556-y. [DOI] [PubMed] [Google Scholar]
- 12.Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29(5):1082–1091. doi: 10.1093/ndt/gft277. [DOI] [PubMed] [Google Scholar]
- 13.Laskin BL, Nehus E, Goebel J, Furth S, Davies SM, Jodele S. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2014;20(12):2056–2061. doi: 10.1016/j.bbmt.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddique K, Leonard D, Borders L, Seikaly MG. Validation of the CKiD formulae to estimate GFR in children post renal transplant. Pediatr Nephrol Berl Ger. 2014;29(3):445–451. doi: 10.1007/s00467-013-2660-z. [DOI] [PubMed] [Google Scholar]
- 15.Deng F, Finer G, Haymond S, Brooks E, Langman CB. Applicability of estimating glomerular filtration rate equations in pediatric patients: comparison with a measured glomerular filtration rate by iohexol clearance. Transl Res. 2015;165(3):437–445. doi: 10.1016/j.trsl.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza V, Cochat P, Rabilloud M, et al. Accuracy of different equations in estimating GFR in pediatric kidney transplant recipients. Clin J Am Soc Nephrol CJASN. 2015;10(3):463–470. doi: 10.2215/CJN.06300614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vroling AB, Dorresteijn EM, Cransberg K, de Rijke YB. The impact of estimated glomerular filtration rate equations on chronic kidney disease staging in pediatric renal or heart transplant recipients. Pediatr Nephrol Berl Ger. 2016;31:1145–1155. doi: 10.1007/s00467-016-3312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayestaran FW, Schneider MF, Kaskel FJ, et al. Perceived appetite and clinical outcomes in children with chronic kidney disease. Pediatr Nephrol Berl Ger. 2016;31(7):1121–1127. doi: 10.1007/s00467-016-3321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng DK, Robertson CC, Woroniecki RP, et al. APOL1-associated glomerular disease among African-American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2016 Apr; doi: 10.1093/ndt/gfw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark SL, Denburg MR, Furth SL. Physical activity and screen time in adolescents in the chronic kidney disease in children (CKiD) cohort. Pediatr Nephrol Berl Ger. 2016;31(5):801–808. doi: 10.1007/s00467-015-3287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuttke M, Wong CS, Wühl E, et al. Genetic loci associated with renal function measures and chronic kidney disease in children: the Pediatric Investigation for Genetic Factors Linked with Renal Progression Consortium. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2016;31(2):262–269. doi: 10.1093/ndt/gfv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015;65(6):878–888. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. Progression of Pediatric CKD of Nonglomerular Origin in the CKiD Cohort. Clin J Am Soc Nephrol. 2015;10(4):571–577. doi: 10.2215/CJN.07480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 25.Xie D, Joffe MM, Brunelli SM, et al. A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol CJASN. 2008;3(5):1332–1338. doi: 10.2215/CJN.05631207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhavsar NA, Appel LJ, Kusek JW, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis Off J Natl Kidney Found. 2011;58(6):886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu C, Propert K, Xie D, et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol JASN. 2011;22(10):1931–1937. doi: 10.1681/ASN.2010101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer IH, Sun W, Cleary PA, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol JASN. 2014;25(4):810–818. doi: 10.1681/ASN.2013050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku E, Xie D, Shlipak M, et al. Change in Measured GFR Versus eGFR and CKD Outcomes. J Am Soc Nephrol JASN. 2016;27(7):2196–2204. doi: 10.1681/ASN.2015040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol CJASN. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng DKS, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80(4):423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 33.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 34.Li X, Buechner JM, Tarwater PM, Muñoz A. A Diamond-Shaped Equiponderant Graphical Display of the Effects of Two Categorical Predictors on Continuous Outcomes. Am Stat. 2003;57(3):193–199. [Google Scholar]
- 35.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69(11):2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz GJ, Abraham AG, Furth SL, Warady BA, Muñoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77(1):65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol JASN. 2012;23(2):351–359. doi: 10.1681/ASN.2011070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: Simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest. 2010;70(2):65–70. doi: 10.3109/00365511003642535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas D, Stram D, Dwyer J. Exposure measurement error: influence on exposure-disease. Relationships and methods of correction. Annu Rev Public Health. 1993;14:69–93. doi: 10.1146/annurev.pu.14.050193.000441. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(4 Suppl):1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 41.Ng DK, Jacobson LP, Brown TT, et al. HIV therapy, metabolic and cardiovascular health are associated with glomerular hyperfiltration among men with and without HIV infection. AIDS Lond Engl. 2014;28(3):377–386. doi: 10.1097/QAD.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]


