Abstract
Background
Studies in chronic kidney disease populations suggest that the non-GFR determinants of serum levels of the low molecular weight (LMW) protein filtration markers cystatin C, beta-2 microglobulin (B2M) and beta trace protein (BTP) are less affected by age, sex and ethnicity than those of creatinine.
Study Design
Cross-sectional study.
Setting & Participants
Predominantly elderly participants selected from the Age, Gene/Environment Susceptibility Kidney Study [AGES-Kidney, N=683, mean (SD) age 79 (4), GFR 62 (17) ml/min/1.73 m2] and from the Multi-Ethnic Study of Atherosclerosis Kidney Study [MESA-Kidney, N=273, mean (SD) age 70.5 (9), GFR 73 (19) ml/min/1.73 m2].
Predictors
Demographic and clinical factors hypothesized to be associated with conditions affecting non-GFR determinants of the filtration markers.
Outcomes
mGFR and eGFRs based on creatinine, cystatin C, B2M and BTP (eGFRcr, eGFRcys, eGFRB2M and eGFRBTP, respectively). Residual associations of factors with eGFR after accounting for mGFR as the parameter of interest.
Results
eGFRcys, eGFRB2M and eGFRBTP had significantly less strong residual associations with age and sex than eGFRcr in both AGES-Kidney and MESA-Kidney, and were not associated with ethnicity (black vs. white) in MESA-Kidney. After adjusting for age, sex and ethnicity, residual associations with most clinical factors were smaller than observed with age and sex. eGFRcys, eGFRB2M, but not eGFRBTP had significant residual associations with CRP in both studies.
Limitations
Small sample size may limit power to detect associations. Participants may be healthier than the general population.
Conclusions
Similar to previous studies in chronic kidney disease, in community-dwelling elders, cystatin C, B2M and BTP are less affected than creatinine by age and sex and are not affected by ethnicity. Both cystatin C and B2M may be affected by inflammation. These findings are important for the development and use of GFR estimating equations based on LMW serum proteins throughout the range in GFR.
Index words: filtration markers, glomerular filtration rate (GFR), GFR estimation, elderly, creatinine, cystatin C, β2-microglobulin (B2M), beta-trace protein (BTP), kidney function, biomarker
Serum levels of filtration markers, including the metabolite creatinine and the low molecular weight (LMW) proteins cystatin C, β2-microglobulin (B2M), and beta-trace protein (BTP), are influenced both by the glomerular filtration rate (GFR) and physiological processes other than GFR (“non-GFR determinants”). Non-GFR determinants of the serum levels of filtration markers introduce bias and imprecision into estimates of GFR, and knowledge of the non-GFR determinants can facilitate interpretation of GFR estimates when applied to an individual patient.
Prior work has reported on demographic and clinical factors associated with creatinine, cystatin C, B2M and BTP after adjustment for associations with measured GFR in chronic kidney disease (CKD) populations and suggests differential effects of non-GFR determinants on the serum concentrations of these filtration markers.1,2 In particular, age, sex and ethnicity appear to affect the non-GFR determinants of the LMW serum protein filtration markers less than those of creatinine, while CVD and some CVD risk factor conditions may affect their non-GFR determinants more than those of creatinine. However, it is not known whether these findings would be generalizable to community-dwelling elderly populations. The goal of the current study was to evaluate demographic and clinical characteristics as potential factors associated with non-GFR determinants of creatinine, cystatin C, B2M and BTP in two ancillary studies from established community-based cohort studies of elderly adults in Iceland and the United States in which GFR was measured.
Methods
Study Population
Age, Gene/Environment Susceptibility Kidney Study (AGES-Kidney)
AGES-Kidney is an ancillary sub-study on kidney function in the elderly in Iceland drawn from the AGES-Reykjavik Study, a long-term follow up study of the Reykjavik Study, a prospective cohort study conducted between 1967 and 1996 in Iceland to study cardiovascular disease in a community-based cohort.3 Between 2002 and 2006, 5764 participants from a random sample of survivors of the Reykjavik Study were recruited for the AGES-Reykjavik Study. Between 2007 and 2011, 3411 participants returned for a second AGES-Reykjavik Study visit; in 2010–2011, participants were recruited and enrolled into AGES-Kidney, of whom 805 completed determination of measured GFR (mGFR).4 For the present analysis the AGES-Kidney study sample included 683 participants after excluding participants with missing serum filtration marker measurements or missing data on factors hypothesized to be associated with non-GFR determinants. AGES-Kidney was approved by Icelandic Bioethics Committee; approval number VSN-00-063 and the Institutional Review Boards for the National Institute on Aging and Tufts Medical Center and participants provided written informed consent.
Multi-Ethnic Study of Atherosclerosis Kidney Study (MESA-Kidney)
MESA-Kidney is an ancillary study of older black and white participants from MESA, a community-based cohort designed to study subclinical cardiovascular disease in four race and ethnic groups in the United States who were free of clinical cardiovascular disease at the baseline visit (2000–2002).5 MESA participants underwent their 5th visit between April 2010 and December 2011; Among 746 MESA-Kidney participants who completed the 5th or earlier visits from the Johns Hopkins University, Baltimore, Maryland MESA field center, 307 were recruited between May 2012 and April 2014 and 294 completed determination of mGFR.6 For the present analysis the MESA-Kidney study sample included 275 participants after excluding participants with missing serum filtration marker measurements or with missing data on factors hypothesized to be associated with non-GFR determinants. MESA-Kidney was approved by Johns Hopkins Medicine Institutional Review Board; approval number 99-11-10-06 and participants provided written informed consent.
Outcome Assessment: Measured GFR and Estimated GFR Based on Each Serum Filtration Marker
GFR was measured using plasma clearance of iohexol and expressed with indexing per 1.73 m2 body surface area in both AGES-Kidney and MESA-Kidney, as previously described.4,7 Serum levels of creatinine, cystatin C, B2M and BTP were measured at the University of Minnesota using stored samples collected during the AGES-Kidney and MESA-Kidney study visits.
Creatinine was assayed by the Roche enzymatic method on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) (CV 2.3%). The method is calibrated using a National Institute of Standards and Technology (NIST) standard traceable to reference material SRM 909b (Isotope Dilution Mass Spectroscopy (IDMS).8 Cystatin C was assayed by the Gentian turbidimetric method (Gentian AS, Moss, Norway) on the Roche COBAS 6000 chemistry analyzer (Roche Diagnostics) (CV 4.3% at 0.75 mg/L and 3.2% at 3.83 mg/L). This method is traceable to NIST methods).8, 9 B2M was measured on the Roche COBAS 6000 chemistry analyzer (Roche Diagnostics) (CV 3.2% at 1.63 mg/L and 4.3% at 0.63 mg/L). BTP was assayed using Immunonephelometric methods on the Siemens ProSpec nephelometer (Siemens Healthcare Diagnostics, Newark, DE) (CV 10.6% at 0.618 mg/L and 7.4% at 1.852 mg/L.
Predictor Variable Assessment
Demographic and clinical factors hypothesized to be associated with conditions affecting non-GFR determinants were selected based on previous studies in CKD cohorts1, 2 and in the general US population.5 Demographic factors included age and sex in both studies and, in MESA-Kidney only, race/ethnicity; these factors are associated with muscle mass. Clinical factors of interest included the following: body mass index (a measure of adiposity); smoking status, serum albumin, and serum C-reactive protein (measures of inflammation); serum HDL cholesterol and serum triglycerides (measures of dyslipidemia), cardiovascular disease (CVD), and diabetes. Weight and height were ascertained during the AGES-Kidney or MESA-Kidney visit. Other clinical factors were ascertained during the most recent prior AGES or MESA visit using standard protocols as previous described.4, 7 CRP was measured using the Immunoturbidimetric Roche methods on the COBAS 6000 chemistry analyzer (Roche Diagnostics) (CV 6.7% at 3.12 mg/L and 5.1% at 1.05 mg/L). Albumin was measured using the Bromcresol Purple method on the COBAS 6000 chemistry analyzer (Roche Diagnostics) (CV 2.6% at 3.90 g/dL and 2.2% at 2.68 g/dL).
Statistical Analyses
For analyses, serum creatinine, cystatin C, B2M and BTP were transformed to the GFR scale using each filtration marker to estimate mGFR (after log transformation of markers and mGFR) to allow for comparisons across study-specific single-marker eGFRs (eGFRcr, eGFRcys, eGFRB2M and eGFRBTP), thus each eGFR is related to the reciprocal of the serum concentration of the filtration marker. Other factors typically used in estimation equations (e.g. age, sex, and race/ethnicity) were not incorporated into the study-specific eGFRs, so we could evaluate their roles as factors associated with non-GFR determinants in analyses. We did not use previously developed estimating equations based on B2M and BTP as they were developed in CKD populations and have not been evaluated in elderly populations.
Analyses were performed separately in AGES-Kidney and MESA-Kidney due to regional and demographic differences of the two study cohorts. Participant characteristics and the distribution of mGFR and filtration markers in the two study samples are described using means (standard deviations) for non-skewed continuous variables, medians [interquartile range] for skewed continuous variables, and N (%) for categorical variables. Pearson correlation coefficients and partial Pearson correlation coefficients adjusted for mGFR were used to evaluate correlations among mGFR and eGFR based on each filtration marker.
Generalized estimating equations (GEE) were used to evaluate the unadjusted associations of each GFR with age, sex, and ethnicity as well as age-, sex-, and ethnicity adjusted associations of the factors with mGFR and each eGFR and were used to evaluate residual associations of each factor with each eGFR after accounting for mGFR. Each stacked GEE model included five linear regression models with mGFR and each eGFR as outcomes and each factor of interest, an indicator variable for GFR measurement type, and the interaction of the factor of interest and the GFR indicator variable.10 These interaction terms reflect the difference (residual association) between the regression coefficients for the associations of the factor of interest with eGFR vs. mGFR. Models were also performed with eGFRcr as the reference group for the GFR measurement type variable such that the interaction term in the GEE models compare the residual associations of each factor with eGFRcys, eGFRB2M and eGFRBTP to the residual association of the factor with those of eGFRcr. Generalized estimating equations were performed assuming an exchangeable correlation for the covariance matrix and a sandwich estimator to obtain robust estimates of the standard error.
Continuous predictors were standardized to their study-specific interquartile range. mGFR and eGFRs were expressed as natural log transformations such that the beta coefficients could be interpreted as a percent difference. The direction of associations of categorical and continuous variables is generally described for lower mGFR and eGFR. Analyses were performed in Stata, Version 12.1 (StataCorp. 2011. Stata Statistical Software: Release 12.1 College Station, TX: StataCorp LP).
Results
Participant Characteristics
Characteristics of participants from the two study cohorts are presented in Table 1. In AGES-Kidney, participants were on average 79 years old (SD 4 years, range 71 to 91 years) with a mean mGFR of 62 ml/min/1.73m2; 57% were women, 10% had diabetes, 13% had prevalent CVD, 61% had hypertension, and 5% were current smokers. In MESA-Kidney, participants were on average 70 years old (SD 9 years, range 55 to 91 years) with a mean mGFR of 73 ml/min/1.73m2; 48% were women, 45% were black, 25% had diabetes, 5% had prevalent CVD, 65% had hypertension, and 9% were current smokers.
Table 1.
Participant Characteristics in AGES-Kidney and MESA-Kidney.
| AGES-Kidney | MESA-Kidney | |
|---|---|---|
| N | 683 | 275 |
| Age (year) | 79 (4) | 70 (9) |
| Female | 57% | 48% |
| Black Ethnicity | 0% | 45% |
| Body Mass Index (kg/m2) | 27 (4) | 30 (5) |
| Diabetes | 10% | 25% |
| Current Smoking | 5% | 9% |
| Cardiovascular Disease | 13% | 5% |
| Hypertension | 61% | 65% |
| Serum Albumin (g/dL) | 3.7 (0.2) | 3.8 (0.3) |
| Serum HDL Cholesterol (mg/dL) | 62 (17) | 56 (18) |
| Serum Triglycerides (mg/dL) | 95 [73, 121] | 92 [68, 124] |
| Serum C-Reactive Protein (g/dL)* | 2 [1, 3] | 2 [1, 4] |
| Urinary Albumin-Creatinine ratio (mg/g) | 8 [5, 19] | 10 [6, 21] |
| Serum Creatinine (mg/dL) | 1.0 (0.4) | 0.9 (0.3) |
| Serum Cystatin C (mg/L) | 1.2 (0.4) | 1.0 (0.3) |
| Serum BTP (mg/L) | 0.9 (0.4) | 0.6 (0.3) |
| Serum B2M (mg/L) | 2.9 (1.3) | 2.2 (1.0) |
| mGFR (ml/min/1.73m2) | 62 (17) | 73 (19) |
| eGFRcr (ml/min/1.73m2)** | 61 (14) | 71 (11) |
| eGFRcys (ml/min/1.73m2)** | 62 (15) | 72 (15) |
| eGFRB2M (ml/min/1.73m2)** | 62 (14) | 72 (14) |
| eGFRBTP (ml/min/1.73m2)** | 61 (14) | 72 (13) |
Unless otherwise noted, continuous characteristics are presented as mean (standard deviation); categorical values are presented as count (percent). Conversion factors for units: serum HDL cholesterol in mg/dL to mmol/L, ×0.02586; serum triglycerides in mg/dL to mmol/L, ×0.01129; serum creatinine in mg/dL to μmol/L, ×88.4. eGFR, estimated glomerular filtration rate; eGFRcr, serum creatinine–based eGFR, eGFRcys, cystatin C–based eGFR, eGFRB2M, β2-microglobulin–based eGFR, eGFRBTP, beta trace protein–based eGFR
Median [25th, 75th percentile]
Study specific eGFR based on each filtration marker (without including age, sex or ethnicity)
Correlations Among mGFR and Filtration Markers
In AGES-Kidney, correlations of mGFR with eGFR were significant and ranged from 0.84 for eGFRcys to 0.72 for eGFRcr (Table S1A). Correlations of mGFR with eGFR in MESA-Kidney were significant but not as high as in AGES-Kidney, ranging from 0.75 for eGFRcys to 0.47 for eGFRcr (Table S2A). Partial correlations among eGFRs after adjusting for mGFR ranged from 0.50 and 0.72, respectively, for eGFRcys with eGFRB2M to 0.27 and 0.26, respectively, for eGFRcr with eGFRB2M (Tables S1B and S2B), indicating stronger correlations of cystatin C and B2M due to non-GFR determinants compared to correlations of creatinine and B2M.
Associations with Age, Sex and Ethnicity
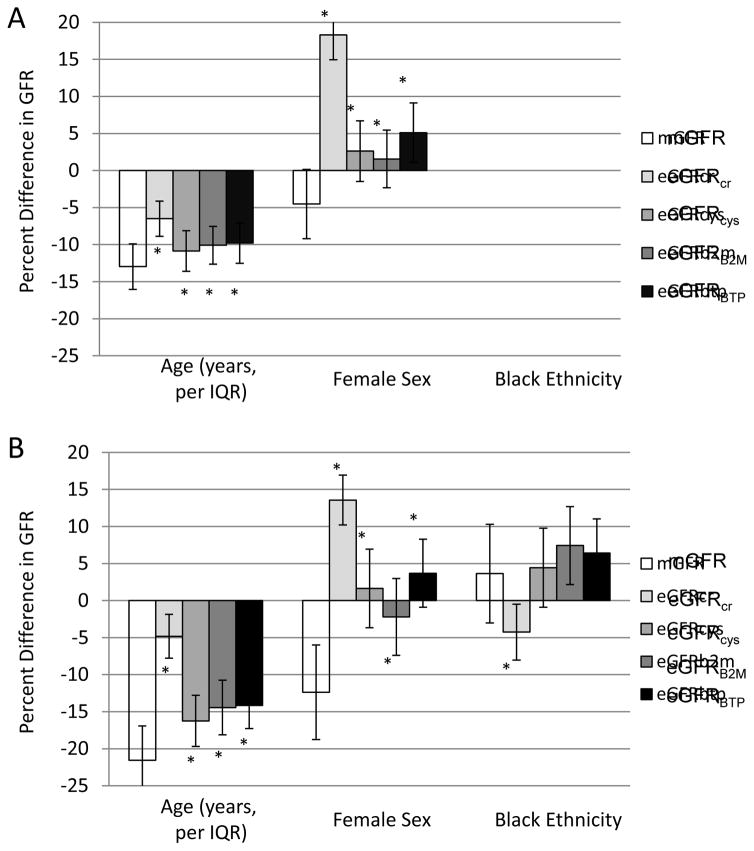
Associations of age, sex and ethnicity with mGFR and each eGFR are shown in Figure 1 and residual associations and their comparisons after accounting for mGFR are shown in Table 2. In both AGES-Kidney and MESA-Kidney, older age and female sex were associated with lower mGFR (Figure 1). Before accounting for mGFR, older age was associated with lower eGFRcr, eGFRcys, eGFRB2M and eGFRBTP (higher serum concentration of all filtration markers) (Figure 1). After accounting for mGFR, older age had a significant positive residual association with each eGFR (Table 2), indicating younger age is associated with lower eGFR (higher serum concentration of filtration markers) after accounting for the association of age with mGFR. Residual associations were significantly smaller for eGFRcys, eGFRB2M and eGFRBTP than for eGFRcr (Table 2). Before accounting for mGFR, female sex was associated with higher eGFRcr only (lower serum creatinine concentration) (Figure 1). After accounting for mGFR, female sex was associated with significant positive residual associations with each eGFR (Table 2), indicating male sex is associated is associated with lower eGFR (higher serum concentration of filtration markers) after accounting for the association of sex with mGFR. Residual associations were significantly smaller for eGFRcys, eGFRB2M and eGFRBTP than for eGFRcr (Table 2).
Figure 1. Unadjusted, study-specific associations of age, sex, and ethnicity with mGFR and eGFR in (A) AGES-Kidney and (B) MESA-Kidney.
Ethnicity estimates not reported in AGES because all participants are white and of Icelandic descent. The difference in the height of the bars for eGFR in comparison to mGFR reflects the residual association with eGFR accounting for mGFR. * p<0.05 vs. mGFR
Table 2.
Unadjusted residual associations of age, sex, and ethnicity with eGFR based on each of the four filtration markers in AGES-Kidney and MESA-Kidney
| Factor | IQR | Residual risk associations after accounting for mGFR | |||
|---|---|---|---|---|---|
| eGFRcr | eGFRcys | eGFRB2M | eGFRBTP | ||
| AGES-Kidney (N=683) | |||||
| Age (year) | 5 | 6.5 (4.5, 8.4) | 2.1 (0.6, 3.6)* | 2.9 (1.3, 4.5)* | 3.2 (1.5, 4.9)* |
| Female (vs. Male) | - | 22.8 (20.4, 25.3) | 7.1 (5.0, 9.3)* | 6.1 (3.7, 8.4)* | 9.6 (7.1, 12.2)* |
| Black (vs. not Black) | NA | - | - | - | - |
| MESA-Kidney (N=275) | |||||
| Age (year) | 15 | 16.7 (12.6, 20.8) | 5.3 (2.1, 8.6)* | 7.1 (3.8, 10.5) * | 7.4 (3.7, 11.1)* |
| Female (vs. Male) | - | 25.9 (21.5, 30.4) | 14.0 (10.5, 17.5)* | 10.2 (6.4, 14.0)* | 16.1 (11.8, 20.3)* |
| Black (vs. not Black) | - | −7.9 (−13.2, −2.5) | 0.8 (−3.1, 4.7)* | 3.8 (−0.2, 7.8) * | 2.8 (−1.9, 7.5)* |
Note: Unadjusted residual associations estimated using stacked generalized estimating equations. Creatinine, cystatin C, BTP, and B2M were transformed to the eGFR scale using study- specific equations based on each serum filtration marker. Associations can be interpreted as a percent difference since mGFR and eGFR are expressed as natural log transformations.
Residual association vs. residual association for eGFRcr p<0.05
In MESA-Kidney, black ethnicity was not associated with lower mGFR (Figure 1). Before accounting for mGFR, black ethnicity was associated with lower eGFRcr and higher eGFRB2M and eGFRBTP. After accounting for mGFR, black ethnicity had a significant negative residual association with eGFRcr but not with eGFRcys, eGFRB2M or eGFRBTP (Table 2), indicating black ethnicity is associated only with lower eGFRcr (higher serum creatinine concentration) after accounting for the association of ethnicity with mGFR. Residual associations of black ethnicity were significantly smaller for eGFRcys, eGFRB2M and eGFRBTP than for eGFRcr (Table 2).
Associations with Clinical Factors
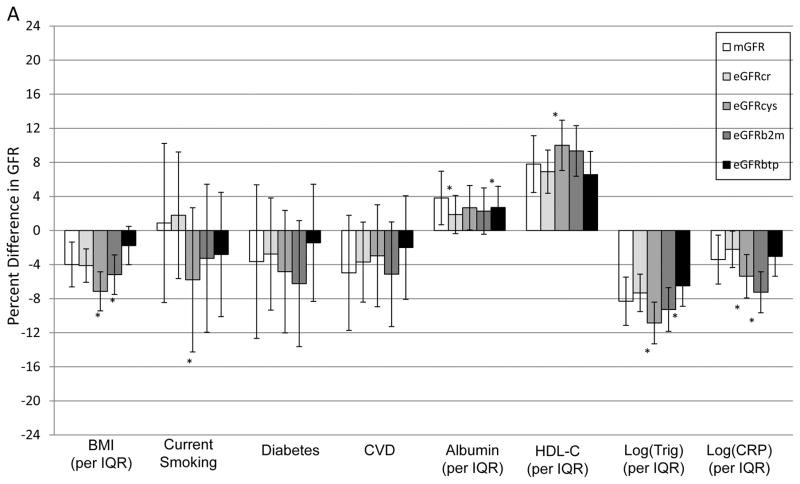
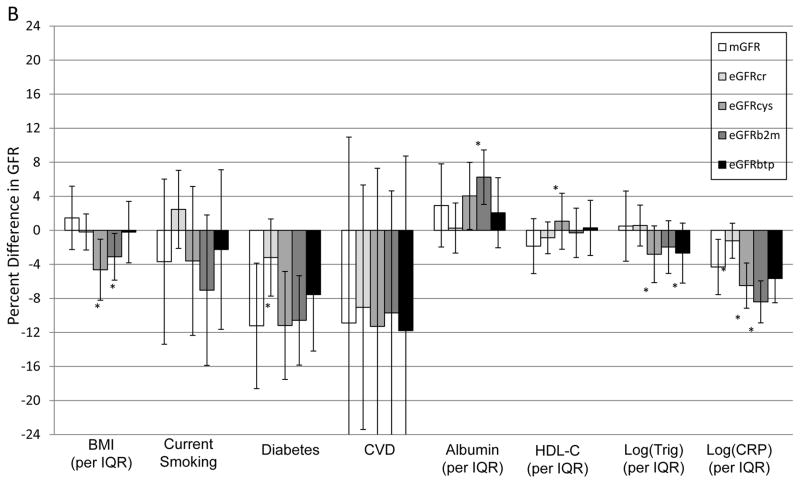
Associations of clinical factors with mGFR and each eGFR after adjustment for age, sex and ethnicity are shown in Figure 2 and residual associations and their comparisons after accounting for mGFR are shown in Table 3. Many factors had residual associations with eGFR using one or more filtration markers, but most associations were smaller than observed with age and sex. A residual association with lower eGFRcr was observed for higher serum albumin in both cohorts; and for non-diabetic status and lower CRP in MESA only. Residual associations with lower eGFRcys were observed for higher BMI, lower HDL cholesterol, higher triglycerides and higher CRP in both cohorts; and with current smoking status in AGES only. Residual associations with lower eGFRB2M were observed for higher CRP in both cohorts; higher serum albumin in AGES-Kidney only; and higher BMI, lower serum albumin and higher triglycerides in MESA-Kidney only. Residual associations with lower eGFRBTP were observed for lower BMI and higher triglycerides in AGES-Kidney only; and lower triglycerides in MESA-Kidney only. No significant residual associations with any eGFR were observed for CVD. Residual associations for eGFRcys, eGFRB2M and eGFRBTP with several factors were significantly different from those observed for eGFRcr.
Figure 2. Study-specific age, sex, and ethnicity-adjusted associations with mGFR and eGFR in (A) AGES-Kidney and (B) MESA-Kidney.
AGES results are age- and sex-adjusted because all participants are white and of Icelandic descent. The difference in the height of the bars for eGFR in comparison to mGFR reflects the residual association with eGFR accounting for mGFR. * p<0.05 vs. mGFR
Table 3.
Age-, sex-, and ethnicity-adjusted residual associations with eGFR based on each of the four filtration markers in AGES-Kidney and MESA-Kidney using stacked generalized estimating equations.
| Factor | IQR | Residual associations after accounting for mGFR | |||
|---|---|---|---|---|---|
| eGFRcr | eGFRcys | eGFRB2M | eGFRBTP | ||
| AGES-Kidney | |||||
| Body Mass Index (kg/m2) | 4.8 | −0.1 (−1.5, 1.3) | −3.2 (−4.4, −1.9) | −1.2 (−2.5, 0.1) | 2.2 (0.7, 3.7) |
| Diabetes (Yes vs. No) | - | 0.9 (−2.8, 4.6) | −1.2 (−5.1, 2.7) | −2.6 (−6.8, 1.6) | 2.2 (−2.6, 7.0) |
| Current Smoking | - | 0.9 (−3.7, 5.5) | −6.7 (−11.8, −1.6)* | −4.1 (−9.5, 1.2) | −3.7 (−9.0, 1.6) |
| Cardiovascular Disease | - | 1.3 (−2.2, 4.7) | 2.0 (−0.9, 4.9) | −0.2 (−3.4, 3.1) | 3.0 (−1.1, 7.0) |
| Albumin (g/dL) | 0.3 | −2.0 (−3.5, −0.4) | −1.2 (−2.9, 0.6) | −1.5 (−3.1, 0.0) | −1.1 (−2.7, 0.5) |
| HDL Cholesterol (mg/dL) | 24 | −0.9 (−2.6, 0.8) | 2.2 (0.5, 3.9)* | 1.5 (−0.2, 3.3)* | −1.2 (−3.2, 0.7) |
| Log triglycerides (mg/dL) | 0.5 | 1.0 (−0.4, 2.3) | −2.5 (−4.0, −1.1)* | −1.0 (−2.5, 0.5)* | 1.8 (0.1, 3.5) |
| Log C-Reactive Protein (g/dL) | 1.2 | 1.2 (−0.3, 2.7) | −2.0 (−3.3, −0.6)* | −3.8 (−5.2, −2.4)* | 0.4 (−1.2, 1.9) |
| MESA-Kidney | |||||
| Body Mass Index (kg/m2) | 7.0 | −1.6 (−4.2, 0.9) | −6.1 (−8.2, −4.0)* | −4.6 (−6.8, −2.3)* | −1.7 (−4.4, 1.1) |
| Diabetes (Yes vs. No) | - | 8.0 (3.7, 12.3) | 0.1 (−3.9, 4.0)* | 0.6 (−3.5, 4.8)* | 3.7 (−1.1, 8.4)* |
| Current Smoking | - | 6.2 (−0.9, 13.3) | 0.1 (−5.6, 5.8) | −3.3 (−10.7, 4.0)* | 1.4 (−7.0, 9.8) |
| Cardiovascular Disease | - | 1.9 (−7.6, 11.3) | −0.4 (−8.8, 8.0) | 1.2 (−5.6, 8.0) | −0.9 (−11.5, 9.7) |
| Albumin (g/dL) | 0.4 | −2.7 (−5.3, 0.0) | 1.1 (−1.3, 3.6)* | 3.3 (0.9, 5.7)* | −0.9 (−3.9, 2.2) |
| HDL Cholesterol (mg/dL) | 19 | 1.0 (−1.0, 3.0) | 2.9 (1.1, 4.8)* | 1.6 (−0.2, 3.4) | 2.1 (−0.3, 4.6) |
| Log triglycerides (mg/dL) | 0.6 | 0.1 (−2.3, 2.5) | −3.3 (−5.6, −1.0)* | −2.5 (−5.0, 0.0)* | −3.2 (−6.0, −0.4)* |
| Log C-Reactive Protein (g/dL) | 1.1 | 3.1 (1.0, 5.2) | −2.2 (−4.2, −0.2)* | −4.1 (−6.1, −2.1)* | −1.3 (−3.7, 1.0)* |
Note: Residual associations estimated using stacked generalized estimating equations. Creatinine, cystatin C, BTP, and B2M were transformed to the eGFR scale using study-specific equations based on each filtration marker. Associations can be interpreted as a percent difference since mGFR and eGFR are expressed as natural log transformations.
Conversion factors for units: serum HDL cholesterol in mg/dL to mmol/L, ×0.02586; serum triglycerides in mg/dL to mmol/L, ×0.01129.
AGES results are age- and sex-adjusted because all participants are white and of Icelandic descent.
Residual association vs. residual association for eGFRcr p<0.05
Discussion
In two community-based cohort studies of predominantly elderly persons, we demonstrate differential residual associations of demographic and clinical factors with serum levels of the LMW protein filtration markers cystatin C, B2M and BTP (expressed as eGFRs) after accounting for mGFR compared to residual associations with the metabolite creatinine. First, we found that age and sex had stronger residual associations with creatinine than with cystatin C, B2M and BTP, consistent with results from previous studies in younger patients with CKD.1, 2 Second, we observed that ethnicity (black vs. white) had no significant residual associations with either cystatin C, B2M or BTP. Third, after accounting for age, sex and ethnicity, we found differential residual associations of some common clinical factors with LMW protein filtration markers, but most associations were smaller than their associations with age and sex. In particular, both cystatin C and B2M, but not BTP, had significant residual associations with CRP in both studies. Neither CVD nor diabetes was associated with cystatin C, B2M or BTP. These findings suggest age, sex and common clinical conditions may affect the non-GFR determinants of LMW serum proteins throughout the range of GFR and have implications for the development and use of GFR estimating equations based on LMW serum proteins.
Creatinine generation by muscle is generally assumed to be the main source of variation in serum levels, independent of GFR. Cystatin C is thought to be less affected by muscle mass than creatinine.11 The smaller residual associations of age, sex and ethnicity with B2M and BTP, as well as cystatin C, compared to creatinine observed in this study are similar to results observed in low GFR populations and suggests that muscle mass has weaker effects on generation of B2M and BTP than on creatinine at higher GFR levels and in the elderly.1, 2, 12 These findings suggest that eGFRB2M and eGFRBTP, similar to eGFRcys, may be more accurate than eGFRcr in patients with alterations in muscle mass, such as limb amputations, eating disorders, neuromuscular disorders or malnutrition.13, 14 On the other hand, significant residual associations of cystatin C and B2M but not creatinine with BMI suggests that eGFRcys and eGFRB2M may be less accurate than eGFRcr in obesity.
Residual associations of age and sex with cystatin C, B2M and BTP were significant and in the same direction as previously reported in patients with CKD.2 Residual associations with ethnicity were not significant, in contrast to the previously reported study in patients with CKD, 13 which showed significantly lower BTP in blacks than whites after adjusting for mGFR.2 The findings regarding cystatin C are consistent with recent GFR estimating equations based on cystatin C, which include coefficients for sex and/or age, but not ethnicity.8, 15–18 The findings regarding B2M and BTP can be compared with GFR estimating equations based on these markers that were developed in CKD populations.12 Coefficients for age and sex were included in the equations based on BTP, but not the equations based on B2M alone or in combination with BTP. Whether coefficients for age and sex are required in estimating equations based on BTP and B2M for use in elderly populations with higher levels of GFR is not yet determined. In CKD populations, a coefficient for ethnicity was not included in the equations based on either BTP or B2M in CKD, and our findings suggest that ethnicity may not be required in elderly populations of blacks and whites with higher levels of GFR. Lack of inclusion of ethnicity in GFR estimating equations could potentially facilitate their use in racial-ethnic groups other than blacks and whites, in geographical regions outside of North America, Europe and Australia, in mixed ethnicity patients and in patients in whom information on ethnicity is not available.19
Conditions affecting the non-GFR determinants of LMW serum proteins are less well characterized than for creatinine. All three LMW serum proteins have multiple biological functions and prior studies have suggested associations with CVD and CVD risk factors, including obesity, smoking, diabetes, inflammation, dyslipidemia and proteinuria independent of mGFR, although the mechanisms for these associations are not understood.1, 10, 20–22 Cystatin C is a 120 amino acid protein cysteine protease inhibitor, which is generated in all nucleated cells, modulates the immune system, has antibacterial and antiviral activities, and modifies the response to brain injury.23 B2M is a 100 amino acid protein component of class I major histocompatibility molecules, which is found on the surface of nucleated cells, and is used clinically as a marker for tumor burden in some lymphoproliferative and plasma cell disorders.24, 25 BTP is a 168 amino acid glycoprotein enzyme, which is produced in the central nervous system, promotes the conversion of prostaglandin H2 to prostaglandin D, and is used clinically as a marker for leakage of cerebrospinal fluid into nasal secretions.26 As previously reported in patients with CKD,2 both cystatin C and B2M had small residual associations with CRP in both AGES-Kidney and MESA-Kidney suggesting that both markers are affected by inflammation throughout the range in GFR, which should be considered in interpreting eGFRcys and eGFRB2M. Other associations, such as the previously reported associations of smoking and BMI with cystatin C,2 were less consistent, but may contribute to errors in eGFR in some patients. Nonetheless, these markers may be useful in combination, as has been shown previously for eGFRcr-cys compared with eGFRcr and eGFRcys, likely because combining markers reduces the influence of conditions affecting the non-GFR determinants of each marker compared with the influence of these conditions when using either marker alone.17, 19, 27, 28 In CKD populations, eGFRB2M, eGFRBTP and in combination were not more accurate than eGFRcr-cys.12 Whether combinations using B2M and BTP can improve GFR estimation further in higher GFR populations has not been determined.
Similar to the prior study in CKD,2 we found moderate to high correlations of GFRB2M and eGFRBTP with mGFR, but after accounting for mGFR, we observed higher correlations between eGFRcys and eGFRB2M than between eGFRcys and eGFRBTP, suggesting more conditions in common which affect the non-GFR determinants of cystatin C and B2M than conditions which affect the non-GFR determinants of cystatin C and BTP. In addition, we found no consistent significant residual associations with BTP in both cohorts. Possibly, factors other than those that we studied may be associated with the non-GFR determinants of BTP to account for the lower correlation with mGFR of eGFRBTP compared with eGFRcys and eGFRB2M. Alternatively, measurement of BTP was less precise than for other LMW serum protein and may have precluded detection of such associations.
Our study has several strengths that warrant mention. AGES-Kidney and MESA-Kidney are drawn from well-characterized elderly community-based cohorts from Iceland and the United States, respectively, allowing evaluation of many important demographic and clinical characteristics that may influence LMW serum filtration marker levels in the elderly. Our study sample includes both white (MESA and AGES) and black (MESA only) participants, increasing the potential generalizability of our findings. We have utilized a statistical approach that takes into account measurement error in our gold standard assessment of mGFR. We have also evaluated each filtration marker on the eGFR scale using single-marker eGFR equations developed within each study rather than relying on current estimating equations. While published GFR estimating equations for creatinine, cystatin C, B2M and BTP are available, equations using creatinine and cystatin C include ethnicity and/or age and sex, which would preclude direct evaluation of these factors; equations using B2M and BTP were developed in CKD populations, leading to the potential for bias if we had used them in these analyses. Our study also has limitations to inferences about the relationships of underlying conditions with non-GFR determinants of the filtration markers. Our cohorts are smaller than the study of Liu et al,2 thus we may not have had sufficient power to detect some associations. Only a small fraction of eligible participants were included, so selection bias must also be considered. Similar to previous studies, our study is cross-sectional, so we could not assess temporality of the observed associations. Not all participants were elderly, and volunteer participants in clinical research are healthier on average than their peers, so our findings may not apply well to frail or institutionalized elders. Filtration markers were measured in previously frozen, single samples collected during each study’s respective examination. As an observational study, there is always potential for residual confounding that would influence the magnitude of observed associations.
In summary, in a cross-sectional study drawn from two elderly community-based cohorts, we showed that the non-GFR determinants of cystatin C, B2M and BTP are less affected by age and sex than those of creatinine and are unaffected by ethnicity. After adjusting for age, sex and ethnicity, the non-GFR determinants of both cystatin C and B2M are more affected by markers of adiposity, dyslipidemia, and inflammation than creatinine, but many of the effects appear to be small. The non-GFR determinants of BTP may be less affected by these factors than by cystatin C and B2M. The appropriate use of B2M and BTP in GFR estimating equations requires further study.
Supplementary Material
Supplementary Table S1 (PDF). Pearson and partial Pearson correlation coefficients adjusted for mGFR in AGES-Kidney.
Supplementary Table S2 (PDF). Pearson and partial Pearson correlation coefficients adjusted for mGFR in MESA-Kidney.
Acknowledgments
We acknowledge assistance of Andrew L. Simon, ScM in manuscript preparation.
Support: AGES-Kidney was supported by a grant from National Institutes of Health (R01 DK082447), contract from the National Institute on Aging (N01-AG-1-2100 and HHSN27120120022C), the Icelandic Heart Association (Hjartavernd) and the Icelandic Parliament (Althingi), and the American Recovery Act (3R01DK082447-01A1S1). MESA-Kidney was supported by R01DK087961. MESA was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. CKD-EPI was supported by NIH grant R01DK097020. This work was supported by a research grant from Dialysis Clinic, Inc. (DCI Research Grant #S-2607). The funding agencies had no role in the study design; collection, analysis and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure: Drs Levey and Inker report funding to Tufts Medical Center for research and contracts with the NIH, National Kidney Foundation, Amgen, Pharmalink AB, Gilead Sciences, Siemens, and has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014 –“Precise estimation of glomerular filtration rate from multiple biomarkers” PCT/US2015/044567. The technology is not licensed in whole or in part to any company. Tufts Medical Center, John Hopkins University and Metabolon Inc have a collaboration agreement to develop a product to estimate GFR from a panel of markers. Dr Shafi reports consulting fees from Siemens. Gary F. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, Servier and Philips Healthcare and is funded by research grants from Novartis and the National Institutes of Health. Dr Shlipak receives funding from the NIH and serves as an Advisor to Cricket Health and to Tai Diagnostics. The remaining authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: MF, ASL, LAI, MGS; Data acquisition: AO, TS; Data analysis/interpretation: MF, ASL, LAI, TS, LF, VG, RK, GFM, RP, WSP, MGS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Table S1. Pearson and partial Pearson correlation coefficients adjusted for mGFR in AGES-Kidney.
Table S2. Pearson and partial Pearson correlation coefficients adjusted for mGFR in MESA-Kidney.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney international. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Foster MC, Tighiouart H, et al. Non-GFR Determinants of Low Molecular Weight Serum Protein Filtration Markers in CKD [Google Scholar]
- 3.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inker LA, Okparavero A, Tighiouart H, et al. Midlife Blood Pressure and Late-Life GFR and Albuminuria: An Elderly General Population Cohort. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 6.Inker LA, Shafi T, Okparavero A, et al. Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. 2016;68:743–751. doi: 10.1053/j.ajkd.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Inker LA, Shafi T, Okparavero A, et al. Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367:s20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clinical chemistry and laboratory medicine. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 10.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney international. 2013;83:1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filler G, Bökenkamp A, Hoffmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis. 2016;67:40–48. doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurlow JS, Abbott KC, Linberg A, Little D, Fenderson J, Olson SW. SCr and SCysC concentrations before and after traumatic amputation in male soldiers: a case-control study. Am J Kidney Dis. 2014;63:167–170. doi: 10.1053/j.ajkd.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Little DJ, Yuan CM, Thurlow JS, et al. Effects of Traumatic Amputation on beta-Trace Protein and beta2-Microglobulin Concentrations in Male Soldiers. Am J Nephrol. 2015;42:436–442. doi: 10.1159/000443775. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Annals of internal medicine. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 18.Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clinical chemistry. 2014;60:974–986. doi: 10.1373/clinchem.2013.220707. [DOI] [PubMed] [Google Scholar]
- 19.Teo BW, Xu H, Wang D, et al. Estimating glomerular filtration rates by use of both cystatin C and standardized serum creatinine avoids ethnicity coefficients in Asian patients with chronic kidney disease. Clinical chemistry. 2012;58:450–457. doi: 10.1373/clinchem.2011.172346. [DOI] [PubMed] [Google Scholar]
- 20.Mathisen UD, Melsom T, Ingebretsen OC, et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol. 2011;22:927–937. doi: 10.1681/ASN.2010050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney international. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 22.Keddis MT, Amer H, Voskoboev N, Kremers WK, Rule AD, Lieske JC. Creatinine-Based and Cystatin C-Based GFR Estimating Equations and Their Non-GFR Determinants in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2016;11:1640–1649. doi: 10.2215/CJN.11741115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon GM, Waikar SS. Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62:165–178. doi: 10.1053/j.ajkd.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schardijn GH, Statius van Eps LW. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney international. 1987;32:635–641. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]
- 25.Ploegh HL, Orr HT, Strominger JL. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981;24:287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- 26.White CA, Ghazan-Shahi S, Adams MA. beta-Trace Protein: A Marker of GFR and Other Biological Pathways. Am J Kidney Dis. 2015;65:131–146. doi: 10.1053/j.ajkd.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney international. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 28.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney international. 2007;72:1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 (PDF). Pearson and partial Pearson correlation coefficients adjusted for mGFR in AGES-Kidney.
Supplementary Table S2 (PDF). Pearson and partial Pearson correlation coefficients adjusted for mGFR in MESA-Kidney.