Abstract
Adenoviruses are respiratory, ocular and enteric pathogens that form complex capsids, which are assembled from seven different structural proteins and composed of several core proteins that closely interact with the packaged dsDNA genome. The recent near-atomic resolution structures revealed that the interlacing continuous hexagonal network formed by the protein IX (IX) molecules is conserved among different human adenoviruses (HAdVs), but not in non-human adenoviruses (non-HAdVs). In this report we propose a distinct role for the hexon protein as a “molecular mold” in enabling the formation of such hexagonal IX-network that has been shown to preserve the stability, infectivity of HAdVs.
Keywords: Adenovirus structure, hexon, protein IX, molecular mold, protein network
Graphical abstract
Adenoviruses (AdVs) are large, complex and nonenveloped dsDNA viruses that cause respiratory, ocular and gastroenteric infections in humans and various vertebrate species [1, 2]. Additionally, AdVs top the list of reagents that are being used as gene and vaccine delivery vectors in the clinic [3, 4]. The details of structural and organizational diversity of the capsid proteins in AdV virions provide better understanding of the protein-protein interactions that stabilize the capsid, insights into AdV assembly as well as suggest ways to affect their cell targeting specificities.
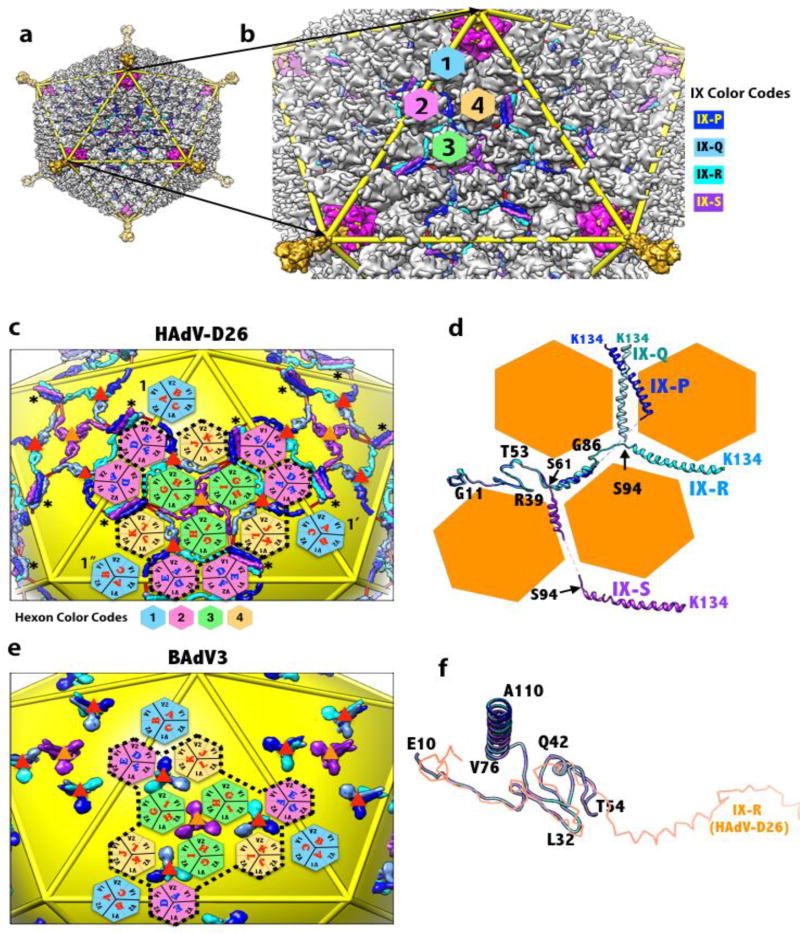
The adenovirus capsid is formed by 3 major proteins (hexon, penton base and fiber) and 4 minor proteins (IIIa, VI, VIII, IX) (Fig. 1a, b) [5]. In addition, several core proteins that associate with the dsDNA genome are packaged together on the capsid interior. Based on the recent near-atomic resolution structures of human adenoviruses (HAdVs) [6, 7], it is apparent that the interactions between the hexon trimers (termed hexons) are mediated by the association of minor proteins. In turn the hexons play an important role in the organization of the minor proteins, in particular of the outer minor protein IX (IX) composed of 110–140 residues and present in 240 copies (Fig. 1b, c). Of note, IX is uniquely present (conserved) in primate adenoviruses that belong to the genus Mastadenovirus in the family Adenoviridae [8]. While the N-terminal triskelion-forming region of IX (NTT; residues 1–62 in HAdV-D26) and the C-terminal coiled-coil forming region (CTC; residues 95–134 in HAdV-D26) are highly conserved, the length and composition of the linker region connecting the two signature structural elements of IX differs between the human and nonhuman AdVs (non-HAdVs) [9–11] (Supplementary Fig. 1). Consistent with the previous observations [9–11], we suggest that the presence or absence of a (~20 aa) stretch of Ala and Ser residues in the linker region appears to determine whether or not the C-termini of IX molecules form the characteristic tetrameric coiled-coils seen in HAdVs or trimeric coiled-coils in non-HAdVs. The structural superposition of the conserved NTT regions of four unique IX molecules in HAdVs revealed two locations (S61 and S94) at which the linker region bends at an angle of 120°, in congruence with the corners of the hexagonal-shaped base of the hexon subunits (Fig 1d). Here, we propose that the hexon subunits are responsible for enforcing such elbow shaped bends in the linker region of IX in compliance with their organization on a pseudo T=25 icosahedral lattice (Fig. 1b). The four structurally unique IX molecules (P, Q, R and S) in HAdVs display distinct and extended conformations that result in the formation of similar trimeric triskelions, but tetrameric coiled-coils located at the boundaries of group of nine hexons (GONs), approximately tangential to the virion surface (Fig. 1c, d, Supplementary Fig. 2b). In contrast, the singular conformation displayed by the four structurally distinct IX molecules in non-HAdVs results in the formation of compact and equivalent IX-trimers comprising intertwining triskelions at the bottom capped with protruding (vertical) trimeric coiled-coils (Fig. 1e, f, Supplementary Fig. 2a) [10]. Remarkably, the tetrameric coiled-coils, also known as 4-helix bundle (4-HLXB), seen in HAdVs are composed of 3 parallel helices, one each from three IX molecules (Q, R and S) that originate from the same facet and an anti-parallel helix from IX-P (dark blue) that comes from the neighboring facet (Fig. 1c) [6, 7, 12]. Of note, the 4-HLXB is formed by the four structurally unique IX molecules that belong to four different IX-trimers that are located at spatially distinct locations on the AdV capsid (Fig. 1c). The icosahedral symmetry related copies of IX (4x60=240) in HAdVs form a continuous interlacing hexagonal IX network, which not only stabilizes the hexons within the GON facets through the triskelion structures, but also extends over and straps the GON-facets together, thereby stabilizing the whole virion, via the 4-HLXBs (Fig. 1c) [6]. Whereas the compact IX-trimers seen in non-HAdVs stabilize the hexons locally within the GON-facets (Fig. 1e) [10].
Figure 1.
Organization of protein IX (IX) molecules in human and non-human adenoviruses. a) Organization of capsid proteins in HAdV-D26, a view down the icosahedral 3-fold axis, derived from the cryo-EM structure of human adenovirus D26 (HAdV-D26) [6]. Hexon, penton base and fiber subunits are shown in gray, magenta and gold colors respectively. Icosahedral cage is shown as yellow rods. b) A zoomed-in view of part of the HAdV capsid. The network formed by the outer minor capsid protein IX is shown in surface/tube representation as indicated by the color scheme shown on the right (P, blue; Q, light blue; R, cyan and S, purple). Of note, only the IX-R molecule is ordered in full. The disordered linker regions in P, Q and S molecules are shown as red colored ribbons. The four unique hexons (1–4) are identified by differently colored hexagons. c) A zoomed-in view of the continuous hexagonal network of the IX molecules in HAdV-D26 is shown on top of an icosahedron (yellow). The four structurally distinct IX molecules are shown in the same differently colored surface/tube representations indicated in panel b. The locations of the hexon trimers within the facet are identified differently colored hexagons according to the color scheme shown at the bottom. In addition, the locations of the constituent subunits of each hexon (e.g., A, B and C) and corresponding V1 and V2 jelly roll β-barrel domains are identified. The same colored IX molecules and hexons (hexagons) are related to each other by the icosahedral symmetry. The IX-triskelions located at the quasi (local) 3-fold (Q3-IX) and icosahedral (strict) 3-fold (I3-IX) axes are identified by red and orange triangles, respectively. The locations of the four helical bundles (4-HLXB) are identified by the asterisks. The boundary depicted by the black dashed line circumscribes the GON (group of nine hexons) in the icosahedral facet. d) Superposition of 4 structurally distinct IX molecules (P, Q, R and S) in HAdV-D26 highlights the similarity in the N-terminal triskelion (NTT) forming regions and differences in the linker and CTC (C-terminal coiled-coil) regions. The disordered linker regions are identified by the dotted lines. It is striking to notice that the angle of deviation between different segments, identified by black arrows, is ~120° in congruence with the corners of the hexon bases that resemble the shape of hexagons. The angle of deviation for the CTC of the IX-P molecule (blue) that forms the anti-parallel helix appears to be different from the rest of CTCs. Selected residues relative to the bifurcation points are labeled. e) Organization of IX molecules in the bovine adenovirus type 3 (BAdV3) (PDB-ID: 3ZIF) [10]. The compact and segregated arrangement of IX molecules in BAdV3 is apparent. The black dashed line circumscribes the GON in the icosahedral facet. The IX-trimers located at the quasi (local) 3-fold (Q3-IX) and icosahedral (strict) 3-fold (I3-IX) axes are identified by red and orange triangles, respectively f) Superposition of the 4 structurally distinct IX molecules in BAdV3 illustrates identical conformations. Selected residues of the protein IX in BAdV3 are identified (in black). For comparison, one of the IX molecules (R) of HAdV-D26 is shown in backbone trace representation (orange). Different diagrams in the figure were generated using the graphics program, UCSF Chimera [18].
The hexagonal IX-network observed in HAdVs is formed by two distinct IX-trimers with their NTTs (triskelions) located at the local 3-fold axis (Q3-IX) and at the icosahedral 3-fold axis (I3-IX), respectively. Even though the conformation and organization of the NTTs are nearly identical between the two types of IX-timers, the linker regions and the CTCs are arranged differently among the 4 structurally distinct IX molecules, which are likely influenced by the hexagonal shaped base of the hexon subunits (Fig. 1d, Supplementary Fig. 2b). While both kinds of IX-triskelions of the I3-IX and Q3-IX trimers are located at the 3-fold junctions formed by the three V2 barrels of the hexon subunits, the 120° elbow bends occur at two distinct 3-fold junctions. The first elbow bend immediately following the triskelion structures always occurs at the 3-fold junction formed by the three V1 barrels, while the second bend just before the CT-helix occurs at the junction involving two V2 domains and one V1 domain (Fig. 1c). It is noteworthy that the linker regions of the IX molecules Q and S pass through the same 3-fold junction formed by the V1 domains of the hexons 3, 3’ (symmetry related) and 4 (Fig. 1c). The linker helix of IX-Q passes at ~10Å higher radius than its counterpart in IX-S.
Furthermore, the 3-fold related interfaces (not junctions) of hexons that interact with the triskelions and linker regions of IX are formed by the V1 and V2 barrels from the same subunit on one side while the V1 and V2 domains from two adjacent subunits on the opposite side (Fig. 1c). However, the interface, where the coiled-coils (4-HLXB) located is formed by V1 and V2 barrels that belong to two adjacent hexon subunits on both sides of the interface, which are related by local 2-fold axis. This organization appears to be true for any 2-fold related interface in the AdV capsid (Fig 1c). Notably, the inter-hexon interfaces where the V1 and V2 barrels from the same subunit line up on either side of an interface are found at the GON-GON interfaces, but not within GON-facet.
In addition, while the Q3-IX forming molecules (P, Q, and R) differ from each other only in the arrangement of the CTCs, the linker regions of the I3-IX forming S-molecules make a 120° bend from the linker regions of the P, Q and R molecules (Fig. 1d). In contrast, the different IX-molecules in non-HAdVs display identical conformations and abide by near perfect 3-fold symmetry, upholding the principles of quasi-equivalence (Fig. 1f, Supplementary Fig. 2a) [13]. Whereas only the NTT regions of the IX-molecules in HAdVs obey the quasi 3-fold symmetry, while the linker regions and CTCs deviate significantly from any such symmetry compliance (Fig. 1d, Supplementary Fig. 2b).
The unique arrangement of the CTCs (homo polypeptides) of IX in the formation of the 4-HLXB with 3 parallel and 1 anti-parallel is remarkable and is directed by their close association with the hexon subunits. In spite of minor differences in the packing of helices in 4-HLXB, the overall arrangement of helices – 3 parallel and 1 anti-parallel – appears to be conserved among different HAdVs [6]. These 4-HLXB structures are indeed responsible for forming the continuous hexagonal network of IX molecules in HAdVs by linking the IX-trimers within the facet with those in the neighboring facets primarily through non-covalent leucine zipper interactions between the C-terminal helices [6, 7, 14, 15]. Of note, the IX-network in HAdVs primarily stabilizes the 9 hexons that represent the GON-facet and also mediates interactions between the adjacent GONs (Fig. 1c), while the peripentonal hexons (1, 1’ and 1”) are stabilized by the proteins IIIa and VIII on the capsid interior (not shown). Remarkably, the IX-molecules – R and P – of the network together with the 4-HLXB and their 2-fold related counterparts bring the two symmetry related hexon-2s (pink hexagons), located at the icosahedral 2-fold axis, into unique “tight” association that is different from any two neighboring hexons in the HAdV capsid, tying them together (Fig. 1c). The continuous IX hexagonal network though dispensable for the formation of HAdV capsids, it is known to significantly stabilize the HAdV capsids [16, 17]. All things being equal, based on the noted differences in the organization and interactions of the IX molecules, we hypothesize that HAdVs could potentially be more stable than non-HAdVs.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01AI070771 and R21AI103692.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wold WSM, Horwitz MS. Fields virology. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. Adenoviruses. [Google Scholar]
- 2.Gordon YJ, Aoki K, Kinchington PR. Adenovirus keratoconjunctivitis. St. Louis, PA: Mosby; 1996. [Google Scholar]
- 3.Schenk-Braat EA, van Mierlo MM, Wagemaker G, Bangma CH, Kaptein LC. An inventory of shedding data from clinical gene therapy trials. J Gene Med. 2007;9:910–21. doi: 10.1002/jgm.1096. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H. Adenovirus vectormediated gene transfer into stem cells. Mol Pharm. 2006;3:95–103. doi: 10.1021/mp0500925. [DOI] [PubMed] [Google Scholar]
- 5.San Martin C. Latest insights on adenovirus structure and assembly. Viruses. 2012;4:847–77. doi: 10.3390/v4050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Veesler D, Campbell MG, Barry ME, Asturias FJ, Barry MA, et al. Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci Adv. 2017;3:e1602670. doi: 10.1126/sciadv.1602670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Jin L, Koh SB, Atanasov I, Schein S, Wu L, et al. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329:1038–43. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrach B, Benko M, Both GW, Brown M, Davison AJ, Echavarria M, et al. 9th Report of the International Committee on Taxonomy of Viruses. ed. London, UK: Academic Press, Elsevier; 2012. Adenoviridae. [Google Scholar]
- 9.Schoehn G, El Bakkouri M, Fabry CM, Billet O, Estrozi LF, Le L, et al. Three-dimensional structure of canine adenovirus serotype 2 capsid. Journal of virology. 2008;82:3192–203. doi: 10.1128/JVI.02393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L, Huang X, Li X, Xiong W, Sun W, Yang C, et al. Cryo-EM structures of two bovine adenovirus type 3 intermediates. Virology. 2014;450–451:174–81. doi: 10.1016/j.virol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Hackenbrack N, Rogers MB, Ashley RE, Keel MK, Kubiski SV, Bryan JA, et al. Evolution and Cryo-electron Microscopy Capsid Structure of a North American Bat Adenovirus and Its Relationship to Other Mastadenoviruses. Journal of virology. 2017;91 doi: 10.1128/JVI.01504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of alpha-helices in a 6- angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. Journal of virology. 2006;80:12049–59. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspar DLD, Klug A. Physical principles in the construction of regular viruses. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 14.Rosa-Calatrava M, Grave L, Puvion-Dutilleul F, Chatton B, Kedinger C. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. Journal of virology. 2001;75:7131–41. doi: 10.1128/JVI.75.15.7131-7141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vellinga J, van den Wollenberg DJ, van der Heijdt S, Rabelink MJ, Hoeben RC. The coiled-coil domain of the adenovirus type 5 protein IX is dispensable for capsid incorporation and thermostability. Journal of virology. 2005;79:3206–10. doi: 10.1128/JVI.79.5.3206-3210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colby WW, Shenk T. Adenovirus type 5 virions can be assembled in vivo in the absence of detectable polypeptide IX. Journal of virology. 1981;39:977–80. doi: 10.1128/jvi.39.3.977-980.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol Ther. 2004;9:942–54. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.